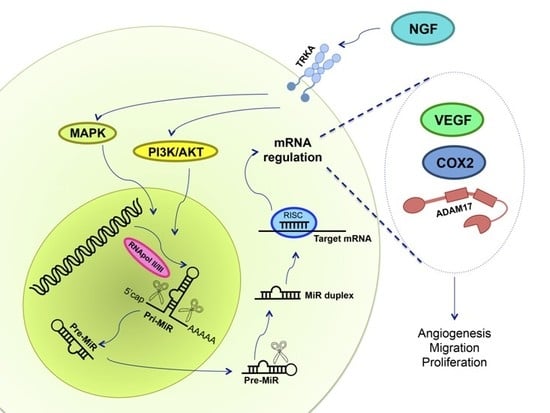
Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer
Abstract
:1. Introduction
2. Ovarian Cancer
3. Epithelial Ovarian Cancer
4. Neurotrophins and Their Role in Ovarian Cancer
5. microRNAs and Cancer
6. Role of miRs in Ovarian Cancer
7. NGF and miRs in Ovarian Cancer
8. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| EOC | Epithelial ovarian cancer |
| NGF | Nerve growth factor |
| VEGF | Vascular endothelial growth factor |
| miR | MicroRNA |
| TRKA | Tyrosine kinase A receptor |
| ADAM17 | Disintegrin and metalloproteinase domain-containing protein 17 |
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer. Int. J. Cancer. 2008, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, K.; Ichimura, A.; Sato, F.; Shimizu, K.; Tsujimoto, G. Sustained activation ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. FEBS J. 2009, 276, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Fujita, Y.; Kojima, T.; Kitamoto, A.; Akao, Y.; Nozawa, Y.; Ito, M. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem. Int. 2012, 60, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ruvkun, G. Molecular biology: Glimpses of a tiny RNA world. Science 2001, 294, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Pope, C.; Botha, J.L. Patients’ help-seeking experiences and delay in cancer presentation: A qualitative synthesis. Lancet 2005, 366, 3–9. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975-2013; National Cancer Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- Brown, P.O.; Palmer, C. The preclinical natural history of serous ovarian cancer: Defining the target for early detection. PLoS Med. 2009, 6, e1000114. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.M.; Bastos, E.; Berchuck, A. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J. Natl. Cancer Inst. 1997, 89, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hunn, J.; Rodriguez, G. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Kjær, S.K.; Dehlendorff, C.; Chang-Claude, J.; Andersen, K.K.; Høgdall, E.; Webb, P.; Jordan, S.; Rossing, M.A.; Doherty, J.A.; et al. Cigarette smoking and risk of ovarian cancer: A pooled analysis of 21 case-control studies. Cancer Cause Control 2013, 24, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Nagle, C.M.; Whiteman, D.C.; Ness, R.; Pearce, C.L.; Pike, M.C.; Rossing, M.A.; Terry, K.L.; Wu, A.H.; Australian Cancer Study (Ovarian Cancer); et al. Obesity and risk of ovarian cancer subtypes: Evidence from the ovarian cancer association consortium. Endocr. Related Cancer 2013, 20, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.; Thiel, F.C.; Bayer, C.M.; Fasching, P.A.; Häberle, L.; Lux, M.P.; Renner, S.P.; Jud, S.M.; Schrauder, M.G.; Müller, A.; et al. Hormone replacement therapy and prognosis in ovarian cancer patients. Eur. J. Cancer Prev. 2013, 22, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Zeppernick, F.; Meinhold-Heerlein, I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Archiv. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Basso, O.; Sampalis, J.; Karp, I.; Martins, C.; Feng, J.; Piedimonte, S.; Quintal, L.; Ramanakumar, A.V.; Takefman, J. Assessment of symptomatic women for early diagnosis of ovarian cancer: Results from the prospective DOvE pilot project. Lancet Oncol. 2012, 13, 285–291. [Google Scholar] [CrossRef]
- Das, P.M.; Bast, R.B. Early detection of ovarian cancer. Biomark. Med. 2008, 2, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Zhao, P.; Yemelyanova, A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: Assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol. Oncol. 2011, 120, 470–473. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Available online: http://www.nice.org.uk/guidance/CG122 (accessed on 20 December 2016).
