Medication Event Monitoring System for Infectious Tuberculosis Treatment in Morocco: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morocco Guidelines for Tuberculosis Treatment
2.2. Smart Pillbox and Medication Event Monitoring System with E-Promms
2.3. Operation Oversight and Study Population
2.4. Definition of Treatment Outcomes
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Participants
3.2. Comparison of Treatment Results between the Medication Event Monitoring System Group and Control Group
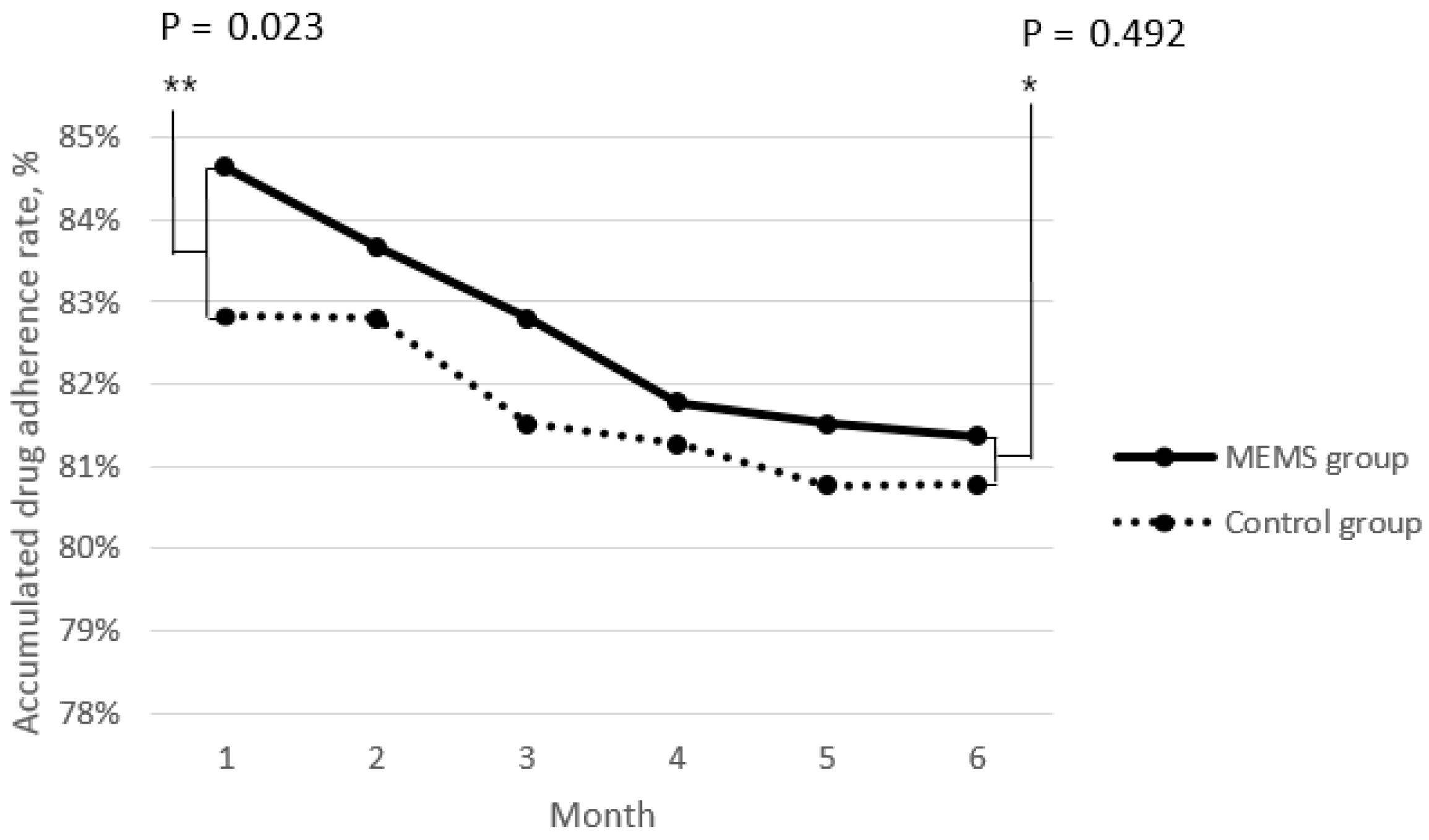
3.3. Time Series Analysis of the Efficacy of Medication Event Monitoring System
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2015; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/191102/9789241565059_eng.pdf?sequence=1&isAllowed=y (accessed on 9 August 2018).
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official american thoracic society/centers for disease control and prevention/infectious diseases society of america clinical practice guidelines: Treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef] [PubMed]
- Alistair, S.; Malcolm, C. Tuberculosis Case Management and Cohort Review; Royal College of Nursing: London, UK, 2012; Available online: https://www.rcn.org.uk/professional-development/publications/pub-004204 (accessed on 9 August 2018).
- World Health Organization. Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care (2017 Update); World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf;jsessionid=E5261E6AB1691ADF8FC18FE4D2D9B92D?sequence=1 (accessed on 9 August 2018).
