Omalizumab Is Equally Effective in Persistent Allergic Oral Corticosteroid-Dependent Asthma Caused by Either Seasonal or Perennial Allergens: A Pilot Study
Abstract
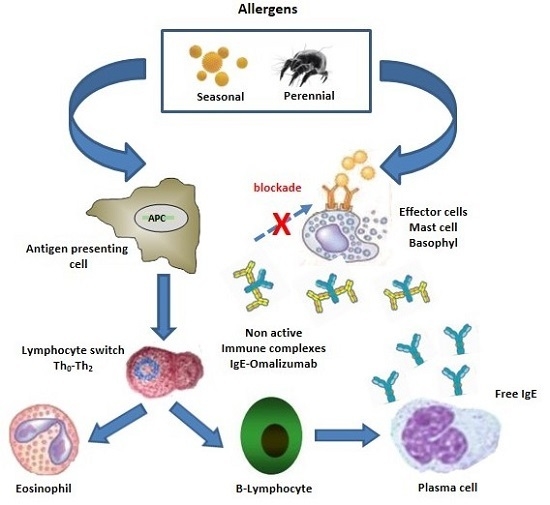
:1. Introduction
2. Results
2.1. Changes in the Number of Exacerbations
2.2. Changes in Oral Corticosteroid Dose
2.3. Changes in Eosinophils, FeNO, and IgE Concentration
2.4. PFTs Evolution
2.5. Side Effects
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Population
4.3. Size Calculation
4.4. Procedures
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available online: http://www.ginasthma.org/documents (accessed on 22 June 2015).
- Domingo, C.; Pacheco, A.; Hinojosa, M.; Bosque, M. The relevance of IgE in the pathogenesis of allergy: The effect of an anti-IgE drug in asthma and other diseases. Recent Pat. Inflamm. Allergy Drug Discov. 2007, 1, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C. Omalizumab for severe asthma: Efficacy beyond the atopic patient? Drugs 2014, 74, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Pomares, X.; Angrill, N.; Rudi, N.; Amengual, M.; Mirapeix, R.M. Effectiveness of omalizumab in non-allergic severe asthma. J. Biol. Regul. Homeost. Agents 2013, 27, 45–53. [Google Scholar] [PubMed]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Vennera Mdel, C.; Pérez De Llano, L.; Bardagí, S.; Ausin, P.; Sanjuas, C.; González, H.; Gullón, J.A.; Martínez-Moragón, E.; Carretero, J.A.; Vera, E.; et al. Omalizumab therapy in severe asthma: Experience from the Spanish registry—Some new approaches. J. Asthma 2012, 49, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Braunstahl, G.-J.; Chen, C.-W.; Maykut, R.; Georgiou, P.; Peachey, G.; Bruce, J. The eXpeRience registry: The “real-world” effectiveness of omalizumab in allergic asthma. Respir. Med. 2013, 107, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Menzies-Gow, A.; Mansur, A.H.; Spencer, D.; Percival, F.; Radwan, A.; Niven, R. Effectiveness of omalizumab in severe allergic asthma: A retrospective UK real-world study. J. Asthma 2013, 50, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Walker, S.; Milan, S.J.; Walters, E.H.; Nair, P. Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev. 2014, 13, CD003559. [Google Scholar]
- Powe, D.G.; Jagger, C.; Kleinjan, A.; Carney, A.S.; Jenkins, D.; Jones, N.S. “Entopy”: Localized mucosal allergic disease in the absence of systemic responses for atopy. Clin. Exp. Allergy 2003, 33, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Guía Española Para el Manejo del Asma-GEMA 4.0. Available online: http://www.semg.es/documentos-semg/guias/1164-gema-4-0-2015.html (accessed on 22 September 2015).
