Developing a Smartphone Application That Promotes Responsible Short-Acting Beta2-Agonist Use in People with Asthma: A Participatory Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedure
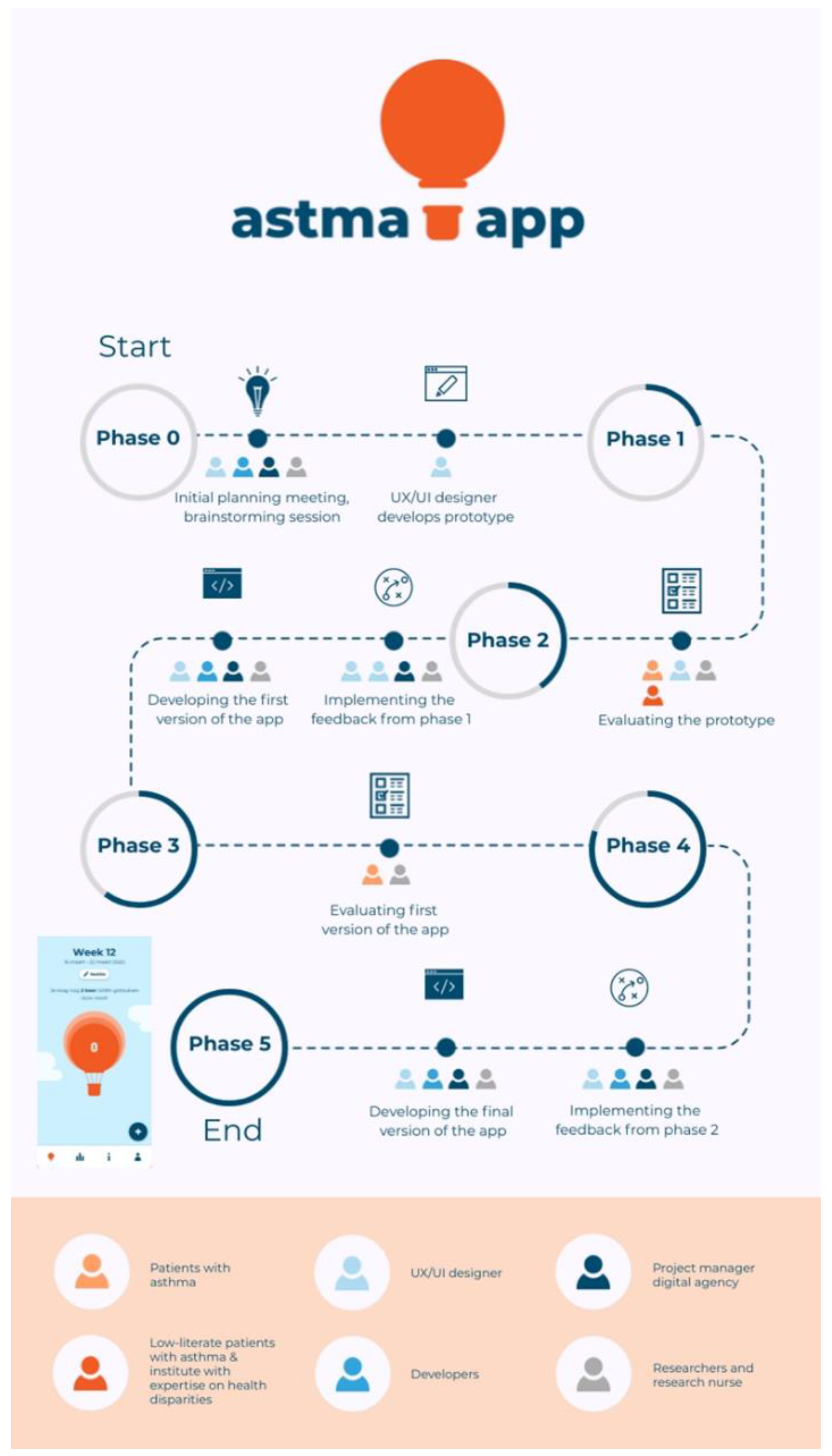
2.3. App Development Process
2.4. Phase 0—Identifying the Problem and Developing the Prototype
2.5. Phase 1.1—Evaluating the Prototype (Qualitative Test Sessions)
2.6. Phase 1.2—Evaluating the Prototype (Institute with Expertise on Health Disparities)
2.7. Phase 2—Implementing Feedback from Phase 1
2.8. Phase 3—Evaluating the First Version of the App
2.9. Phase 4—Implementing Feedback from Phase 2
2.10. Phase 5—Final Version of the App
3. Results
3.1. First Iterative Cycle
Pharos Session
3.2. Second Iterative Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Remarks Prototype | Frequency | Category 1 |
|---|---|---|
| Registering SABA (task: add one SABA inhalation) | ||
| I would use the app immediately after using SABA; otherwise, I would forget to register the used SABA | 3 | 1,2 |
| The homepage is accessible | 1 | 1,3 |
| I always play with new apps first to see how they work | 1 | 2 |
| It is great that the date is prominent on the homepage | 1 | 1,3 |
| Balloon | ||
| Warning that you have taken too much SABA is appropriate | 3 | 1,3 |
| Bursting of the balloon looks like a little sun, later it became clear that it means that the balloon has burst | 1 | 1,3 |
| It is a nice design, the app can be used anywhere because others do not immediately see that the app is about asthma | 1 | 1 |
| It seems like an app for children (a lot of gamification) | 1 | 4 * |
| The use of a traffic light would also be pleasant; if you have taken less SABA than twice a week, the color could be green | 1 | 4 ** |
| Graph | ||
| Triggers are clear in the overview | 4 | 1,3 |
| Swiping from the sixth to the second week felt natural | 1 | 1,3 |
| Questionnaire (not available in app prototype) | ||
| Registering symptoms in combination with registering SABA use is a good idea | 3 | 1 |
| Pulling apart fatigue/restriction | 1 | 2 *** |
| Answers “would you use the app?” | ||
| Yes, I would use the app, because it takes too much time to keep track of my medication at the moment | 3 | 1,2 |
| Yes, it is useful to take the app to my general practitioner/pulmonologist to show the results in the graph | 3 | 1,2 |
| I would definitely use the app! At the end of the week, you have your results. Where can I find the peak flow? It would help me to change my behavior | 1 | 1,2 *** |
| Yes, this would increase my energy distribution and I would love to do this without taking medication | 1 | 1,2 |
| I do not need any insight into my medication, because I am a nurse. I would rather know what I need or when I should take more medication | 1 | 3 **** |
| I can look up my symptoms and share this insight with my physiotherapist | 1 | 1,2 |
| Missing aspects or wanted changes in the app | ||
| Make the date more prominent | 1 | 3 |
| Being able to add SABA daily | 1 | 3 |
