Efficacy of an Internet-Based Program to Promote Physical Activity and Exercise after Inpatient Rehabilitation in Persons with Multiple Sclerosis: A Randomized, Single-Blind, Controlled Study
Abstract
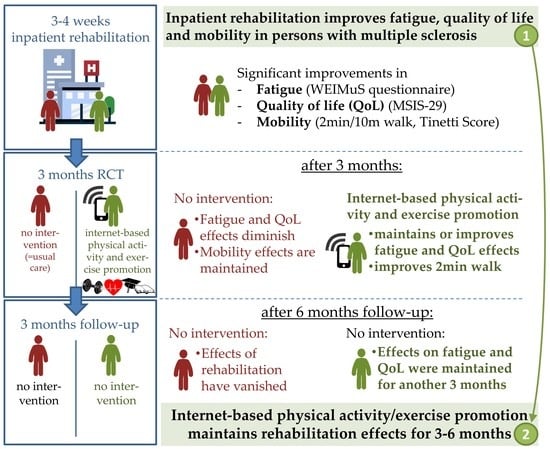
:1. Introduction
2. Patients and Methods
3. Results
3.1. Primary Outcome: Fatigue (WEIMuS Questionnaire)
3.2. Health-Related Quality of Life (MSIS-29)
3.3. Gait and Balance Measures
3.4. Training Statistics and Adherence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sterz, C.; Ellenberger, D.; Friede, T.; Flachenecker, P. Employment-associated factors in multiple sclerosis—Results of a cross-sectional study in Germany. Edorium J. Disabil. Rehabil. 2016, 2, 24–33. [Google Scholar]
- Veauthier, C.; Hasselmann, H.; Gold, S.M.; Paul, F. The Berlin Treatment Algorithm: Recommendations for tailored innovative therapeutic strategies for multiple sclerosis-related fatigue. EPMA J. 2016, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Rommer, P.S.; Eichstädt, K.; Ellenberger, D.; Flachenecker, P.; Friede, T.; Haas, J.; Kleinschnitz, C.; Pöhlau, D.; Rienhoff, O.; Stahmann, A.; et al. Symptomatology and symptomatic treatment in multiple sclerosis: Results from a nationwide MS registry. Mult. Scler. J. 2019, 25, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.A.; Greenlee, T.A.; Motl, R.W.; Nickrent, M.S.; Petruzzello, S.J. Effects of exercise training on fatigue in multiple sclerosis: A meta-analysis. Psychosom. Med. 2013, 75, 575–580. [Google Scholar] [CrossRef]
- Andreasen, A.K.; Stenager, E.; Dalgas, U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult. Scler. J. 2011, 17, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Amatya, B. Rehabilitation in Multiple Sclerosis: A Systematic Review of Systematic Reviews. Arch. Phys. Med. Rehabil. 2017, 98, 353–367. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Platta, M.E.; Motl, R.W.; Latimer-Cheung, A.E. The safety of exercise training in multiple sclerosis: A systematic review. J. Neurol. Sci. 2014, 343, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, J.H. Getting beyond the plateau: Bridging the gap between rehabilitation and community-based exercise. PM R 2012, 4, 857–861. [Google Scholar] [CrossRef]
- Tallner, A.; Tzschoppe, R.; Peters, S.; Mäurer, M.; Pfeifer, K. Internetgestützte Bewegungsförderung bei Personen mit Multipler Sklerose. Neurol. Rehab. 2013, 19, 35–46. [Google Scholar]
- Tallner, A.; Streber, R.; Hentschke, C.; Morgott, M.; Geidl, W.; Mäurer, M.; Pfeifer, K. Internet-Supported Physical Exercise Training for Persons with Multiple Sclerosis—A Randomised, Controlled Study. Int. J. Mol. Sci. 2016, 17, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis. 2005 revisions to the “McDonald criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flachenecker, P.; König, H.; Meissner, H.; Müller, G.; Rieckmann, P. Fatigue bei Multipler Sklerose: Validierung des “Würzburger Erschöpfungs-Inventars bei Multipler Sklerose (WEIMuS) ”. Neurol. Rehabil. 2008, 14, 299–306. [Google Scholar]
- Flachenecker, P.; Müller, G.; König, H.; Meissner, H.; Toyka, K.V.; Rieckmann, P. “Fatigue” bei Multipler Sklerose: Entwicklung und Validierung des "Würzburger Erschöpfungs-Inventar bei Multipler Sklerose” (WEIMuS). Nervenarzt 2006, 77, 165–172. [Google Scholar] [CrossRef]
- Pfeifer, K.; Sudeck, G.; Geidl, W.; Tallner, A. Bewegungsförderung und Sport in der Neurologie—Kompetenzorientierung und Nachhaltigkeit. Neurol. Rehabil. 2013, 19, 7–19. [Google Scholar]
- Sudeck, G.; Pfeifer, K. Physical activity-related health competence as an integrative objective in exercise therapy and health sports—Conception and validation of a short questionnaire. Sportwissenschaft 2016, 46, 74–87. [Google Scholar] [CrossRef]
- Deci, E.L.; Ryan, R.M. Self-determination theory: A macrotheory of human motivation, development and health. Can. Psychol. 2008, 49, 182–185. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Preparing People for Change; Guilford Press: New York, NY, USA, 2003. [Google Scholar]
- Gawlik, A.; Streber, R.; Flachenecker, P.; Gusowski, K.; Geidl, W.; Tallner, A.; Pfeifer, K. Konzept eines internetbasierten Programms zur Bewegungsförderung für Personen mit Multipler Sklerose. Neurol. Rehabil. 2018, 24, 171–182. [Google Scholar]
- Mäurer, M.; Schuh, K.; Seibert, S.; Baier, M.; Hentschke, C.; Streber, R.; Tallner, A.; Pfeifer, K. A randomized study to evaluate the effect of exercise on fatigue in people with relapsing-remitting multiple sclerosis treated with fingolimod. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318756688. [Google Scholar] [CrossRef] [Green Version]
