The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after nearly Two Decades of Epidemiological Research?
Abstract
:1. Introduction
2. Bidirectionality
2.1. Children
2.2. Middle-Aged and Older Adults
3. Objective vs. Subjective Sleep Duration
3.1. Brief Overview
3.2. Subjective Sleep Duration
3.3. Objective Sleep Duration
3.4. Verdict on Subjective vs. Objective Methods
4. Size, Consistency and Clinical Significance of Effects
4.1. Size and Consistency of Effects
4.1.1. Children
4.1.2. Adults and Older Adults
4.2. Clinical Significance of Effects
5. Causality
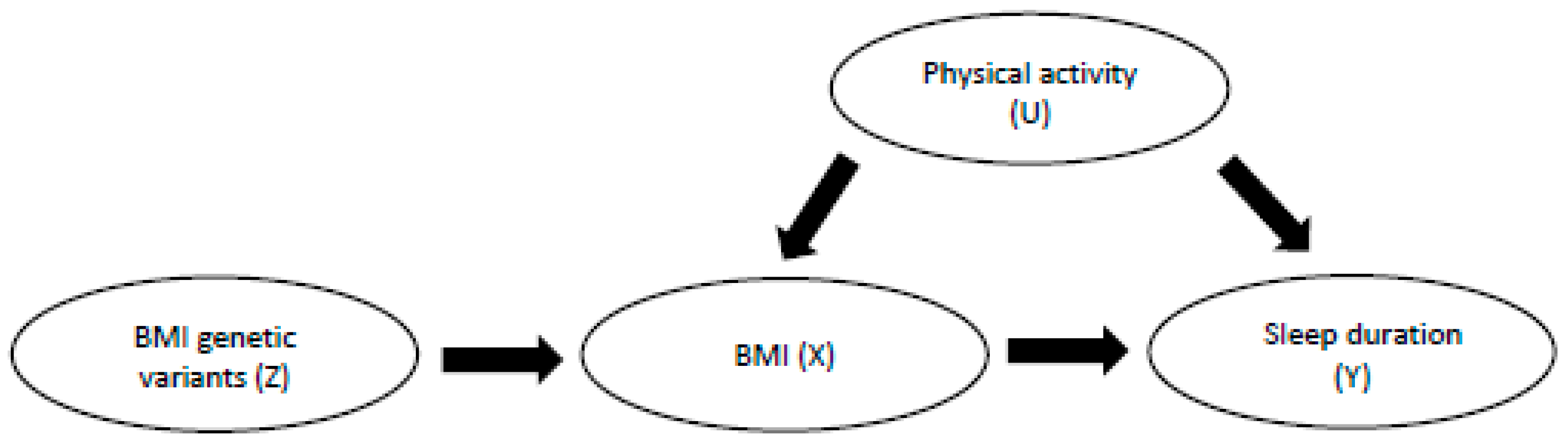
5.1. Findings from Mendelian Randomisation (MR) Studies
5.2. Findings from Experimental Studies
5.3. Consensus on Causality
6. Conclusions
Funding
Conflicts of Interest
References
- Vioque, J.; Torres, J.; Quiles, A. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int. J. Obes. 2000, 24, 1683–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Beydoun, M.A.; Wang, Y. Is Sleep Duration Associated With Childhood Obesity? A Systematic Review and Meta-analysis. Obesity 2008, 16, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity (Silver Spring) 2008, 16, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, A.; Li, L. Sleep duration and overweight/obesity in children: Review and implications for pediatric nursing. J. Spec. Pediatr. Nurs. 2012, 17, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.A.R.; Mamun, A.A. Longitudinal impact of sleep on overweight and obesity in children and adolescents: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2015, 16, 137–149. [Google Scholar] [CrossRef]
- Ruan, H.; Xun, P.; Cai, W.; He, K.; Tang, Q. Habitual Sleep Duration and Risk of Childhood Obesity: Systematic Review and Dose-response Meta-analysis of Prospective Cohort Studies. Sci. Rep. 2015, 5, 16160. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, S.; Huang, Y.; Chen, K. Sleep duration and obesity in children: A systematic review and meta-analysis of prospective cohort studies. J. Paediatr. Child Health 2017, 53, 378–385. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Q.; Zou, Z.; Li, H.; Zhang, X. Short sleep duration and obesity among children: A systematic review and meta-analysis of prospective studies. Obes. Res. Clin. Pract. 2017, 11, 140–150. [Google Scholar] [CrossRef]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; Ji, C.; Cappuccio, F.P. Sleep duration and incidence of obesity in infants, children, and adolescents: A systematic review and meta-analysis of prospective studies. Sleep 2018, 41, zsy018. [Google Scholar] [CrossRef] [PubMed]
- Amagai, Y.; Ishikawa, S.; Gotoh, T.; Doi, Y.; Kayaba, K.; Nakamura, Y.; Kajii, E. Sleep Duration and Mortality in Japan: The Jichi Medical School Cohort Study. J. Epidemiol. 2004, 14, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M. Interactions between sleep normative data and sociocultural characteristics in the elderly. J. Psychosom. Res. 2004, 56, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Vecchierini, M.-F. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep 2005, 28, 981–989. [Google Scholar] [PubMed]
- Lauderdale, D.S.; Knutson, K.L.; Yan, L.L.; Rathouz, P.J.; Hulley, S.B.; Sidney, S.; Liu, K. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA study. Am. J. Epidemiol. 2006, 164, 5–16. [Google Scholar] [CrossRef]
- Spruyt, K.; Molfese, D.L.; Gozal, D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 2011, 127, e345–e352. [Google Scholar] [CrossRef]
- Klingenberg, L.; Christensen, L.B.; Hjorth, M.F.; Zangenberg, S.; Chaput, J.P.; Sjödin, A.; Mølgaard, C.; Michaelsen, K.F. No relation between sleep duration and adiposity indicators in 9-36 months old children: The SKOT cohort. Pediatr. Obes. 2013, 8, 14–18. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Bernabe-Ortiz, A.; Miranda, J.J. Short sleep duration and childhood obesity: Cross-sectional analysis in Peru and patterns in four developing countries. PLoS ONE 2014, 9, e112433. [Google Scholar] [CrossRef]
- Mcneil, J.; Tremblay, M.S.; Leduc, G.; Boyer, C.; Bélanger, P.; Leblanc, A.G.; Borghese, M.M.; Chaput, J.P. Objectively-measured sleep and its association with adiposity and physical activity in a sample of Canadian children. J. Sleep Res. 2015, 24, 131–139. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, Y.; Zhou, J.; Jia, F.; Li, X.; Tang, Y.; Zheng, H. The association between sleep patterns and overweight/obesity in Chinese children: A cross-sectional study. Neuropsychiatr. Dis. Treat. 2015, 11, 2209–2216. [Google Scholar] [CrossRef]