| Disseminate the app via pulmonary nurses and perhaps engage them in testing the app | 1 | 2 *** |
| Remarks First Version of the App | Frequency | Category 1 |
|---|---|---|
| First impression | ||
| It is great that you can change the maximum amount of SABA based on the advice of your doctor | 2 | 1,3 |
| The hot air balloon is a beneficial change compared to the bursting balloon | 1 | 1,3 |
| It is a nice and clear app. The app can also be used by less literate people. It is obvious how to use the app | 1 | 1 |
| Great that I can add triggers, this way I get an overview when symptoms worsen | 1 | 1,3 |
| Evaluation app usage | ||
| When you open the app, it is immediately clear how much SABA you have used and you can easily add SABA | 2 | 1,3 |
| The information used in the app was clear, such as the different asthma types | 2 | 1,3 |
| It is useful that you can fill in the CARAT questionnaire once a week. The questions are very clear | 1 | 1,3 |
| It is clear that you can change the maximum amount of SABA use under “profile” | 1 | 1,3 |
| The feedback “Great, you needed less SABA than recommended” is pleasant, because it sounds positive | 1 | 1,3 |
| The link to the website www.inhalatorgebruik.nl is very helpful | 1 | 1,3 |
| This app came into my life at the right time and place, due to COVID-19 I am unable to go to my pulmonary rehabilitation | 1 | 1,2 |
| It is very pleasant that the app is solely focused on asthma and that there are no advertisements in the app | 1 | 1,3 |
| The app does not contain too much information, if you want to know more, you should contact your pulmonary nurse or doctor | 1 | 1,2 |
| The dashboard is really convenient. In the graph you get an overview of your symptoms. It is a lot harder to get this overview by writing it down in a notebook, because you sometimes forget to write it down. For example, this week I do not remember how I have slept last week | 1 | 1,3 |
| Ease of use functionalities | ||
| All four icons and the matching functionalities were easy to find and easy to use | 5 | 1,3 |
| The graphical overview was easy to interpret | 5 | 1,3 |
| The questionnaire about symptoms and triggers was clear and quickly to fill out | 1 | 1,3 |
| The weekly and monthly graphical overview were both clear | 1 | 1,3 |
| Notes work well as some sort of weekly report | 1 | 1,3 |
| The information section was clear | 1 | 1,3 |
| Goal | ||
| Yes, it is clear which information you want from me, namely is my asthma controlled/how stable am I at the moment? | 1 | 1 |
| Yes, I think it is clear | 1 | 1 |
| The goal is clear: how to use your SABA and how you can decrease your SABA use | 1 | 1,3 |
| Using the app after release | ||
| Yes, because it shows you an overview of your used SABA and the app is user-friendly | 2 | 1,3 |
| Yes, because it becomes clear when my asthma is fluctuating and when I have a worsen period. Moreover, I can show the app to my pulmonary nurse | 1 | 1,2 |
References