- Hobart, J.; Lamping, D.; Fitzpatrick, R.; Riazi, A.; Thompson, A. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient- based outcome measure. Brain 2001, 124, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.H.; Ford, D.V.; Jones, P.A.; John, A.; Middleton, R.M.; Lockhart-Jones, H.; Peng, J.; Osborne, L.A.; Noble, J.G. The physical and psychological impact of multiple sclerosis using the MSIS-29 via the web portal of the UK MS Register. PLoS ONE 2013, 8, e55422. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, D.; Dalgas, U.; Romberg, A.; de Groot, V.; Bethoux, F.; Vaney, C.; Gebara, B.; Medina, C.S.; Maamagi, H.; Rasova, K.; et al. Which walking capacity tests to use in multiple sclerosis? A multicentre study providing the basis for a core set. Mult. Scler. J. 2012, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Heine, M.; van de Port, I.; Rietberg, M.B.; van Wegen, E.E.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015, 9, CD009956. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 1, CD012732. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Motl, R.W. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J. Neurol. Sci. 2013, 331, 102–107. [Google Scholar] [CrossRef]
- Moss-Morris, R.; Harrison, A.M.; Safari, R.; Norton, S.; van der Linden, M.L.; Picariello, F.; Thomas, S.; White, C.; Mercer, T. Which behavioural and exercise interventions targeting fatigue show the most promise in multiple sclerosis? A systematic review with narrative synthesis and meta-analysis. Behav. Res. Ther. 2019, article in press, 103464. [Google Scholar] [CrossRef]
- Riazi, A.; Hobart, J.C.; Lamping, D.L.; Fitzpatrick, R.; Thompson, A.J. Evidence-based measurement in multiple sclerosis: The psychometric properties of the physical and psychological dimensions of three quality of life rating scales. Mult. Scler. J. 2003, 9, 411–419. [Google Scholar] [CrossRef]
- Schaffler, N.; Schonberg, P.; Stephan, J.; Stellmann, J.P.; Gold, S.M.; Heesen, C. Comparison of patient-reported outcome measures in multiple sclerosis. Acta Neurol. Scand. 2013, 128, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Motl, R.W.; Gosney, J.L. Effect of exercise training on quality of life in multiple sclerosis: A meta-analysis. Mult. Scler. J. 2008, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348. [Google Scholar] [CrossRef]
- Charron, S.; McKay, K.A.; Tremlett, H. Physical activity and disability outcomes in multiple sclerosis: A systematic review (2011–2016). Mult. Scler. Relat. Disord. 2018, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Langeskov-Christensen, M.; Stenager, E.; Riemenschneider, M.; Hvid, L.G. Exercise as Medicine in Multiple Sclerosis-Time for a Paradigm Shift: Preventive, Symptomatic, and Disease-Modifying Aspects and Perspectives. Curr. Neurol. Neurosci. Rep. 2019, 19, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Tallner, A.; Waschbisch, A.; Wenny, I.; Schwab, S.; Hentschke, C.; Pfeifer, K.; Mäurer, M. Multiple sclerosis relapses are not associated with exercise. Mult. Scler. J. 2012, 18, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motl, R.W.; Dlugonski, D.; Wojcicki, T.R.; McAuley, E.; Mohr, D.C. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult. Scler. J. 2011, 17, 116–128. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Dlugonski, D.; Sandroff, B.M.; Klaren, R.E.; Motl, R.W. Internet-delivered lifestyle physical activity intervention improves body composition in multiple sclerosis: Preliminary evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2014, 95, 1283–1288. [Google Scholar] [CrossRef]
- Romberg, A.; Virtanen, A.; Ruutiainen, J. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J. Neurol. 2005, 252, 839–845. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Finlayson, M.; McAuley, E.; Morrison, S.; Motl, R.W. Home-based exercise program and fall-risk reduction in older adults with multiple sclerosis: Phase 1 randomized controlled trial. Clin. Rehabil. 2014, 28, 254–263. [Google Scholar] [CrossRef]
- Ertekin, O.; Ozakbas, S.; Idiman, E.; Algun, Z.C. Quality of life, fatigue and balance improvements after home-based exercise program in multiple sclerosis patients. Arch. Neuropsychiatry 2012, 49, 33–38. [Google Scholar]
- Schmidt, S.; Wonneberger, M. Long-term endurance exercise improves aerobic capacity in patients with relapsing-remitting multiple sclerosis: Impact of baseline fatigue. J. Neurol. Sci. 2014, 336, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gronseth, G.S.; Cox, J.; Gloss, D.; Merillat, S.; Dittman, J.; Armstrong, M.J.; Getchius, T.S.D.; the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Clinical Practice Guideline Process Manual, 2017th ed.; The American Academy of Neurology: Minneapolis, MN, USA, 2017; Available online: https://www.aan.com/siteassets/home-page/policy-and-guidelines/guidelines/about-guidelines/17guidelineprocman_pg.pdf (accessed on 23 April 2020).




