Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Free and Cued Selective Reminding Test, Rey–Osterrieth Complex Figure Test and Judgement of Line Orientation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Free and Cued Selective Reminding Test
2.2.2. Rey–Osterrieth Complex Figure
2.2.3. Judgement of Line Orientation
2.3. Procedure
2.4. Statistical Analysis
2.4.1. Calculation of Normative Data
2.4.2. Comparing Normative Data Sets
3. Results
3.1. Calculation of Normative Data
3.2. Comparing Normative Data Sets
3.3. Free and Cued Selective Reminding Test
3.4. Rey–Osterrieth Complex Figure
3.5. Judgement of Line Orientation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Ageing 2009; United Nations: New York, NY, USA, 2009. [Google Scholar]
- Instituto Nacional de Estadística Proporción de Personas Mayores de Cierta Edad Por Comunidad Autónoma. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=1451#!tabs-tabla (accessed on 13 March 2021).
- De Pedro-Cuesta, J.; Virués-Ortega, J.; Vega, S.; Seijo-Martínez, M.; Saz, P.; Rodríguez, F.; Rodríguez-Laso, A.; Reñé, R.; de las Heras, S.P.; Mateos, R.; et al. Prevalence of Dementia and Major Dementia Subtypes in Spanish Populations: A Reanalysis of Dementia Prevalence Surveys, 1990–2008. BMC Neurol. 2009, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ronchi, D.; Berardi, D.; Menchetti, M.; Ferrari, G.; Serretti, A.; Dalmonte, E.; Fratiglioni, L. Occurrence of Cognitive Impairment and Dementia after the Age of 60: A Population-Based Study from Northern Italy. Dement. Geriatr. Cogn. Disord. 2005, 19, 97–105. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, E.; Mora-Simón, S.; Patino-Alonso, M.C.; García-García, R.; Escribano-Hernández, A.; García-Ortiz, L.; Perea- Bartolomé, M.V.; Gómez-Marcos, M.A. Prevalence of Cognitive Impairment in Individuals Aged over 65 in an Urban Area: DERIVA Study. BMC Neurol. 2011, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Tola-Arribas, M.A.; Yugueros, M.I.; Garea, M.J.; Ortega-Valín, F.; Cerón-Fernández, A.; Fernández-Malvido, B.; San José-Gallegos, A.; González-Touya, M.; Botrán-Velicia, A.; Iglesias-Rodríguez, V.; et al. Prevalence of Dementia and Subtypes in Valladolid, Northwestern Spain: The DEMINVALL Study. PLoS ONE 2013, 8, e77688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellinger, K.A.; Attems, J. Prevalence of Dementia Disorders in the Oldest-Old: An Autopsy Study. Acta Neuropathol. 2010, 119, 421–433. [Google Scholar] [CrossRef]
- Vega Alonso, T.; Miralles Espí, M.; Mangas Reina, J.M.; Castrillejo Pérez, D.; Rivas Pérez, A.I.; Gil Costa, M.; López Maside, A.; Arrieta Antón, E.; Lozano Alonso, J.E.; Fragua Gil, M. Prevalencia de deterioro cognitivo en España. Estudio Gómez de Caso en redes centinelas sanitarias. Neurología 2018, 33, 491–498. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Contin. Minneap. Minn. 2016, 22, 404–418. [Google Scholar] [CrossRef]
- Villarejo Galende, A.; Eimil Ortiz, M.; Llamas Velasco, S.; Llanero Luque, M.; López de Silanes de Miguel, C.; Prieto Jurczynska, C. Informe de la Fundación del Cerebro. Impacto social de la enfermedad de Alzheimer y otras demencias. Neurología 2021, 36, 39–49. [Google Scholar] [CrossRef]