- The Global Asthma Network. The Global Asthma Report. 2018. Available online: http://www.globalasthmareport.org/ (accessed on 8 September 2020).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available online: https://ginasthma.org/reports/ (accessed on 8 September 2020).
- Nederlands Huisartsen Genootschap. Astma bij Volwassenen (derde Herziening). 2020. Available online: https://www.nhg.org/standaarden/volledig/nhg-standaard-astma-bij-volwassenen (accessed on 12 August 2020).
- Belhassen, M.; Nibber, A.; Van Ganse, E.; Ryan, D.; Langlois, C.; Appiagyei, F.; Skinner, D.; Laforest, L.; Soriano, J.B.; Price, D. Inappropriate asthma therapy-a tale of two countries: A parallel population-based cohort study. Npj Prim. Care Respir. Med. 2016, 26, PA4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoly, P.; Annunziata, K.; Gubba, E.; Adamek, L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur. Respir. Rev. 2012, 21, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosse, R.C.; Murray, E.; Bouvy, M.L.; de Vries, T.W.; Stevenson, F.; Koster, E.S. Potential normalization of an asthma mHealth intervention in community pharmacies: Applying a theory-based framework. Res. Soc. Adm. Pharm. 2020, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, V.; Price, D.; Papi, A.; Infantino, A.; Stallberg, B.; Ryan, D.; Lavorini, F.; Chrystyn, H.; Haugney, J.; Lisspers, K.; et al. A multinational observational study identifying primary care patients at risk of overestimation of asthma control. Npj Prim. Care Respir. Med. 2019, 29, 43. [Google Scholar] [CrossRef]
- Stanford, R.H.; Shah, M.B.; D’Souza, A.O.; Dhamana, A.D.; Schatz, M. Short-acting beta-agonist use and its ability to predict future asthma-related outcomes. Ann. Allergy Asthma Immunol. 2012, 109, 403–407. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Ekström, M.; Hasvold, P.; Wiklund, F.; Telg, G.; Janson, C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: A nationwide cohort study of the global SABINA programme. Eur. Respir. J. 2020, 55, 1901872. [Google Scholar] [CrossRef] [Green Version]
- Rennard, S.I.; Farmer, S.G. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004, 1, 88–92. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Tavakoli, H.; Lynd, L.D.; Al Efraij, K.; Sadatsafavi, M. The impact of inappropriate use of short acting beta agonists in asthma. Respir. Med. 2017, 131, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Anis, A.H.; Lynd, L.D.; Wang, X.H.; King, G.; Spinelli, J.J.; Fitzgerald, M.; Bai, T.; Pare, P. Double trouble: Impact of inappropriate use of asthma medication on the use of health care resources. Can. Med. Assoc. J. 2001, 164, 625–631. [Google Scholar]
- O’Byrne, P.M.; Jenkins, C.; Bateman, E.D. The paradoxes of asthma management: Time for a new approach? Eur. Respir. J. 2017, 50, 1701103. [Google Scholar] [CrossRef] [Green Version]