- Van der Burg, M.E.; van Lent, M.; Buyse, M.; Kobierska, A.; Colombo, N.; Favalli, G.; Lacave, A.J.; Nardi, M.; Renard, J.; Pecorelli, S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N. Engl. J. Med. 1995, 332, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kaye, S.B. New strategies in the treatment of ovarian cancer: Current clinical perspectives and future potential. Clin. Cancer Res. 2012, 19, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Takahashi, F.; Isonishi, S.; Jobo, T.; Aoki, D.; Tsuda, H.; Sugiyama, T.; Kodama, S.; Kimura, E.; et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 1331–1338. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Markman, M.; Markman, J.; Webster, K.; Zanotti, K.; Kulp, B.; Peterson, G.; Belinson, J. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design. J. Clin. Oncol. 2004, 22, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Ilancheran, A.; Ng, J.S. Malignant ovarian germ-cell tumours. Best. Pract. Res. Clin. Obstet. 2012, 26, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Garbi, A.; Achilarre, M.T.; Colombo, N. Ovarian Sex-Cord Tumors. In Ovarian Cancers; Pujade-Lauraine, E., Ray-Coquard, I., Lécuru, F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 261–279. [Google Scholar]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.; Coleman, M.P.; Carreira, H.; Salmerón, D.; Chirlaque, M.D.; Allemani, C. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Ginecol. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.D.; Ponder, B.A. The genetics of ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1 and BRCA2 Associated Hereditary Breast and Ovarian Cancer. In GeneReviews®; Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Ledbetter, N., Mefford, H.C., Smith, R.J.H., Eds.; University of Washington: Seattle, WA, USA, 1998. [Google Scholar]
- Lowe, K.A.; Chia, V.M.; Taylor, A.; O’Malley, C.; Kelsh, M.; Mohamed, M.; Mowat, F.S.; Goff, B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013, 130, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dubeau, L.; Drapkin, R. Coming into focus: The nonovarian origins of ovarian cancer. Ann. Oncol. 2013, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.K.; Conner, M.G.; Landen, C.N. The role of the fallopian tube in the origin of ovarian cancer. Am. J. Obstet. Gynecol. 2013, 209, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef]
- Wong, C.; Wellman, T.L.; Lounsbury, K.M. VEGF and HIF-1α expression are increased in advanced stages of epithelial ovarian cancer. Gynecol. Oncol. 2003, 91, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- The Electronic Medicines Compendium. Avastin 25 mg/mL Concentrate for Solution for Infusion. Available online: https://www.medicines.org.uk/emc/medicine/15748/SPC/Avastin+25mg+ml+concentrate+for+solution+for+infusion/ (accessed on 16 December 2016).
- Gschwind, A.; Fischer, O.M.; Ullrich, A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat. Rev. Cancer 2004, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A. TRK receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett. 2001, 169, 107–114. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Hempstead, B.L. p75 and TRK: A two-receptor system. Trends Neurosci. 1995, 18, 321–326. [Google Scholar] [CrossRef]
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol. 1994, 25, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. TRK receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 2011, 149, 175–181. [Google Scholar] [PubMed]
- Hein, L. The Neuroendocrine Adrenergic System and Cardiovascular Function. In The Cardiovascular Adrenergic System; Springer International Publishing: Cham, Switzerland, 2015; pp. 117–132. [Google Scholar]
- Procaccini, C.; Pucino, V.; de Rosa, V.; Marone, G.; Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Streiter, S.; Fisch, B.; Sabbah, B.; Ao, A.; Abir, R. The importance of neuronal growth factors in the ovary. Mol. Hum Reprod. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.N.; Alves, A.M.; Lima, L.F.; Matos, H.M.; Rodrigues, A.P.; Figueiredo, J.R. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote 2013, 21, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Parrott, J.A.; Skinner, M.K.; Hill, D.F.; Costa, M.E.; Ojeda, S.R. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology 2000, 141, 4736–4750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Du, J.; Yu, Y.; Liang, N.; Liang, M.; Yao, G.; Cui, S.; Huang, H.; Sun, F. NGF promotes mouse granulosa cell proliferation by inhibiting ESR2 mediated down-regulation of CDKN1A. Mol. Cell. Endocrinol. 2015, 406, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Hill, D.F.; Costa, M.E.; Les Dees, C.W.; Lara, H.E.; Ojeda, S.R. A role for TRKA nerve growth factor receptors in mammalian ovulation. Endocrinology 1996, 137, 198–209. [Google Scholar] [PubMed]