- Poblador-Plou, B.; Calderón-Larrañaga, A.; Marta-Moreno, J.; Hancco-Saavedra, J.; Sicras-Mainar, A.; Soljak, M.; Prados-Torres, A. Comorbidity of Dementia: A Cross-Sectional Study of Primary Care Older Patients. BMC Psychiatry 2014, 14, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusik, P.; Tomaszewski, K.; Chmielowska, K.; Nowak, J.; Nowak, W.; Parnicka, A.; Dubiel, M.; Gąsowski, J.; Grodzicki, T. Severe Frailty and Cognitive Impairment Are Related to Higher Mortality in 12-Month Follow-up of Nursing Home Residents. Arch. Gerontol. Geriatr. 2012, 55, 22–24. [Google Scholar] [CrossRef]
- Leyhe, T.; Reynolds, C.F.; Melcher, T.; Linnemann, C.; Klöppel, S.; Blennow, K.; Zetterberg, H.; Dubois, B.; Lista, S.; Hampel, H. A Common Challenge in Older Adults: Classification, Overlap, and Therapy of Depression and Dementia. Alzheimers Dement. 2017, 13, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Shih, P.-Y.; Ku, L.-J.E. Activities of Daily Living Function and Neuropsychiatric Symptoms of People with Dementia and Caregiver Burden: The Mediating Role of Caregiving Hours. Arch. Gerontol. Geriatr. 2019, 81, 25–30. [Google Scholar] [CrossRef]
- Eefsting, J.A.; Boersma, F.; Brink, W.V.D.; Tilburg, W.V. Differences in Prevalence of Dementia Based on Community Survey and General Practitioner Recognition. Psychol. Med. 1996, 26, 1223–1230. [Google Scholar] [CrossRef]
- Valcour, V.G.; Masaki, K.H.; Curb, J.D.; Blanchette, P.L. The Detection of Dementia in the Primary Care Setting. Arch. Intern. Med. 2000, 160, 2964–2968. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2012; ISBN 978-0-19-539552-5. [Google Scholar]
- Munro Collum, C.; Lacritz, L. Neuropsychological Assessment in Dementia. In The American Psychiatric Publishing Textbook of Alzheimer Disease and Other Dementias; American Psychiatric Publishing: Washington, DC, USA, 2009. [Google Scholar]
- Arango-Lasprilla, J.C.; Rivera, D.; Ramos-Usuga, D.; Vergara-Moragues, E.; Montero-López, E.; Adana Díaz, L.A.; Aguayo Arelis, A.; García-Guerrero, C.E.; García De La Cadena, C.; Llerena Espezúa, X.; et al. Trail Making Test: Normative Data for the Latin American Spanish-Speaking Pediatric Population. NeuroRehabilitation 2017, 41, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Ardila, A. Cultural Values Underlying Psychometric Cognitive Testing. Neuropsychol. Rev. 2005, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Ardila, A. The Impact of Culture on Neuropsychological Test Performance. In International Handbook of Cross-Cultural Neuropsychology; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2007; pp. 23–44. [Google Scholar]
- Horton, A.M.; Roberts, C. Demographic Effects on the Trail Making Test in a Drug Abuse Treatment Sample. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2003, 18, 49–56. [Google Scholar] [CrossRef]
- Strauss, E.; Sherman, E.M.S.; Spreen, O.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; ISBN 978-0-19-515957-8. [Google Scholar]
- Hall, J.R.; Balldin, V.H.; Gamboa, A.; Edwards, M.L.; Johnson, L.A.; O’Bryant, S.E. Texas Mexican American Adult Normative Studies: Normative Data for the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Dev. Neuropsychol. 2018, 43, 27–35. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Edwards, M.; Johnson, L.; Hall, J.; Gamboa, A.; O’jile, J. Texas Mexican American Adult Normative Studies: Normative Data for Commonly Used Clinical Neuropsychological Measures for English- and Spanish-Speakers. Dev. Neuropsychol. 2018, 43, 1–26. [Google Scholar] [CrossRef]