- Partridge, M.R.; van der Molen, T.; Myrseth, S.E.; Busse, W.W. Attitudes and actions of asthma patients on regular maintenance therapy: The INSPIRE study. BMC Pulm. Med. 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janson, C.; Menzies-Gow, A.; Nan, C.; Nuevo, J.; Papi, A.; Quint, J.K.; Quirce, S.; Vogelmeier, C.F. SABINA: An Overview of Short-Acting β2-Agonist Use in Asthma in European Countries. Adv. Ther. 2020, 37, 1124–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, D.; Fletcher, M.; van der Molen, T. Asthma control and management in 8,000 European patients: The REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim. Care Respir. Med. 2014, 24, 14009. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.F.; Chaudhuri, R.; Thomson, N.C.; Ramparsad, N.; O’Pray, H.; Barclay, S.; MacBride-Stewart, S.; McCallum, C.; Sharma, V.; McSharry, C.; et al. Insights into frequent asthma exacerbations from a primary care perspective and the implications of UK National Review of Asthma Deaths recommendations. NPJ Prim. Care Respir. Med. 2018, 28, 35. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, H.; FitzGerald, J.M.; Lynd, L.D.; Sadatsafavi, M. Predictors of inappropriate and excessive use of reliever medications in asthma: A 16-year population-based study. BMC Pulm. Med. 2018, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- van Kemenade, G.J.; Paalvast, H.; Uijleman, L.; Duine, A.; ten Have, P.; van Hal, P.; Moen, M.; Bosma, J.; van Asselt, R. Verslag Verdiepingsbijeenkomst Zinnige Zorg Astma. 2020. Available online: https://www.zorginstituutnederland.nl/werkagenda/publicaties/verslag/2020/03/05/zinnige-zorg---verslag-verdiepingsbijeenkomst-astma20 (accessed on 11 April 2022).
- Kaplan, A.; van Boven, J.F.M.; Ryan, D.; Tsiligianni, I.; Bosnic-Anticevich, S.; Grp, R.A.W. GINA 2020: Potential Impacts, Opportunities, and Challenges for Primary Care. J. Allergy Clin. Immunol. Pract. 2021, 9, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Pladevall, M.; Xi, H.; Peterson, E.L.; Joseph, C.; Lafata, J.E.; Ownby, D.R.; Johnson, C.C. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J. Allergy Clin. Immunol. 2004, 114, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Soliman, M.; McIvor, A.; Cave, A.; Cabrera, C. Understanding Patient Perspectives on Medication Adherence in Asthma: A Targeted Review of Qualitative Studies. Patient Prefer. Adherence 2020, 14, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Barrett, M.A.; Humblet, O.; Marcus, J.E.; Henderson, K.; Smith, T.; Eid, N.; Sublett, J.W.; Renda, A.; Nesbitt, L.; Van Sickle, D.; et al. Effect of a mobile health, sensor-driven asthma management platform on asthma control. Ann. Allergy Asthma Immunol. 2017, 119, 415. [Google Scholar] [CrossRef]
- Merchant, R.K.; Inamdar, R.; Quade, R.C. Effectiveness of Population Health Management Using the Propeller Health Asthma Platform: A Randomized Clinical Trial. J. Allergy Clin. Immunol. Pract. 2016, 4, 455–463. [Google Scholar] [CrossRef]