| Measurements | Beginning of Inpatient Rehabilitation T0 | End of Inpatient Rehabilitation T1 | 3 Months after Discharge T2 | 6 Months after Discharge T3 |
|---|---|---|---|---|
| Subjective fatigue (WEIMuS) | Study center | Study center | Study center | Postal survey |
| Quality of life (MSIS-29) | Study center | Study center | Study center | Postal survey |
| Gait (2minWT, 10mWT) | Study center | Study center | Study center | / |
| Balance (Tinetti Score) | Study center | Study center | Study center | / |
| Intervention Group (n = 34) | Control Group (n = 30) | p-Value | |
|---|---|---|---|
| Age [years] mean ± SD | 47.6 ± 9.2 | 46.4 ± 12.2 | 0.328 |
| Women [n] (percent) | 22 (65%) | 17 (57%) | 0.279 * |
| Disease duration [years] mean ± SD | 13.4 ± 7.9 | 9.0 ± 7.5 | 0.015 |
| EDSS median (IQR) | 4.3 (3.5–5.0) | 4.0 (3.0–6.0) | 0.828 |
| RR MS [n] (percent) | 19 (56%) | 20 (67%) | 0.531 * |
| WEIMuS median (IQR) | 45 (38–52) | 39 (36–46) | 0.13 |
| MSIS-29 median (IQR) | 77.5 (67.3–87.3) | 74.0 (63.8–84.3) | 0.492 |
| 2minWT median (IQR) | 179.5 (147.0–199.3) | 179.0 (131.8–200.0) | 0.619 |
| 10mWT median (IQR) | 6.65 (5.85–8.03) | 6.90 (5.60–8.98) | 0.777 |
| Intervention Group (n = 34) | Control Group (n = 30) | |
|---|---|---|
| Baseline–Discharge | 15.5 (5–22) | 18 (11–28) |
| Baseline–Month 3 | 16.5 *** (10–29) | 7 (2–11) |
| Baseline–Month 6 | 22.5 *** (8–30) | 5.5 (1–11) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flachenecker, P.; Bures, A.K.; Gawlik, A.; Weiland, A.-C.; Kuld, S.; Gusowski, K.; Streber, R.; Pfeifer, K.; Tallner, A. Efficacy of an Internet-Based Program to Promote Physical Activity and Exercise after Inpatient Rehabilitation in Persons with Multiple Sclerosis: A Randomized, Single-Blind, Controlled Study. Int. J. Environ. Res. Public Health 2020, 17, 4544. https://doi.org/10.3390/ijerph17124544
Flachenecker P, Bures AK, Gawlik A, Weiland A-C, Kuld S, Gusowski K, Streber R, Pfeifer K, Tallner A. Efficacy of an Internet-Based Program to Promote Physical Activity and Exercise after Inpatient Rehabilitation in Persons with Multiple Sclerosis: A Randomized, Single-Blind, Controlled Study. International Journal of Environmental Research and Public Health. 2020; 17(12):4544. https://doi.org/10.3390/ijerph17124544
Chicago/Turabian StyleFlachenecker, Peter, Anna Karoline Bures, Angeli Gawlik, Ann-Christin Weiland, Sarah Kuld, Klaus Gusowski, René Streber, Klaus Pfeifer, and Alexander Tallner. 2020. "Efficacy of an Internet-Based Program to Promote Physical Activity and Exercise after Inpatient Rehabilitation in Persons with Multiple Sclerosis: A Randomized, Single-Blind, Controlled Study" International Journal of Environmental Research and Public Health 17, no. 12: 4544. https://doi.org/10.3390/ijerph17124544
APA StyleFlachenecker, P., Bures, A. K., Gawlik, A., Weiland, A.-C., Kuld, S., Gusowski, K., Streber, R., Pfeifer, K., & Tallner, A. (2020). Efficacy of an Internet-Based Program to Promote Physical Activity and Exercise after Inpatient Rehabilitation in Persons with Multiple Sclerosis: A Randomized, Single-Blind, Controlled Study. International Journal of Environmental Research and Public Health, 17(12), 4544. https://doi.org/10.3390/ijerph17124544