- Silvestre, G.; Iglesias, R.M.; Silvestre, E. Boston Naming Test Norms for the Dominican Population. Aphasiology 2018, 32, 340–365. [Google Scholar] [CrossRef]
- Heaton, A.; Gooding, A.; Cherner, M.; Umlauf, A.; Franklin, D.R.; Rivera Mindt, M.; Suárez, P.; Artiola i Fortuni, L.; Heaton, R.K.; Marquine, M.J. Demographically-Adjusted Norms for the Grooved Pegboard and Finger Tapping Tests in Spanish-Speaking Adults: Results from the Neuropsychological Norms for the U.S.-Mexico Border Region in Spanish (NP-NUMBRS) Project. Clin. Neuropsychol. 2021, 35, 419–432. [Google Scholar] [CrossRef]
- Peña-Casanova, J.; Blesa, R.; Aguilar, M.; Gramunt-Fombuena, N.; Gómez-Ansón, B.; Oliva, R.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Antúnez, C.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Methods and Sample Characteristics. Arch. Clin. Neuropsychol. 2009, 24, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Rivera, D.; Olabarrieta-Landa, L.; Van Der Elst, W.; Gonzalez, I.; Rodríguez-Agudelo, Y.; Arelis, A.A.; Rodriguez-Irizarry, W.; De La Cadena, C.G.; Arango-Lasprilla, J.C. Normative Data for Verbal Fluency in Healthy Latin American Adults: Letter M, and Fruits and Occupations Categories. Neuropsychology 2019, 33, 287–300. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO—World Health Organization. Active Ageing: A Policy Framework; WHO: Madrid, Spain, 2002. [Google Scholar]
- Lee, P.-L.; Lan, W.; Yen, T.-W. Aging Successfully: A Four-Factor Model. Educ. Gerontol. 2011, 37, 210–227. [Google Scholar] [CrossRef]
- Chaves, M.L.; Camozzato, A.L.; Eizirik, C.L.; Kaye, J. Predictors of Normal and Successful Aging Among Urban-Dwelling Elderly Brazilians. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009, 64B, 597–602. [Google Scholar] [CrossRef]
- George, C.; Ummar, S.A.; Shaji, K.S. Prevention of Cognitive Decline: Lifestyle and Other Issues. J. Geriatr. Ment. Health 2016, 3, 5. [Google Scholar] [CrossRef]
- Arias-Merino, E.D.; Mendoza-Ruvalcaba, N.M.; Arias-Merino, M.J.; Cueva-Contreras, J.; Vazquez Arias, C. Prevalence of Successful Aging in the Elderly in Western Mexico. Curr. Gerontol. Geriatr. Res. 2012, 2012, 460249. [Google Scholar] [CrossRef] [Green Version]
- Hijas-Gómez, A.I.; Ayala, A.; Rodríguez-García, M.P.; Rodríguez-Blázquez, C.; Rodríguez-Rodríguez, V.; Rojo-Pérez, F.; Fernández-Mayoralas, G.; Rodríguez-Laso, A.; Calderón-Larrañaga, A.; Forjaz, M.J. The WHO Active Ageing Pillars and Its Association with Survival: Findings from a Population-Based Study in Spain. Arch. Gerontol. Geriatr. 2020, 90, 104114. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballesteros, R.; Robine, J.M.; Walker, A.; Kalache, A. Active Aging: A Global Goal. Curr. Gerontol. Geriatr. Res. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Olivera, J.; Andreoli, F.; Leist, A.K.; Chauvel, L. Inequality in Old Age Cognition across the World. Econ. Hum. Biol. 2018, 29, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Buschke, H.; Fuld, P.A. Evaluating Storage, Retention, and Retrieval in Disordered Memory and Learning. Neurology 1974, 24, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschke, H. Selective Reminding for Analysis of Memory and Learning. J. Verbal Learn. Verbal Behav. 1973, 12, 543–550. [Google Scholar] [CrossRef]