- Gibson, P.G.; Powell, H.; Coughlan, J.; Wilson, A.J.; Abramson, M.; Haywood, P.; Bauman, A.; Hensley, M.J.; Walters, E.H. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst. Rev. 2003, 1, CD001117. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, H. Supported self-management for asthma. Breathe (Sheff) 2015, 11, 98–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinnock, H.; Parke, H.L.; Panagioti, M.; Daines, L.; Pearce, G.; Epiphaniou, E.; Bower, P.; Sheikh, A.; Griffiths, C.J.; Taylor, S.J.; et al. Systematic meta-review of supported self-management for asthma: A healthcare perspective. BMC Med. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- James, D.R.; Lyttle, M.D. British guideline on the management of asthma: SIGN Clinical Guideline 141, 2014. Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbert, N.J.; van Os-Medendorp, H.; van Renselaar, W.; Ekeland, A.G.; Hakkaart-van Roijen, L.; Raat, H.; Nijsten, T.E.C.; Pasmans, S.G.M.A. Effectiveness and Cost-Effectiveness of eHealth Interventions in Somatic Diseases: A Systematic Review of Systematic Reviews and Meta-Analyses. J. Med. Internet Res. 2014, 16, 182–204. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.R.; Ritter, P.L.; Laurent, D.D.; Plant, K. Internet-based chronic disease self-management—A randomized trial. Med. Care 2006, 44, 964–971. [Google Scholar] [CrossRef]
- Miller, L.; Schuz, B.; Walters, J.; Walters, E.H. Mobile Technology Interventions for Asthma Self-Management: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2017, 5, e57. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.; Wyke, S.; Agur, K.; Cameron, E.J.; Docking, R.I.; MacKenzie, A.M.; McConnachie, A.; Raghuvir, V.; Thomson, N.C.; Mair, F.S. Digital Asthma Self-Management Interventions: A Systematic Review. J. Med. Internet Res. 2014, 16, e51. [Google Scholar] [CrossRef] [Green Version]
- Krebs, P.; Duncan, D.T. Health App Use Among US Mobile Phone Owners: A National Survey. JMIR Mhealth Uhealth 2015, 3, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, L.; Seaton, P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J. Med. Internet Res. 2016, 18, e97. [Google Scholar] [CrossRef]
- Van Gemert-Pijnen, J.E.; Nijland, N.; van Limburg, M.; Ossebaard, H.C.; Kelders, S.M.; Eysenbach, G.; Seydel, E.R. A holistic framework to improve the uptake and impact of eHealth technologies. J. Med. Internet Res. 2011, 13, e111. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.; Jepson, R.; Hoskins, G.; Wilson, C.; Pinnock, H.; Sheikh, A.; Wyke, S. Understanding what helps or hinders asthma action plan use: A systematic review and synthesis of the qualitative literature. Patient Educ. Couns. 2011, 85, E131–E143. [Google Scholar] [CrossRef] [PubMed]
- Hsia, B.; Mowrey, W.; Keskin, T.; Wu, S.; Aita, R.; Kwak, L.; Ferastraoarou, D.; Rosenstreich, D.; Jariwala, S.P. Developing and pilot testing ASTHMAXcel, a mobile app for adults with asthma. J. Asthma 2020, 58, 834–847. [Google Scholar] [CrossRef]
- Pagliari, C. Design and evaluation in eHealth: Challenges and implications for an interdisciplinary field. J. Med. Internet Res. 2007, 9, e15. [Google Scholar] [CrossRef]