- Stranges, S.; Cappuccio, F.P.; Kandala, N.B.; Miller, M.A.; Taggart, F.M.; Kumari, M.; Ferrie, J.E.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: The Whitehall II Study. Am. J. Epidemiol. 2008, 167, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, D.S.; Knutson, K.L.; Rathouz, P.J.; Yan, L.L.; Hulley, S.B.; Liu, K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: The CARDIA Sleep Study. Am. J. Epidemiol. 2009, 170, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.; Scalzo, K.; Canterford, L.; Wake, M. Sleep duration and body mass index in 0-7-year olds. Arch. Dis. Child. 2011, 96, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, C.; Hashimoto, H. Sleep duration and weight gain: Reconsideration by panel data analysis. J. Epidemiol. 2014, 24, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Basta, M. Obesity and sleep: A bidirectional association? Sleep 2010, 33, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Collings, P.J.; Ball, H.L.; Santorelli, G.; West, J.; Barber, S.E.; McEachan, R.R.; Wright, J. Sleep Duration and Adiposity in Early Childhood: Evidence for Bidirectional Associations from the Born in Bradford Study. Sleep 2017, 40, zsw054. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; McDonald, L.; van Jaarsveld, C.H.; Llewellyn, C.; Fildes, A.; Schrempft, S.; Wardle, J. Sleep and energy intake in early childhood. Int. J. Obes. 2014, 38, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jansen, W.; Boere-Boonekamp, M.M.; Vlasblom, E.; L’Hoir, M.P.; Beltman, M.; van Grieken, A.; Raat, H. Sleep and body mass index in infancy and early childhood (6-36 mo): A longitudinal study. Pediatr. Obes. 2019, 14, e12506. [Google Scholar] [CrossRef]
- Carter, P.J.; Taylor, B.J.; Williams, S.M.; Taylor, R.W. Longitudinal analysis of sleep in relation to BMI and body fat in children: The FLAME study. BMJ 2011, 342, d2712. [Google Scholar] [CrossRef]
- Garfield, V.; Llewellyn, C.H.; Steptoe, A.; Kumari, M. Investigating the Bidirectional Associations of Adiposity with Sleep Duration in Older Adults: The English Longitudinal Study of Ageing (ELSA). Sci. Rep. 2017, 7, 40250. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Breeze, E.; Banks, J.; Nazroo, J. Cohort profile: The English longitudinal study of ageing. Int. J. Epidemiol. 2013, 42, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, C.M.; Koolhaas, C.M.; Kocevska, D.; Te Lindert, B.H.; Erler, N.S.; Franco, O.H.; Luik, A.I.; Tiemeier, H. Objectively measured sleep and body mass index: A prospective bidirectional study in middle-aged and older adults. Sleep Med. 2019, 57, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Manni, R.; Terzaghi, M.; Repetto, A. The FLEP scale in diagnosing nocturnal frontal lobe epilepsy, NREM and REM parasomnias: Data from a tertiary sleep and epilepsy unit. Epilepsia 2008, 49, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; Delia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef]
- Vanable, P.A.; Aikens, J.E.; Tadimeti, L.; Caruana-Montaldo, B.; Mendelson, W.B. Sleep latency and duration estimates among sleep disorder patients: Variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep 2000, 23, 71–79. [Google Scholar] [CrossRef]
- Lauderdale, D.S.; Knutson, K.L.; Yan, L.L.; Liu, K.; Rathouz, P.J. Self-reported and measured sleep duration: How similar are they? Epidemiology 2008, 19, 838–845. [Google Scholar] [CrossRef]
- Van Den Berg, J.F.; Van Rooij, F.J.; Vos, H.; Tulen, J.H.; Hofman, A.; Miedema, H.M.; Neven, A.K.; Tiemeier, H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J. Sleep Res. 2008, 17, 295–302. [Google Scholar] [CrossRef]
- Arora, T.; Broglia, E.; Pushpakumar, D.; Lodhi, T.; Taheri, S. An Investigation into the Strength of the Association and Agreement Levels between Subjective and Objective Sleep Duration in Adolescents. PLoS ONE 2013, 8, e72406. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed]