- Buschke, H. Cued Recall in Amnesia. J. Clin. Neuropsychol. 1984, 6, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Osterrieth, P.A. Le Test de Copie d’une Figure Complexe; Contribution à l’étude de La Perception et de La Mémoire. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Rey, A. L’examen Psychologique Dans Les Cas d’encéphalopathie Traumatique. (Les Problems.). Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Benton, A.; Hannay, H.J.; Varney, N.R. Visual Perception of Line Direction in Patients with Unilateral Brain Disease. Neurology 1975, 25, 907–910. [Google Scholar] [CrossRef]
- Benton, A.; Sivan, A.B.; Hamsher, K.; Varney, N.R.; Spreen, O. Contributions to Neuropsychological Assessment: A Clinical Manual; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Benton, A.L.; Varney, N.R.; Hamsher, K.D. Visuospatial Judgment. A Clinical Test. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef]
- Palomo, R.; Casals-Coll, M.; Sanchez-Benavides, G.; Quintana, M.; Manero, R.M.; Rognoni, T.; Calvo, L.; Aranciva, F.; Tamayo, F.; Pena-Casanova, J. Spanish Normative Studies in Young Adults (NEURONORMA Young Adults Project): Norms for the Rey-Osterrieth Complex Figure (Copy and Memory) and Free and Cued Selective Reminding Test. Neurol. Barc. Spain 2013, 28, 226–235. [Google Scholar] [CrossRef]
- Peña-Casanova, J.; Gramunt-Fombuena, N.; Quiñones-Ubeda, S.; Sanchez-Benavides, G.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Payno, M.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Rey-Osterrieth Complex Figure (Copy and Memory), and Free and Cued Selective Reminding Test. Arch. Clin. Neuropsychol. 2009, 24, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Calvo, L.; Casals-Coll, M.; Sánchez-Benavides, G.; Quintana, M.; Manero, R.M.; Rognoni, T.; Palomo, R.; Aranciva, F.; Tamayo, F.; Peña-Casanova, J. Spanish Normative Studies in Young Adults (NEURONORMA Young Adults Project): Norms for the Visual Object and Space Perception Battery and Judgment of Line Orientation Tests. Neurol. Engl. Ed. 2013, 28, 153–159. [Google Scholar] [CrossRef]
- Narushima, M.; Liu, J.; Diestelkamp, N. Lifelong Learning in Active Ageing Discourse: Its Conserving Effect on Wellbeing, Health and Vulnerability. Ageing Soc. 2018, 38, 651–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonete López, B.; Oltra-Cucarella, J.; Marín, M.; Antón, C.; Balao, N.; López, E.; Sitges Maciá, E. Validation and Norms for a Recognition Task for the Spanish Version of the Free and Cued Selective Reminding Test. Arch. Clin. Neuropsychol. 2021, 36, 954–964. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.; Coben, L.A.; Martin, R.L. A New Clinical Scale for the Staging of Dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Lawton, M.; Brody, E. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Iñesta, C.; Oltra-Cucarella, J.; Bonete-López, B.; Calderón-Rubio, E.; Sitges-Maciá, E. Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Digit Span, Letters and Numbers, Trail Making Test and Symbol Digit Modalities Test. Int. J. Environ. Res. Public. Health 2021, 18, 9958. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-52629-2. [Google Scholar]