- Nijland, N.; van Gemert-Pijnen, J.; Boer, H.; Steehouder, M.F.; Seydel, E.R. Evaluation of Internet-Based Technology for Supporting Self-Care: Problems Encountered by Patients and Caregivers When Using Self-Care Applications. J. Med. Internet Res. 2008, 10, e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabangwe, R.; Edison, H.; Duc, A.N. Software engineering process models for mobile app development: A systematic literature review. J. Syst. Softw. 2018, 145, 98–111. [Google Scholar] [CrossRef]
- Van der Kleij, R.M.J.J.; Kasteleyn, M.J.; Meijer, E.; Bonten, T.N.; Houwink, I.J.F.; Teichert, M.; van Luenen, S.; Vedanthan, R.; Evers, A.; Car, J.; et al. SERIES: eHealth in primary care. Part 1: Concepts, conditions and challenges. Eur. J. Gen. Pract. 2019, 25, 179–189. [Google Scholar] [CrossRef]
- Licskai, C.J.; Sands, T.W.; Ferrone, M. Development and pilot testing of a mobile health solution for asthma self-management: Asthma action plan smartphone application pilot study. Can. Respir. J. 2013, 20, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; Peters, D.; Calvo, R.A.; Sawyer, S.M.; Foster, J.M.; Smith, L. “Kiss myAsthma”: Using a participatory design approach to develop a self-management app with young people with asthma. J. Asthma 2018, 55, 1018–1027. [Google Scholar] [CrossRef]
- Clemensen, J.; Larsen, S.B.; Kyng, M.; Kirkevold, M. Participatory design in health sciences: Using cooperative experimental methods in developing health services and computer technology. Qual. Health Res. 2007, 17, 122–130. [Google Scholar] [CrossRef]
- Eyles, H.; Jull, A.; Dobson, R.; Firestone, R.; Whittaker, R.; Te Morenga, L.; Goodwin, D.; Mhurchu, C.N. Co-design of mHealth Delivered Interventions: A Systematic Review to Assess Key Methods and Processes. Curr. Nutr. Rep. 2016, 5, 160–167. [Google Scholar] [CrossRef]
- Vandekerckhove, P.; de Mul, M.; Bramer, W.M.; de Bont, A.A. Generative Participatory Design Methodology to Develop Electronic Health Interventions: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e13780. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.; Correia-de-Sousa, J.; Bousquet, J.; Bugalho-Almeida, A.; Del Giacco, S.R.; Demoly, P.; Haahtela, T.; Jacinto, T.; Garcia-Larsen, V.; van der Molen, T.; et al. Control of Allergic Rhinitis and Asthma Test (CARAT): Dissemination and applications in primary care. Prim. Care Respir. J. 2013, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Carat. The Control of Allergic Rhinitis and Asthma Test (CARAT). 2020. Available online: http://www.astma-hooikoortstest.nl/carat/index.php (accessed on 24 May 2021).
- Fasoglio, D.; de Jong, K.; Trimbos, B.; Tuin, D.; Beeker, A. Taalprofielen 2015: Herziene versie van Taalprofielen 2004. 2015. Available online: https://www.slo.nl/publish/pages/2890/taalprofielen-2015.pdf (accessed on 9 April 2022).
- Lung Foundation Netherlands. Prikkels. 2021. Available online: https://www.longfonds.nl/longziekten/astma/prikkels (accessed on 20 September 2021).