- Casale, T.B.; Condemi, J.; LaForce, C.; Nayak, A.; Rowe, M.; Watrous, M.; McAlary, M.; Fowler-Taylor, A.; Racine, A.; Gupta, N.; et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: A randomized controlled trial. JAMA 2001, 9, 2956–2967. [Google Scholar] [CrossRef]
- Babu, K.S.; Polosa, R.; Morjaria, J.B. Anti-IgE emerging opportunities for omalizumab. Expert Opin. Biol. Ther. 2013, 13, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Moreno, A.; Jose Amengual, M.; Monton, C.; Suarez, D.; Pomares, X. Omalizumab in the management of oral corticosteroid-dependent IGE-mediated asthma patients. Curr. Med. Res. Opin. 2011, 27, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Haldar, P.; Bradding, P.; Wardlaw, A.J. Mepolizumab in refractory eosinophilic asthma. Thorax 2010, 65, 370. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.; Casalle, T.; Wenzell, S.; Bousquet, J.; Deniiz, Y.; Reiisner, C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Ortega, J.; Quirce, S.; Delgado, J.; Dávila, I.; Martí-Guadaño, E.; Valero, A. Diagnostic and therapeutic approaches in respiratory allergy are different depending on the profile of aeroallergen sensitization. Allergol. Immunopathol. 2014, 42, 11–18. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Stanziola, A.; Sanduzzi, A.; Liccardi, G.; Salzillo, A.; Vitale, C.; Molino, A.; Vatrella, A.; D’Amato, M. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): A review. Multidiscip. Respir. Med. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]






| All | Perennial | Seasonal | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Male/Female | 9/19 | 32.1/67.9 | 8/19 | 42.1/57.9 | 1/9 | 11.1/88.9 |
| Median | IQR | Median | IQR | Median | IQR | |
| Exacerbation (year/rate) | 2.67 | 3.17 | 2.67 | 2.67 | 2.67 | 4.00 |
| Omalizumab dose | 300 | 450 | 300 | 300 | 300 | 300 |
| Corticoids | 120 | 224 | 120 | 224 | 120 | 168 |
| IgE | 178.5 | 254.8 | 138 | 173.1 | 229 | 184 |
| FeNO | 15 | 32 | 14 | 25 | 17 | 26 |
| Mean | SD | Mean | SD | Mean | SD | |
| Body Mass Index | 27.43 | 5.75 | 27.68 | 4.25 | 26.91 | 8.39 |
| EoS (%) | 5.31 | 3.53 | 5.2 | 3.79 | 5.29 | 2.93 |
| EoS | 429.29 | 313.83 | 410.00 | 328.67 | 440.00 | 274.10 |
| FVC | 2.69 | 0.78 | 2.71 | 0.90 | 2.67 | 0.52 |
| FVC% | 77.21 | 17.99 | 76.32 | 20.38 | 79.11 | 12.35 |
| FEV1 | 1.71 | 0.59 | 1.65 | 0.63 | 1.85 | 0.49 |
| FEV1% | 64.93 | 20.66 | 62.21 | 22.29 | 70.67 | 16.36 |
| FEV1/FVC% | 63.43 | 13.34 | 60.74 | 13.08 | 69.11 | 12.75 |
| Perennial allergens (n = 20) | Seasonal allergens (n = 10) | ||
|---|---|---|---|
| House dust mites | 13 | Grass pollens | 2 |
| Moulds | 2 | Grass Pollens + cat epithelium | 1 |
| House dust mites + cat and dog epithelium + grass pollen | 2 | Cupressus Pollens + cat epithelium | 1 |
| House dust mites + grass pollen | 1 | Grass Pollens + cat and dog epithelium | 2 |
| House dust mites + cat epithelium + moulds | 1 | Grass Pollen + bird feathers | 1 |
| House dust mites + cat epithelium + grass pollen + moulds | 1 | Grass Pollen + rabbit epithelium | 1 |
| Grass Pollen + house dust mites | 1 | ||
| Parietaria Pollen + house dust mites | 1 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, C.; Pomares, X.; Navarro, A.; Rudi, N.; Sogo, A.; Dávila, I.; Mirapeix, R.M. Omalizumab Is Equally Effective in Persistent Allergic Oral Corticosteroid-Dependent Asthma Caused by Either Seasonal or Perennial Allergens: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 521. https://doi.org/10.3390/ijms18030521
Domingo C, Pomares X, Navarro A, Rudi N, Sogo A, Dávila I, Mirapeix RM. Omalizumab Is Equally Effective in Persistent Allergic Oral Corticosteroid-Dependent Asthma Caused by Either Seasonal or Perennial Allergens: A Pilot Study. International Journal of Molecular Sciences. 2017; 18(3):521. https://doi.org/10.3390/ijms18030521
Chicago/Turabian StyleDomingo, Christian, Xavier Pomares, Albert Navarro, Núria Rudi, Ana Sogo, Ignacio Dávila, and Rosa M. Mirapeix. 2017. "Omalizumab Is Equally Effective in Persistent Allergic Oral Corticosteroid-Dependent Asthma Caused by Either Seasonal or Perennial Allergens: A Pilot Study" International Journal of Molecular Sciences 18, no. 3: 521. https://doi.org/10.3390/ijms18030521
APA StyleDomingo, C., Pomares, X., Navarro, A., Rudi, N., Sogo, A., Dávila, I., & Mirapeix, R. M. (2017). Omalizumab Is Equally Effective in Persistent Allergic Oral Corticosteroid-Dependent Asthma Caused by Either Seasonal or Perennial Allergens: A Pilot Study. International Journal of Molecular Sciences, 18(3), 521. https://doi.org/10.3390/ijms18030521