- de Andrade Moral, R.; Diaz-Orueta, U.; Oltra-Cucarella, J. Logistic versus Linear Regression-Based Reliable Change Index: Implications for Clinical Studies with Diverse Sample Sizes. PsyArXiv 2021. [Google Scholar] [CrossRef]
- Campo, P.; Morales, M. Normative Data and Reliability for a Spanish Version of the Verbal Selective Reminding Test. Arch. Clin. Neuropsychol. 2004, 19, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Ivnik, R.J.; Smith, G.E.; Lucas, J.A.; Tangalos, E.G.; Kokmen, E.; Petersen, R.C. Free and Cued Selective Reminding Test: MOANS Norms. J. Clin. Exp. Neuropsychol. 1997, 19, 676–691. [Google Scholar] [CrossRef]
- Koss, E.; Haxby, J.V.; DeCarli, C.; Schapiro, M.B.; Friedland, R.P.; Rapoport, S.I. Patterns of Performance Preservation and Loss in Healthy Aging. Dev. Neuropsychol. 1991, 7, 99–113. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; Faulkner, P.; Hubley, A.M. Effects of Age on the Rey-Osterrieth and Taylor Complex Figures: Test-Retest Data Using an Intentional Learning Paradigm. J. Clin. Exp. Neuropsychol. 1992, 14, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. What Is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Amieva, H.; Carcaillon, L.; Rouze L’Alzit-Schuermans, P.; Millet, X.; Dartigues, J.F.; Fabrigoule, C. Cued and uncued memory tests: Norms in elderly adults from the 3 Cities epidemiological study. Rev. Neurol. 2007, 163, 205–221. [Google Scholar] [CrossRef]
- Grober, E.; Buschke, H.; Crystal, H.; Bang, S.; Dresner, R. Screening for Dementia by Memory Testing. Neurology 1988, 38, 900–903. [Google Scholar] [CrossRef]
- Ouvrard, C.; Berr, C.; Meillon, C.; Ribet, C.; Goldberg, M.; Zins, M.; Amieva, H. Norms for Standard Neuropsychological Tests from the French CONSTANCES Cohort. Eur. J. Neurol. 2019, 26, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Frasson, P.; Ghiretti, R.; Catricalà, E.; Pomati, S.; Marcone, A.; Parisi, L.; Rossini, P.M.; Cappa, S.F.; Mariani, C.; Vanacore, N.; et al. Free and Cued Selective Reminding Test: An Italian Normative Study. Neurol. Sci. 2011, 32, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Stokholm, J.; Andreasen, R.; Henriksen, B.D.; Brønniche, V.; Madsen, G.J.; Gustafsson, M.; Overgaard, S.; Guldberg, A.-M.; Jørgensen, K. Psychometric Properties and Reference Data for Danish Versions of Free and Cued Selective Reminding Test, Category Cued Memory Test and Logical Memory. Scand. J. Psychol. 2018, 59, 496–502. [Google Scholar] [CrossRef]
- Girtler, N.; De Carli, F.; Amore, M.; Arnaldi, D.; Bosia, L.E.; Bruzzaniti, C.; Cappa, S.F.; Cocito, L.; Colazzo, G.; Ghio, L.; et al. A Normative Study of the Italian Printed Word Version of the Free and Cued Selective Reminding Test. Neurol. Sci. 2015, 36, 1127–1134. [Google Scholar] [CrossRef]
- Mokri, H.; Ávila-Funes, J.A.; Meillon, C.; Gutiérrez Robledo, L.M.; Amieva, H. Normative Data for the Mini-Mental State Examination, the Free and Cued Selective Reminding Test and the Isaacs Set Test for an Older Adult Mexican Population: The Coyoacán Cohort Study. Clin. Neuropsychol. 2013, 27, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Derby, C.; LeValley, A.; Katz, M.J.; Verghese, J.; Lipton, R.B. Education Delays Accelerated Decline on a Memory Test in Persons Who Develop Dementia. Neurology 2007, 69, 1657–1664. [Google Scholar] [CrossRef]