- Romero, C.; Paredes, A.; Dissen, G.A.; Ojeda, S.R. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology 2002, 143, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Schmidt, T.; Kimmina, S.; Kozian, D.; Augustin, H.G. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab. Investig. 1998, 78, 1385–1394. [Google Scholar] [PubMed]
- Zimmermann, RC.; Xiao, E.; Husami, N.; Sauer, M.V.; Lobo, R.; Kitajewski, J.; Ferin, M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J. Clin. Endocrinol. Metab. 2001, 86, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.-P.; Nguyen, T.-N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Konishi, I.; Tsuruta, Y.; Nanbu, K.; Mandai, M.; Kuroda, H.; Matsushita, K.; Hamid, A.A.; Yura, Y.; Mori, T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol. Endocrinol. 1997, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G. Gonadotropic control of ovarian follicular growth and development. Mol. Cel. Endocrinol. 2001, 179, 39–46. [Google Scholar] [CrossRef]
- Cantarella, G.; Lempereur, L.; Presta, M.; Ribatti, D.; Lombardo, G.; Lazarovici, P.; Zappala, G.; Pafumi, C.; Bernardini, R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002, 16, 1307–1309. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Giardino, L.; Giuliani, A.; Aloe, L.; Levi-Montalcini, R. Nerve growth factor control of neuronal expression of angiogenic and vasoactive factors. Proc. Natl. Acad. Sci. USA 2001, 98, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Julio-Pieper, M.; Valladares, M.; Pommer, R.; Vega, M.; Mastronardi, C.; Kerr, B.; Ojeda, S.R.; Lara, H.E.; Romero, C. Nerve growth factor-dependent activation of TRKA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J. Clin. Endocrinol. Metab. 2006, 91, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor TRKA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Zhang, W.; Tang, B.; Zhang, J.; Song, H.; Yao, D.; Tang, Y.; Chen, X.; Yang, Z.; et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004, 24, 1973–1979. [Google Scholar] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.; Vanhecke, E.; Saule, P.; Mougel, A.; Page, A.; Romon, R.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008, 68, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Walch, E.T.; Marchetti, D. Role of neurotrophins and neurotrophin receptors in the in vitro invasion and heparanase production of human prostate cancer cells. Clin. Exp. Metastasis 1999, 17, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Adriaenssens, E.; Yazidi-Belkoura, I.E.; Bourhis, X.L.; Nurcombe, V.; Hondermarck, H. Nerve growth factor receptors and signaling in breast cancer. Curr. Cancer Drug Targets 2004, 4, 463–470. [Google Scholar] [CrossRef] [PubMed]
- McGregor, L.M.; McCune, B.K.; Graff, J.R.; McDowell, P.R.; Romans, K.E.; Yancopoulos, G.D.; Ball, D.W.; Baylin, S.B.; Nelkin, B.D. Roles of TRK family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc. Natl. Acad. Sci. USA 1999, 96, 4540–4545. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Greco, S.; Mariotta, S.; Felici, L.; Bronzetti, E.; Cavazzana, A.; Barbolini, G. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respir. Cell Mol. Biol. 2001, 25, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Okumura, T.; Ito, T.; Mori, Y.; Soma, T.; Watanabe, G.; Kaganoi, J.; Itami, A.; Sakai, Y.; Shimada, Y. Significance of nerve growth factor overexpression and its autocrine loop in oesophageal squamous cell carcinoma. Br. J. Cancer 2006, 95, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (TRKA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, U.; Tapia, V.; Geraldo, M.P.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor stimulates cellular proliferation of human epithelial ovarian cancer. Horm. Metab. Res. 2012, 44, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Hurtado, I.; Garrido, M.; Selman, A.; Vega, M. The expression of coclooxigenase-2 is increased by nerve growth factor in epithelial ovarian cancer. In Proceedings of the 24th Biennial Congress of the European Associtaion for Cancer Research, Manchester, UK, 9–12 July 2016.