- Del Giacco, S.R.; Firinu, D.; Bjermer, L.; Carlsen, K.H. Exercise and asthma: An overview. Eur. Clin. Respir. J. 2015, 2, 27984. [Google Scholar] [CrossRef]
- Ahmed, B.; Dannhauser, T.; Philip, N. A systematic review of reviews to identify key research opportunities within the field of eHealth implementation. J. Telemed. Telecare 2019, 25, 276–285. [Google Scholar] [CrossRef]
- Canonica, G.W.; Paggiaro, P.; Blasi, F.; Musarra, A.; Richeldi, L.; Rossi, A.; Papi, A. Manifesto on the overuse of SABA in the management of asthma: New approaches and new strategies. Ther. Adv. Respir. Dis. 2002, 15, 17534666211042534. [Google Scholar] [CrossRef]
- Barnum, C. The ‘magic number 5’: Is it enough for web-testing? Inf. Des. J. 2002, 11, 160–170. [Google Scholar] [CrossRef]
- Versluis, A.; van Luenen, S.; Meijer, E.; Honkoop, P.J.; Pinnock, H.; Mohr, D.C.; Neves, A.L.; Chavannes, N.H.; van der Kleij, R.M.J.J. SERIES: eHealth in primary care. Part 4: Addressing the challenges of implementation. Eur. J. Gen. Pract. 2020, 26, 140–145. [Google Scholar] [CrossRef]


| Remarks Prototype | Frequency | Feasibility 1 |
|---|---|---|
| Registering SABA (task: add one SABA inhalation) | ||
| The amount of SABA advised is personal (“I need to use SABA more than once a day already”) | 2 | + |
| The icon on the top left seems to be for a calendar | 1 | + |
| Is the data automatically sent to the physician? | 1 | − |
| Balloon | ||
| Bursting balloon does not deter | 3 | +/− |
| I am feeling punished when I am allowed to only use SABA twice per week, as I already need two inhalations in the morning | 1 | + |
| The balloon is clear, but the bursting is stigmatizing | 1 | +/− |
| A balloon that is getting bigger is not equal to getting less air. I also have a negative association with the trees in the background because of hay fever | 1 | +/− |
| Graph | ||
| Possibility to view the data (graph) per 6 months | 3 | − |
| Possibility to view the data (graph) per month | 2 | + |
| The graph is not clear, what is the Y-axis? Lower seems to be worse, but it is not clear | 2 | +/− |
| Possibility to view the data (graph) per season | 1 | − |
| Questionnaire (not available in app prototype) | ||
| I want to receive a notification to fill in the questionnaire at the start of the week, and receive a reminder on Wednesday and Friday | 1 | − |
| I want to receive a reminder daily because after two days I do not know anymore that I need to fill out a questionnaire | 1 | − |
| Can you see the items that you have filled in? So actually being able to view the questionnaire each time you completed it (for example, which answer did I give on having a cold that week) | 1 | − |
| I expect a different questionnaire per asthma type | 1 | − |
| Missing to fill in pain | 1 | − |
| Information (not available in the app prototype) | ||
| About inhalation use or medication (explaining short-and long-acting respiratory inhaling) | 4 | + |
| How do you properly take your medication | 3 | + |
| News items or YouTube videos | 2 | − |
| Adding information from a specific website about prescribing and pharmacology | 1 | − |
| Extra information about the app/how does the app work | 1 | + |
| Answers “would you use the app?” | ||
| Only relevant when I can register all asthma medication | 4 | − |
| Missing aspects or wanted changes in the app | ||
| Add a note to fill in details, such as having the flu | 4 | + |
| The maximum amount of SABA should be adjustable per user | 3 | + |
| A counter is helpful | 3 | − |
| What if you use a combination of different medicines? | 3 | − |
| Matching prescription and maximum dosage in-app | 2 | + |
| Adding the word “week” in the graph, what does 1/2/3 et cetra mean? | 2 | + |
| I want to know all the side effects of each type of medication | 2 | − |
| Different types of asthma are important | 2 | + |
| A lower score in the graph in combination with a higher SABA use does not seem logical | 1 | +/− |
| Adding details per day | 1 | − |
| Adding change of medication and taking notes of different side effects, which can be forwarded to the physician | 1 | − |
| Receive a reminder at the end of the day and being able to notify the app about the severity of the asthma | 1 | − |
| Keeping the notification casual | 1 | + |
| Remarks First Version of the App | Frequency | Feasibility 1 |
|---|---|---|
| First impression | ||
| I want to fill in the CARAT more often than once a week | 2 | − |
| The app is very much focused on SABA use, I would prefer to add all asthma medication | 2 | − |
| The score of the CARAT was not completely clear (“When filling out the questionnaire, there was a bit of a confusion whether the red score was okay or not”) | 1 | +/− |