- Le Carret, N.; Lafont, S.; Mayo, W.; Fabrigoule, C. The Effect of Education on Cognitive Performances and Its Implication for the Constitution of the Cognitive Reserve. Dev. Neuropsychol. 2003, 23, 317–337. [Google Scholar] [CrossRef]
- Stern, Y.; Gurland, B.; Tatemichi, T.K.; Tang, M.X.; Wilder, D.; Mayeux, R. Influence of Education and Occupation on the Incidence of Alzheimer’s Disease. JAMA 1994, 271, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Talarico, J.N.; Caramelli, P.; Nitrini, R.; Chaves, E.C. The Influence of Schooling on Working Memory Performance in Elderly Individuals without Cognitive Decline. Dement. Neuropsychol. 2007, 1, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Ostrosky-Solis, F.; Ardila, A.; Rosselli, M.; Lopez-Arango, G.; Uriel-Mendoza, V. Neuropsychological Test Performance in Illiterate Subjects. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 1998, 13, 645–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.T.R.; Allen, R.S.; Schmitt, F.A. Rey-Osterrieth Complex Figure: Psychometric Characteristics in a Geriatric Sample. Clin. Neuropsychol. 1991, 5, 143–153. [Google Scholar] [CrossRef]
- Lu, P.; Boone, K.; Cozolino, L.; Mitchell, C. Effectiveness of the Rey-Osterrieth Complex Figure Test and the Meyers and Meyers Recognition Trial in the Detection of Suspect Effort. Clin. Neuropsychol. 2003, 17, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.-P.; Potvin, O.; Callahan, B.L.; Belleville, S.; Gagnon, J.-F.; Caza, N.; Ferland, G.; Hudon, C.; Macoir, J. Normative Data for the Rey-Osterrieth and the Taylor Complex Figure Tests in Quebec-French People. Arch. Clin. Neuropsychol. 2015, 30, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, D.; Perrin, P.B.; Morlett-Paredes, A.; Galarza-del-Angel, J.; Martínez, C.; Garza, M.T.; Saracho, C.P.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Rábago, B.; et al. Rey–Osterrieth Complex Figure—Copy and Immediate Recall: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 677–698. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Lorenzana, A.; Núñez-Fernández, S.; Adana-Díaz, L.; Mascialino, G.; Ponce, T.Y.; Rivera, D.; Arango-Lasprilla, J.C. Normative Data for Test of Learning and Memory in an Ecuadorian Adult Population. Clin. Neuropsychol. 2020, 34, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Allen, H.A. Perception and Cognition in the Ageing Brain: A Brief Review of the Short- and Long-Term Links between Perceptual and Cognitive Decline. Front. Aging Neurosci. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Lucas, J.A.; Ivnik, R.J.; Smith, G.E.; Ferman, T.J.; Willis, F.B.; Petersen, R.C.; Graff-Radford, N.R. Mayo’s Older African Americans Normative Studies: Norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, WRAT-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. Clin. Neuropsychol. 2005, 19, 243–269. [Google Scholar] [CrossRef] [PubMed]