- Tordjman, C.; Coge, F.; Andre, N.; Rique, H.; Spedding, M.; Bonnet, J. Characterisation of cyclooxygenase 1 and 2 expression in mouse resident peritoneal macrophages in vitro; interactions of non steroidal anti-inflammatory drugs with COX2. Biochim. Biophys. Acta 1995, 1256, 249–256. [Google Scholar] [CrossRef]
- Shen, H.; Li, L.; Zhou, S.; Yu, D.; Yang, S.; Chen, X.; Wang, D.; Zhong, S.; Zhao, J.; Tang, J. The role of ADAM17 in tumorigenesis and progression of breast cancer. Tumour Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Girardi, S.; Tapia, V.; Kohan, K.; Contreras, H.; Gabler, F.; Selman, A.; Vega, M.; Romero, C. ADAM17 and TRKA receptor are involved in epithelial ovarian cancer progression. In Proceedings of the 17th World Congress on Advances in Oncology and 15th International Symposium on Molecular Medicine, Hersonissos, Greece, 11–13 October 2012.
- Romero, C.; Vallejos, C.; Gabler, F.; Selman, A.; Vega, M. Activation of TRKA receptor by nerve growth factor induces shedding of p75 receptor related with progression of epithelial ovarian cancer. In Proceedings of the 23rd Biennial Congress of the European Association for Cancer Research, Munich, Germany, 5–8 July 2014; pp. 5119–5120.
- Vera, C.; Tapia, V.; Kohan, K.; Gabler, F.; Ferreira, A.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor induces the expression of chaperone protein calreticulin in human epithelial ovarian cells. Horm. Metab. Res. 2012, 44, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Qader Hamadneh, L.A.; Veerakumarasivam, A.; Abdul Rahman, S.; Shohaimi, S.; Rosli, R. Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell. Int. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chen, C.; Dong, M.; Zhou, J.; Liu, Q.; Dong, Q.; Li, F. Overexpression of calreticulin contributes to the development and progression of pancreatic cancer. J. Cell. Physiol. 2014, 229, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Tomari, Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015, 25, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Zlotorynski, E. Small RNAs: New microRNA-like molecules. Nat. Rev. Mol. Cell Biol. 2016, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Molasy, M.; Walczak, A.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. MicroRNAs in glaucoma and neurodegenerative diseases. J. Hum. Genet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in cardiovascular disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Fang, X.; Ouyang, G. MicroRNAs: Novel regulators in the hallmarks of human cancer. Cancer Lett. 2009, 285, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Croce, C.M. Roles of small RNAs in tumor formation. Trends Mol. Med. 2010, 16, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.; Kasinski, A.; Slack, F. Aberrant regulation and function of microRNAs in cancer. Curr. Biol. 2014, 24, 762–776. [Google Scholar]
- Zhang, Y.; Zhang, D.; Wang, D.; Xu, D.; Guo, Y.; Cui, W. Serum miRNAs panel (miR-16–2*, miR-195, miR-2861, miR-497) as novel non-invasive biomarkers for detection of cervical cancer. Sci. Rep. 2015, 14, 17942. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Taslim, C.; Weng, D.Y.; Brasky, T.M.; Dumitrescu, R.G.; Huang, K.; Kallakury, B.V.; Krishnan, S.; Llanos, A.A.; Marian, C.; McElroy, J.; et al. Discovery and replication of microRNAs for breast cancer risk using genome-wide profiling. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, X.; Wang, J.; Lopez, J.; Zhou, W.; Yang, L.; Wang, S.E.; Raz, D.J.; Kim, J.Y. Circulating miRNA profile in esophageal adenocarcinoma. Am. J. Cancer Res. 2016, 6, 2713–2721. [Google Scholar] [PubMed]
- Shu, X.; Hildebrandt, M.A.; Gu, J.; Tannir, N.M.; Matin, S.F.; Karam, J.A.; Wood, C.G.; Wu, X. MicroRNA profiling in clear cell renal cell carcinoma tissues potentially links tumorigenesis and recurrence with obesity. Br. J. Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Sergiampietri, C.; Lanfredini, N.; Guiggi, I. Micro-RNAs and ovarian cancer: The state of art and perspectives of clinical research. Gynecol. Endocrinol. 2014, 30, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, V.N.; Tripathi, A.K.; Dwivedi, A.; Sankhwar, P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol. Med. 2015, 12, 328–341. [Google Scholar] [PubMed]