| I wanted to change notes from the first week in the second week, but I was unable to do this. I would have preferred to add a few things, such as my other medication use | 1 | +/− |
| Evaluation app usage | ||
| I do not think I would use the app in my daily life. I am alert when I need to take more medication and what the causes are of the extra usage. If I need to take more medication over a longer period of time, I contact my lung consultant. I do not need to put this information in an app. I would only use the app if my lung consultant could also have access to my data | 1 | − |
| I do not know whether SABA is a commonly known term | 1 | + |
| You do not receive an alert when you have taken too much SABA or when you have uncontrolled asthma | 1 | − |
| Maybe add extra information at the CARAT: that these scores need to be taken seriously and that it is your own responsibility | 1 | − |
| It is nice to notice that all my CARAT scores are below the dotted line | 1 | + |
| I needed to get used to the hot air balloon. The add-sign was not immediately clear, I noticed this button on the second day of usage. Maybe you can add a manual at the start of the app? Especially for users who are less technical | 1 | +/− |
| The first question about your SABA intake was unclear. I filled in the amount of SABA I can use per day instead of per week (4 inhalations versus 28 inhalations) | 1 | +/− |
| Ease of use functionalities | ||
| I found out on the second day that I needed to fill out a questionnaire about symptoms | 1 | + |
| There was not a lot of new information in the information section. Maybe add information about the goal of the app? | 1 | + |
| At the start the hot air balloon was not clear, but this is negligible | 1 | − |
| The add-sign is not very clear | 1 | − |
| Aim | ||
| The goal is to not to use too much SABA and otherwise contact your doctor. However, what is the next step when you have used your maximum amount of SABA? So is the app purely for tracking medication use? Is it part of your asthma plan? When should you exactly alarm your doctor? It might be useful to add this information in the app | 1 | − |
| Yes, it is about SABA. However, it would be convenient to know what the goal of the app is before using it. I needed to go through the whole app before finding the goal | 1 | + |
| Missing aspects or wanted changes in the app | ||
| I would want to add more/all medicines next to SABA | 3 | − |
| I would want to revisit the filled-out CARAT, this way I can discuss this with my pulmonary doctor | 1 | +/− |
| Add information about SABA, preferably at the start of the app | 1 | + |
| Add or change information in the notes from the previous week | 1 | − |
| Is it possible to send the data to my doctor or nurse, this way they can check the graphical overview during an e-consult | 1 | − |
| I would add a remark at the notes that users can write down their other medicines over there | 1 | + |
| Using the app after release | ||
| No, I would not use the app. It has no added value until my long consultant has access to my data | 1 | − |
| No, because I want to be able to add all my asthma medication | 1 | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, L.N.; Hallensleben, C.; Chavannes, N.H.; Versluis, A. Developing a Smartphone Application That Promotes Responsible Short-Acting Beta2-Agonist Use in People with Asthma: A Participatory Design. Int. J. Environ. Res. Public Health 2022, 19, 8496. https://doi.org/10.3390/ijerph19148496
van den Berg LN, Hallensleben C, Chavannes NH, Versluis A. Developing a Smartphone Application That Promotes Responsible Short-Acting Beta2-Agonist Use in People with Asthma: A Participatory Design. International Journal of Environmental Research and Public Health. 2022; 19(14):8496. https://doi.org/10.3390/ijerph19148496
Chicago/Turabian Stylevan den Berg, Liselot N., Cynthia Hallensleben, Niels H. Chavannes, and Anke Versluis. 2022. "Developing a Smartphone Application That Promotes Responsible Short-Acting Beta2-Agonist Use in People with Asthma: A Participatory Design" International Journal of Environmental Research and Public Health 19, no. 14: 8496. https://doi.org/10.3390/ijerph19148496
APA Stylevan den Berg, L. N., Hallensleben, C., Chavannes, N. H., & Versluis, A. (2022). Developing a Smartphone Application That Promotes Responsible Short-Acting Beta2-Agonist Use in People with Asthma: A Participatory Design. International Journal of Environmental Research and Public Health, 19(14), 8496. https://doi.org/10.3390/ijerph19148496