- Peña-Casanova, J.; Quintana-Aparicio, M.; Quiñones-Ubeda, S.; Aguilar, M.; Molinuevo, J.L.; Serradell, M.; Robles, A.; Barquero, M.S.; Villanueva, C.; Antunez, C.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Visual Object and Space Perception Battery-Abbreviated, and Judgment of Line Orientation. Arch. Clin. Neuropsychol. 2009, 24, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.A.; Bieliauskas, L.A.; Smith, G.E.; Langellotti, C.; Ivnik, R.J. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Boston Naming Test, the MAE Token Test, and the Judgment of Line Orientation Test. Clin. Neuropsychol. 2005, 19, 280–328. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Backman, L.; Albert, M.; Almkvist, O.; et al. Mild Cognitive Impairment—Beyond Controversies, towards a Consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild Cognitive Impairment: Clinical Characterization and Outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jak, A.J.; Preis, S.R.; Beiser, A.S.; Seshadri, S.; Wolf, P.A.; Bondi, M.W.; Au, R. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J. Int. Neuropsychol. Soc. 2016, 22, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltra-Cucarella, J.; Sánchez-SanSegundo, M.; Lipnicki, D.M.; Sachdev, P.S.; Crawford, J.D.; Pérez-Vicente, J.A.; Cabello-Rodríguez, L.; Ferrer-Cascales, R. Using Base Rate of Low Scores to Identify Progression from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease: Base Rate of Low Scores for MCI Diagnosis. J. Am. Geriatr. Soc. 2018, 66, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Oltra-Cucarella, J.; Sánchez-SanSegundo, M.; Rubio-Aparicio, M.; Arango-Lasprilla, J.C.; Ferrer-Cascales, R. The Association Between the Number of Neuropsychological Measures and the Base Rate of Low Scores. Assessment 2019, 28, 955–963. [Google Scholar] [CrossRef]
- Jak, A.J.; Bondi, M.W.; Delano-Wood, L.; Wierenga, C.; Corey-Bloom, J.; Salmon, D.P.; Delis, D.C. Quantification of Five Neuropsychological Approaches to Defining Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 2009, 17, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Oltra-Cucarella, J.; Sánchez-SanSegundo, M.; Lipnicki, D.M.; Crawford, J.D.; Lipton, R.B.; Katz, M.J.; Zammit, A.R.; Scarmeas, N.; Dardiotis, E.; Kosmidis, M.H.; et al. Visual Memory Tests Enhance the Identification of Amnestic MCI Cases at Greater Risk of Alzheimer’s Disease. Int. Psychogeriatr. 2019, 31, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Oltra-Cucarella, J.; Ferrer-Cascales, R.; Alegret, M.; Gasparini, R.; Díaz-Ortiz, L.M.; Ríos, R.; Martínez-Nogueras, Á.L.; Onandia, I.; Pérez-Vicente, J.A.; Cabello-Rodríguez, L.; et al. Risk of Progression to Alzheimer’s Disease for Different Neuropsychological Mild Cognitive Impairment Subtypes: A Hierarchical Meta-Analysis of Longitudinal Studies. Psychol. Aging 2018, 33, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Polunina, A.; Bryun, E.; Sydniaeva, E.; Golukhova, E. Gender Differences in Cognitive Functions: Retrospective Analysis of the Data of 5 Neuropsychological Studies. Sch. Rep. 2018, 3, 61–75. [Google Scholar]
- Zhang, J.; Zhou, W.; Wang, L.; Zhang, X. Gender Differences of Neuropsychological Profiles in Cognitively Normal Older People without Amyloid Pathology. Compr. Psychiatry 2017, 75, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosselli, M.; Ardila, A. The Impact of Culture and Education on Non-Verbal Neuropsychological Measurements: A Critical Review. Brain Cogn. 2003, 52, 326–333. [Google Scholar] [CrossRef]