- Lipsitch, M.; Levin, B.R. Population dynamics of tuberculosis treatment: Mathematical models of the roles of non-compliance and bacterial heterogeneity in the evolution of drug resistance. Int. J. Tuberc. Lung Dis. 1998, 2, 187–199. [Google Scholar] [PubMed]
- Mundra, A.; Deshmukh, P.R.; Dawale, A. Magnitude and determinants of adverse treatment outcomes among tuberculosis patients registered under revised national tuberculosis control program in a tuberculosis unit, wardha, central india: A record-based cohort study. J. Epidemiol. Glob. Health 2017, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, G. Survival analysis and risk factors for death in tuberculosis patients on directly observed treatment-short course. Indian J. Med. Sci. 2009, 63, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Breen, R.A.; Miller, R.F.; Gorsuch, T.; Smith, C.J.; Schwenk, A.; Holmes, W.; Ballinger, J.; Swaden, L.; Johnson, M.A.; Cropley, I.; et al. Adverse events and treatment interruption in tuberculosis patients with and without hiv co-infection. Thorax 2006, 61, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Kizub, D.; Ghali, I.; Sabouni, R.; Bourkadi, J.E.; Bennani, K.; El Aouad, R.; Dooley, K.E. Qualitative study of perceived causes of tuberculosis treatment default among health care workers in Morocco. Int. J. Tuberc. Lung Dis. 2012, 16, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Morocco Tuberculosis Profile. Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=MA&LAN=EN&outtype=html (accessed on 9 August 2018).
- Mahmud, N.; Rodriguez, J.; Nesbit, J. A text message-based intervention to bridge the healthcare communication gap in the rural developing world. Technol. Health Care 2010, 18, 137–144. [Google Scholar] [PubMed]
- Denkinger, C.M.; Grenier, J.; Stratis, A.K.; Akkihal, A.; Pant-Pai, N.; Pai, M. Mobile health to improve tuberculosis care and control: A call worth making. Int. J. Tuberc. Lung Dis. 2013, 17, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sinkou, H.; Hurevich, H.; Rusovich, V.; Zhylevich, L.; Falzon, D.; de Colombani, P.; Dadu, A.; Dara, M.; Story, A.; Skrahina, A. Video-observed treatment for tuberculosis patients in belarus: Findings from the first programmatic experience. Eur. Respir. J. 2017, 49, 49. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, N.; Jarlsberg, L.; Miller, C.; Linh, N.N.; Falzon, D.; Jaramillo, E.; Nahid, P. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018, 15, e1002595. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Christrup, L.L.; Fabricius, P.E.; Chrostowska, M.; Wronka, M.; Narkiewicz, K.; Hansen, E.H. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: A randomized controlled trial. J. Hypertens. 2010, 28, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Broomhead, S.; Mars, M. Retrospective return on investment analysis of an electronic treatment adherence device piloted in the northern cape province. Telemed. J. E Health 2012, 18, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lewis, J.J.; Zhang, H.; Lu, W.; Zhang, S.; Zheng, G.; Bai, L.; Li, J.; Li, X.; Chen, H.; et al. Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients: A cluster-randomised trial. PLoS Med. 2015, 12, e1001876. [Google Scholar] [CrossRef] [PubMed]
- Tachfouti, N.; Slama, K.; Berraho, M.; Elfakir, S.; Benjelloun, M.C.; El Rhazi, K.; Nejjari, C. Determinants of tuberculosis treatment default in Morocco: Results from a national cohort study. Pan Afr. Med. J. 2013, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé. Manuel de Reference du Systeme D’information Sanitaire du Programme National de Lutte Antituberculeuse. Available online: http://www.sante.gov.ma/Documents/2016/01/Manuel%20de%20r%C3%A9f%C3%A9rence%20du%20SIS%20du%20PNLAT%20v%2013%20janv%202016.pdf (accessed on 9 August 2018).
- Camacho Rodriguez, J.C.; Staubert, S.; Lobe, M. Automated import of clinical data from hl7 messages into openclinica and transmart using mirth connect. Stud. Health Technol. Inform. 2016, 228, 317–321. [Google Scholar] [PubMed]
- Brubaker, C.; Jana, S.; Ray, B.; Khurshid, S.; Shmatikov, V. Using frankencerts for automated adversarial testing of certificate validation in ssl/tls implementations. IEEE Secur. Priv. 2014, 2014, 114–129. [Google Scholar] [PubMed]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis–2013 Revision; World Health Organization: Geneva, Switzerland, 2013; Available online: http://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf?sequence=1 (accessed on 9 August 2018).
- Bruxvoort, K.; Festo, C.; Cairns, M.; Kalolella, A.; Mayaya, F.; Kachur, S.P.; Schellenberg, D.; Goodman, C. Measuring patient adherence to malaria treatment: A comparison of results from self-report and a customised electronic monitoring device. PLoS ONE 2015, 10, e0134275. [Google Scholar] [CrossRef] [PubMed]
- Oshd Department; African Development Bank. Medical Coverage Reform Support Programme—Phase 3 (Parcoum III)—Appraisal Report. Available online: https://www.afdb.org/en/documents/document/morocco-medical-coverage-reform-support-programme-phase-3-parcoum-iii-appraisal-report-45471/ (accessed on 9 August 2018).