- Pollak, C.P.; Tryon, W.W.; Nagaraja, H.; Dzwonczyk, R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep 2001, 24, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of Sleep Parameters from Actigraphy and Polysomnography in Older Women: The SOF Study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Biddle, D.J.; Robillard, R.; Hermens, D.F.; Hickie, I.B.; Glozier, N. Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Health 2015, 1, 214–220. [Google Scholar] [CrossRef]
- Paquet, J.; Kawinska, A.; Carrier, J. Wake detection capacity of actigraphy during sleep. Sleep 2007, 30, 1362–1369. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Hasler, G.; Buysse, D.J.; Klaghofer, R.; Gamma, A.; Ajdacic, V.; Eich, D.; Rössler, W.; Angst, J. The association between short sleep duration and obesity in young adults: A 13-year prospective study. Sleep 2004, 27, 661–666. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Malaspina, D.; Boden-Albala, B.; Heymsfield, S.B. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep 2005, 28, 1289–1296. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Somashekar, D.; Appugliese, D.; Kaciroti, N.; Corwyn, R.F.; Bradley, R.H. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics 2007, 120, 1020–1029. [Google Scholar] [CrossRef]
- Seegers, V.; Petit, D.; Falissard, B.; Vitaro, F.; Tremblay, R.E.; Montplaisir, J.; Touchette, E. Short sleep duration and body mass index: A prospective longitudinal study in preadolescence. Am. J. Epidemiol. 2011, 173, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.E.; Goodwin, J.L.; Parthasarathy, S.; Sherrill, D.L.; Vana, K.D.; Drescher, A.A.; Quan, S.F. Longitudinal Association between Short Sleep, Body Weight, and Emotional and Learning Problems in Hispanic and Caucasian Children. Sleep 2011, 34, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Tyrrell, J.; Wood, A.R.; Beaumont, R.N.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; Hu, Y.; Teder-Laving, M.; Hayward, C.; et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet. 2016, 12, e1006125. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.; Jones, S.E.; Wood, A.R.; Lane, J.; van Hees, V.T.; Wang, H.; Rhodes, J.A.; Song, Y.; Patel, K.; Anderson, S.G.; et al. GWAS in 446,118 European adults identifies 78 genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. bioRxiv 2018, 274977. [Google Scholar] [CrossRef]
- Klingenberg, L.; Sjödin, A.; Holmbäck, U.; Astrup, A.; Chaput, J.P. Short sleep duration and its association with energy metabolism. Obes. Rev. 2012, 13, 565–577. [Google Scholar] [CrossRef]
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Tilling, K.; Smith, G.D. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 2016, 45, 1866–1886. [Google Scholar] [CrossRef]
- Mei, H.; Chen, W.; Jiang, F.; He, J.; Srinivasan, S.; Smith, E.N.; Schork, N.; Murray, S.; Berenson, G.S. Longitudinal Replication Studies of GWAS Risk SNPs Influencing Body Mass Index over the Course of Childhood and Adulthood. PLoS ONE 2012, 7, e31470. [Google Scholar] [CrossRef]
- Llewellyn, C.H.; Trzaskowski, M.; Plomin, R.; Wardle, J. Finding the missing heritability in pediatric obesity: The contribution of genome-wide complex trait analysis. Int. J. Obes. (Lond.) 2013, 37, 1506. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Curado, J.; Church, G.M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009, 25, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Lin, L.; Austin, D.; Young, T. Short Sleep Duration is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garfield, V. The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after nearly Two Decades of Epidemiological Research? Int. J. Environ. Res. Public Health 2019, 16, 4327. https://doi.org/10.3390/ijerph16224327
Garfield V. The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after nearly Two Decades of Epidemiological Research? International Journal of Environmental Research and Public Health. 2019; 16(22):4327. https://doi.org/10.3390/ijerph16224327
Chicago/Turabian StyleGarfield, Victoria. 2019. "The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after nearly Two Decades of Epidemiological Research?" International Journal of Environmental Research and Public Health 16, no. 22: 4327. https://doi.org/10.3390/ijerph16224327
APA StyleGarfield, V. (2019). The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after nearly Two Decades of Epidemiological Research? International Journal of Environmental Research and Public Health, 16(22), 4327. https://doi.org/10.3390/ijerph16224327