| Neuropsychological Test | M | SD | Range |
|---|---|---|---|
| FCSRT-Imm | 44.01 | 4.04 | 26–48 |
| FCSRT-Del | 14.86 | 1.48 | 9–16 |
| ROCF-C | 28.18 | 2.24 | 14–36 |
| ROCF-Imm | 14.97 | 4.90 | 2–26.5 |
| ROCF-Del | 15.04 | 4.80 | 2–25 |
| JLO | 20.55 | 4.94 | 9–30 |
| B | Std. Error | Sig. | R2 | SEE | ||
|---|---|---|---|---|---|---|
| FCSRT-Imm | Intercept | 40.31 | 1.17 | 0.000 | 0.104 | 3.865 |
| EducationMin | 0.288 | 0.111 | 0.011 | |||
| Sex | 1.886 | 0.811 | 0.022 | |||
| FSCRT-Del | Intercept | 11.567 | 0.836 | 0.000 | 0.195 | 1.350 |
| EducationMin | 0.547 | 0.185 | 0.004 | |||
| Sex | 0.667 | 0.283 | 0.021 | |||
| EducationMin2 | −0.021 | 0.010 | 0.030 | |||
| FSCRT-DelSABIEX | Intercept | 9.386 | 0.430 | 0.000 | 0.632 | 0.908 |
| FCSRT-ImmMin | 0.272 | 0.023 | 0.000 | |||
| EducationMin | 0.069 | 0.027 | 0.011 | |||
| ROCF-ImmSABIEX | Intercept | 8.771 | 1.57 | 0.000 | 0.144 | 4.554 |
| ROCF-CMin | 0.872 | 0.044 | 0.000 | |||
| ROCF-DelSABIEX | Intercept | 3.727 | 0.616 | 0.000 | 0.792 | 2.199 |
| ROCF-ImmMin | 0.872 | 0.044 | 0.000 | |||
| JLO | Intercept | 22.252 | 0.958 | 0.000 | 0.208 | 4.446 |
| Sex | −4.263 | 0.933 | 0.000 | |||
| EducationMin2 | 0.014 | 0.007 | 0.038 |
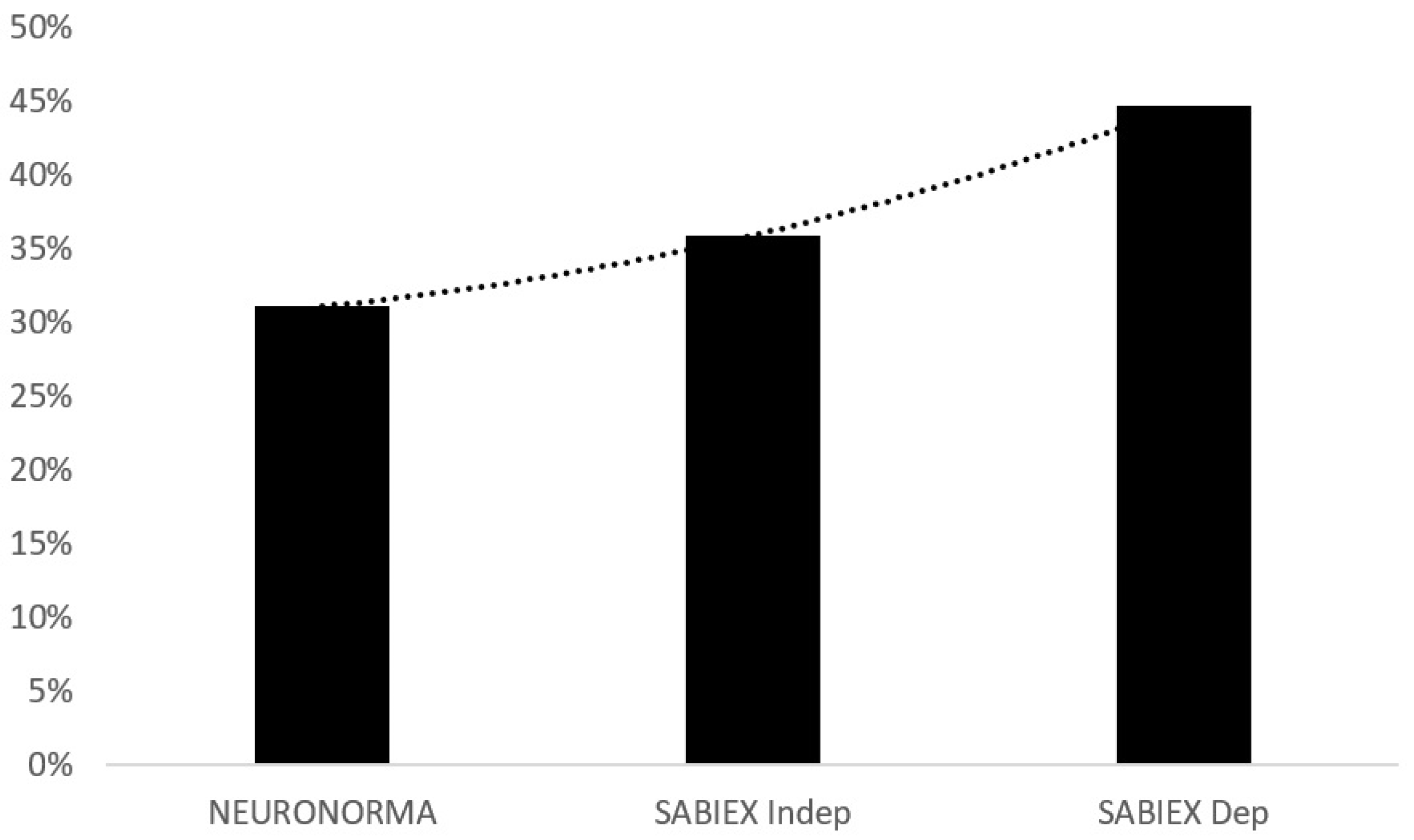
| Low Scores | NEURONORMA | SABIEXINDEP | SABIEXDEP | |||
|---|---|---|---|---|---|---|
| Freq | Cum% | Freq | Cum% | Freq. | Cum% | |
| 0 | 71 | 100 | 66 | 100 | 57 | 100 |
| 1 | 23 | 31.1 | 17 | 35.9 | 31 | 44.7 |
| 2 | 3 | 8.8 | 12 | 19.5 | 12 | 14.6 |
| 3 | 4 | 5.9 | 5 | 7.8 | 2 | 2.9 |
| 4 | 1 | 2 | 2 | 2.9 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 0 | 0 |
| Total | 103 | 103 | 103 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Rubio, E.; Oltra-Cucarella, J.; Bonete-López, B.; Iñesta, C.; Sitges-Maciá, E. Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Free and Cued Selective Reminding Test, Rey–Osterrieth Complex Figure Test and Judgement of Line Orientation. Int. J. Environ. Res. Public Health 2021, 18, 12977. https://doi.org/10.3390/ijerph182412977
Calderón-Rubio E, Oltra-Cucarella J, Bonete-López B, Iñesta C, Sitges-Maciá E. Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Free and Cued Selective Reminding Test, Rey–Osterrieth Complex Figure Test and Judgement of Line Orientation. International Journal of Environmental Research and Public Health. 2021; 18(24):12977. https://doi.org/10.3390/ijerph182412977
Chicago/Turabian StyleCalderón-Rubio, Eva, Javier Oltra-Cucarella, Beatriz Bonete-López, Clara Iñesta, and Esther Sitges-Maciá. 2021. "Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Free and Cued Selective Reminding Test, Rey–Osterrieth Complex Figure Test and Judgement of Line Orientation" International Journal of Environmental Research and Public Health 18, no. 24: 12977. https://doi.org/10.3390/ijerph182412977
APA StyleCalderón-Rubio, E., Oltra-Cucarella, J., Bonete-López, B., Iñesta, C., & Sitges-Maciá, E. (2021). Regression-Based Normative Data for Independent and Cognitively Active Spanish Older Adults: Free and Cued Selective Reminding Test, Rey–Osterrieth Complex Figure Test and Judgement of Line Orientation. International Journal of Environmental Research and Public Health, 18(24), 12977. https://doi.org/10.3390/ijerph182412977