- Kolifarhood, G.; Khorasani-Zavareh, D.; Salarilak, S.; Shoghli, A.; Khosravi, N. Spatial and non-spatial determinants of successful tuberculosis treatment outcomes: An implication of geographical information systems in health policy-making in a developing country. J. Epidemiol. Glob. Health 2015, 5, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Curry International Tuberculosis; Centers for Disease Control and Prevention (U.S.); California Governor; California Health and Human Services Agency; California Department of Public Health. Drug-Resistant Tuberculosis: A Survival Guide for Clinicians; Curry International Tuberculosis Center: San Francisco, CA, USA, 2016.
- World Health Organization. Who Treatment Guidelines for Drug-Resistant Tuberculosis 2016 Update; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 266–267. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 141) | MEMS Group (n = 206) | p Value | |
|---|---|---|---|
| Age (mean, SD) | 35.74 (16.3) | 36.70 (14.9) | 0.5772 |
| Male gender (n, %) | 97 (68.8%) | 147 (71.4%) | 0.6936 |
| Patients with sputum AFB smear +ve (n, %) | 141 (100%) | 206 (100%) | |
| Treatment type | |||
| New case, category I (n, %) | 141 (100%) | 206 (100%) | 0.488 |
| Total follow-up days (mean, SD) | 175.2 (29.4) | 173.4 (18.7) | 0.488 |
| Total medication days, (mean, SD) | 141.87 (29.5) | 140.85 (17.9) | 0.7147 |
| Drug adherence rate, (mean, SD) | 80.77 (9.2) | 81.35 (6.8) | 0.525 |
| Numbers of patients who belong to treatment success (n, %) | 112 (79.5%) | 191 (93.2%) | <0.001 |
| Treatment outcome | |||
| Cured (n, %) | 61 (43.3%) | 128 (62.1%) | 0.001 |
| Treatment completed (n, %) | 51 (36.2%) | 64 (31.1%) | 0.321 |
| Treatment failed (n, %) | 3 (2.1%) | 0 (0.0%) | 0.066 |
| Died (n, %) | 2 (1.4%) | 1 (0.5%) | 0.569 |
| Lost to follow-up (n, %) | 15 (10.6%) | 1 (0.5%) | <0.001 |
| Not evaluated (n, %) | 9 (6.4%) | 12 (5.8%) | 0.831 |
| Treatment success (n, %) | 112 (79.5%) | 192 (93.2%) | <0.001 |
| Variable | Odds Ratio | 95% CI | p Value | |
|---|---|---|---|---|
| Treatment success | MEMS | 4.33 | 2.13–8.81 | <0.001 |
| Controls | Ref. | |||
| Sex, Male | 0.49 | 0.22–1.10 | 0.085 | |
| Age | 0.99 | 0.98–1.02 | 0.657 | |
| Health centers | 0.90 | 0.83–0.98 | 0.012 | |
| Lost to follow-up | MEMS | 0.03 | 0.05–0.24 | 0.001 |
| Controls | Ref. | |||
| Sex, Male | 8.58 | 1.07–68.45 | 0.043 | |
| Age | 1.01 | 0.97–1.04 | 0.825 | |
| Health Centers | 1.11 | 0.97–1.27 | 0.128 | |
| Variable | Drug Adherence Rate (%) Estimate | 95% CI | p Value |
|---|---|---|---|
| MEMS | 1.02 | 0.40–1.66 | 0.002 |
| Controls | Ref. | ||
| Age | −0.02 | −0.04–0.002 | 0.077 |
| Time, Month | −0.62 | −0.80–−0.44 | <0.001 |
| Gender (Male) | 0.86 | 0.18–1.55 | 0.014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Sentissi, I.; Gil, S.J.; Park, W.-S.; Oh, B.; Son, A.R.; Kong, Y.J.; Park, S.; Paek, E.; Park, Y.J.; et al. Medication Event Monitoring System for Infectious Tuberculosis Treatment in Morocco: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 412. https://doi.org/10.3390/ijerph16030412
Park S, Sentissi I, Gil SJ, Park W-S, Oh B, Son AR, Kong YJ, Park S, Paek E, Park YJ, et al. Medication Event Monitoring System for Infectious Tuberculosis Treatment in Morocco: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(3):412. https://doi.org/10.3390/ijerph16030412
Chicago/Turabian StylePark, Seup, Ilham Sentissi, Seung Jae Gil, Won-Seok Park, ByungKwon Oh, Ah Reum Son, Young Ju Kong, Sol Park, Eunseong Paek, Yong Joon Park, and et al. 2019. "Medication Event Monitoring System for Infectious Tuberculosis Treatment in Morocco: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 16, no. 3: 412. https://doi.org/10.3390/ijerph16030412