- Cheng, G. Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy. Adv. Drug Deliv. Rev. 2015, 81, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, Z.; Unruh, A.K.; Ivan, C.; Baggerly, K.A.; Calin, G.A.; Li, Z.; Bast, R.C., Jr.; Le, X.F. Clinically relevant microRNAs in ovarian cancer. Mol. Cancer Res. 2015, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Tropé, C.G.; Reich, R.; Davidson, B. MicroRNAs in ovarian cancer. Hum. Pathol. 2015, 46, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Kinose, Y.; Sawada, K.; Nakamura, K.; Kimura, T. The role of microRNAs in ovarian cancer. Biomed. Res. Int. 2014, 2014, 249393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, J.J. HMGA2 and high-grade serous ovarian carcinoma. J. Mol. Med. 2013, 91, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Helland, Å.; Anglesio, M.S.; George, J.; Cowin, P.A.; Johnstone, C.N.; House, C.M.; Sheppard, K.E.; Etemadmoghadam, D.; Melnyk, N.; Rustgi, A.K.; et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS ONE 2011, 6, e18064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Zhang, L.; Zhao, Y.; Yang, D.; Song, F.; Wen, Y.; Hao, Q.; Hu, Z.; Zhang, W.; Chen, K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS ONE 2013, 8, e77853. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guo, R.; Lin, M.; Zhou, B.; Wang, Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol. Oncol. 2011, 122, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, M.T.; Helleman, J.; Boersma, A.W.; van Kuijk, P.F.; van Ijcken, W.F.; Despierre, E.; Vergote, I.; Mathijssen, R.H.; Berns, E.M.; Verweij, J.; et al. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene 2013, 32, 4284–4293. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Nordeen, S.K.; Richer, J.K. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol. Cancer Ther. 2009, 8, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Dimitrova, I.; Howe, E.N.; Cochrane, D.R.; Jean, A.; Spoelstra, N.S.; Post, M.D.; Lu, X.; Broaddus, R.R.; Spillman, M.A.; et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol. Cancer Ther. 2012, 11, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Megraw, M.S.; Giannakakis, A.; Liang, S.; Naylor, T.L.; Barchetti, A.; Ward, M.R.; et al. microRNAs exhibit high frecuency genomic alteration in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 9136–9141. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Thomas, R.; Breen, M.; Zhang, L.; Crago, A.M.; Singer, S.; Khanin, R.; Maki, R.G.; Mihailovic, A.; Hafner, M.; et al. The miR-17–92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer 2012, 51, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Yang, X.; Wang, F.; Cui, Z.; Huang, Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010, 26, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Tinazzi, A.; Giurici, T.; Simonato, F.; Guzzardo, V.; Ventura, L.; Crescenzi, M.; Chiarelli, S.; Fassina, A. Programmed cell death 4 and miR-21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014, 122, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Báez-Vega, P.M.; Echevarría Vargas, I.M.; Valiyeva, F.; Encarnación-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martínez, M.J.; Vivas-Mejía, P.E. Targeting miR-21–3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Xi, Q.H.; Ge, W.L.; Zhang, X.Q. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Kent, O.A.; McCall, M.N.; Cornish, T.C.; Halushka, M.K. Lessons from miR-143/145: The importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014, 42, 7528–7538. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, L.Z.; Qian, X.; Chen, Q.; Jiang, Y.; Li, D.; Lai, L.; Jiang, B.H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth angiogenesis. Nucleic Acids Res. 2012, 40, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Yu, M.; Wu, N.; Wang, H. MicroRNA-145 function as a cell growth repressor by directly targeting c-Myc in human ovarian cancer. Technol. Cancer Res. Treat. 2014, 13, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mataki, H.; Seki, N.; Mizuno, K.; Nohata, N.; Kamikawaji, K.; Kumamoto, T.; Koshizuka, K.; Goto, Y.; Inoue, H. Dual-strand tumor-suppressor microRNA-145 (miR-145–5p and miR-145–3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget 2016, 7, 72085–72098. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, J.Y.; Meng, X.N.; Xiu, Y.L.; Zong, Z.H. MiR-23b targets cyclin G1 and suppresses ovarian cancer tumorigenesis and progression. J. Exp. Clin. Cancer Res. 2016, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Chen, L.; Zhou, L.; Yao, Y. MicroRNA-23b is an independent prognostic marker and suppresses ovarian cancer progression by targeting runt-related transcription factor-2. FEBS Lett. 2014, 588, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, W.; Ge, H.; Li, P.; Wang, Z. Up-regulation of miR-204 enhaces anoikis sensitivity in ephitehlial ovarian cancer cell line via brain-derived neurotrophic factor pathway in vitro. Int. J. Gynecol. Cancer 2015, 25, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Montalban, E.; Mattugini, N.; Ciarapica, R.; Provenzano, C.; Savino, M.; Scagnoli, F.; Prosperini, G.; Carissimi, C.; Fulci, V.; Matrone, C.; et al. MiR-21 is an NGF-modulated microRNA that supports NGF signaling and regulates neuronal degeneration in PC12 cells. Neuromol. Med. 2014, 16, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Pore, S.; Chancellor, M.; Yoshimura, N.; Tyagi, P. Bladder overactivity involves overexpression of microRNA 132 and nerve growth factor. Life Sci. 2016, 167, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Othumpangat, S.; Walton, C.; Piedimonte, G. MicroRNA-221 modulates RSV replication in human bronchial epithelial by targeting NGF expression. PLoS ONE 2012, 7, e30030. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Roy, S.; Nuovo, G.; Ramaswamy, B.; Miller, T.; Shapiro, C.; Jacob, S.T.; Majumder, S. Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamixofen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J. Biol. Chem. 2011, 286, 42292–42302. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Cheng, B.; Li, P.Y.; Huang, H.J.; Zhao, Q.; Dan, Z.L.; Tian, D.A.; Zhang, P. MicroRNA-143 suppresses gastric cancer cells growth and induces apoptosis by targeting COX2. World J. Gastroenterol. 2013, 19, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Xu, H.; Xu, L.; Li, N. MiR-143 targets CTCF and exerts tumor-suppressing functions in epithelial ovarian cancer. Am. J. Transl. Res. 2016, 8, 2716–2726. [Google Scholar] [PubMed]
- Manek, R.; Pakzamir, E.; Mhawech-Fauceglia, P.; Pejovic, T.; Sowter, H.; Gayther, S.A.; Lawrenson, K. Targeting SRC in endometriosis-associated ovarian cancer. Oncogenesis 2016, 5, e251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, X.; Pan, Z.K.; Huang, S. Integrity of SOS1/EPS8/ABI1 TRI-complex determines ovarian cancer metastasis. Cancer Res. 2016, 70, 9979–9990. [Google Scholar] [CrossRef] [PubMed]
- Fulciniti, M.; Amodio, N.; Bandi, R.L.; Cagnetta, A.; Samur, M.K.; Acharya, C.; Prabhala, R.; D’Aquila, P.; Bellizzi, D.; Passarino, G. miR-23b/SP1/c-Myc forms a feed-forward loop supporting multiple myeloma cell growth. Blood Cancer J. 2016, 15, e380. [Google Scholar] [CrossRef] [PubMed]




| miR-23b EOC-Related Targets | miR-23b NGF-Related Targets | miR-23b EOC and NGF Related Targets |
|---|---|---|
| NOTCH 1 | VAV3 | SRC |
| HMGB2 | KLF10 | SOS1 |
| ZEB1 | SOCS6 | SOD1 |
| VCAM1 | NOTCH1 | PTEN |
| RB1 | - | - |
| CAP1 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retamales-Ortega, R.; Oróstica, L.; Vera, C.; Cuevas, P.; Hernández, A.; Hurtado, I.; Vega, M.; Romero, C. Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 507. https://doi.org/10.3390/ijms18030507
Retamales-Ortega R, Oróstica L, Vera C, Cuevas P, Hernández A, Hurtado I, Vega M, Romero C. Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2017; 18(3):507. https://doi.org/10.3390/ijms18030507
Chicago/Turabian StyleRetamales-Ortega, Rocío, Lorena Oróstica, Carolina Vera, Paula Cuevas, Andrea Hernández, Iván Hurtado, Margarita Vega, and Carmen Romero. 2017. "Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer" International Journal of Molecular Sciences 18, no. 3: 507. https://doi.org/10.3390/ijms18030507
APA StyleRetamales-Ortega, R., Oróstica, L., Vera, C., Cuevas, P., Hernández, A., Hurtado, I., Vega, M., & Romero, C. (2017). Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. International Journal of Molecular Sciences, 18(3), 507. https://doi.org/10.3390/ijms18030507