Targeting the Bacterial Cytoskeleton of the Burkholderia cepacia Complex for Antimicrobial Development: A Cautionary Tale
Abstract
:1. Introduction
2. Results
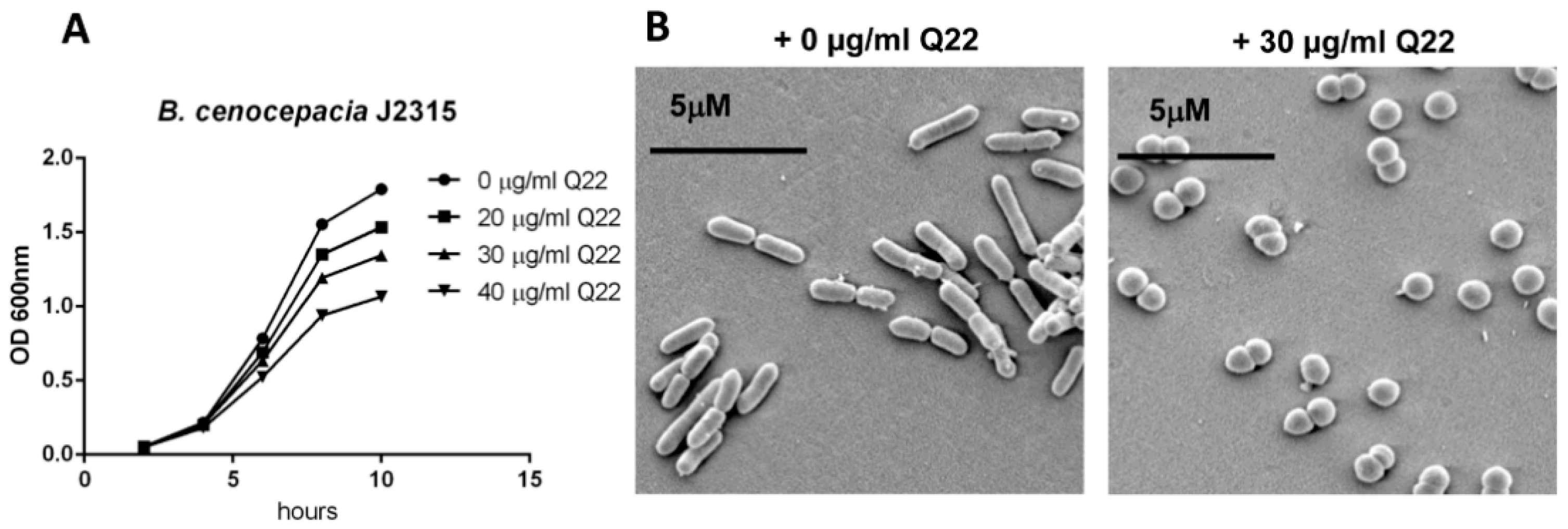
2.1. Q22 Treatment Alters Cell Morphology, Reduces Growth Rate, and Inhibits Growth of B. cenocepacia Species
2.2. Q22 Treatment Alters Ability of B. cenocepacia to Resist H2O2-Induced Oxidative Stress
2.3. Q22 Treatment Does Not Alter Lipopolysaccharide Profile of B. cenocepacia Strains
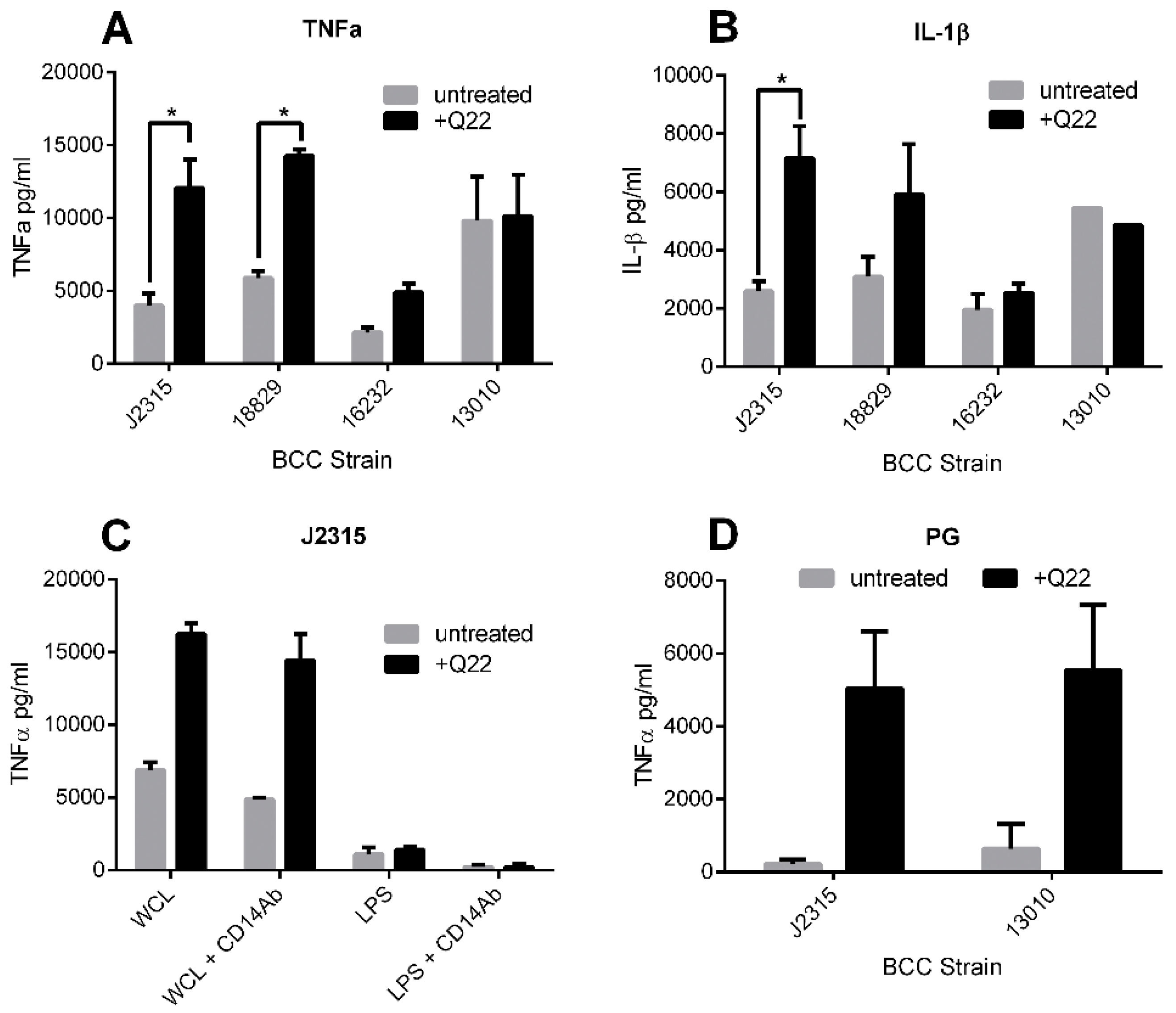
2.4. Q22 Treatment Alters Proinflammatory Potential of BCC Strains
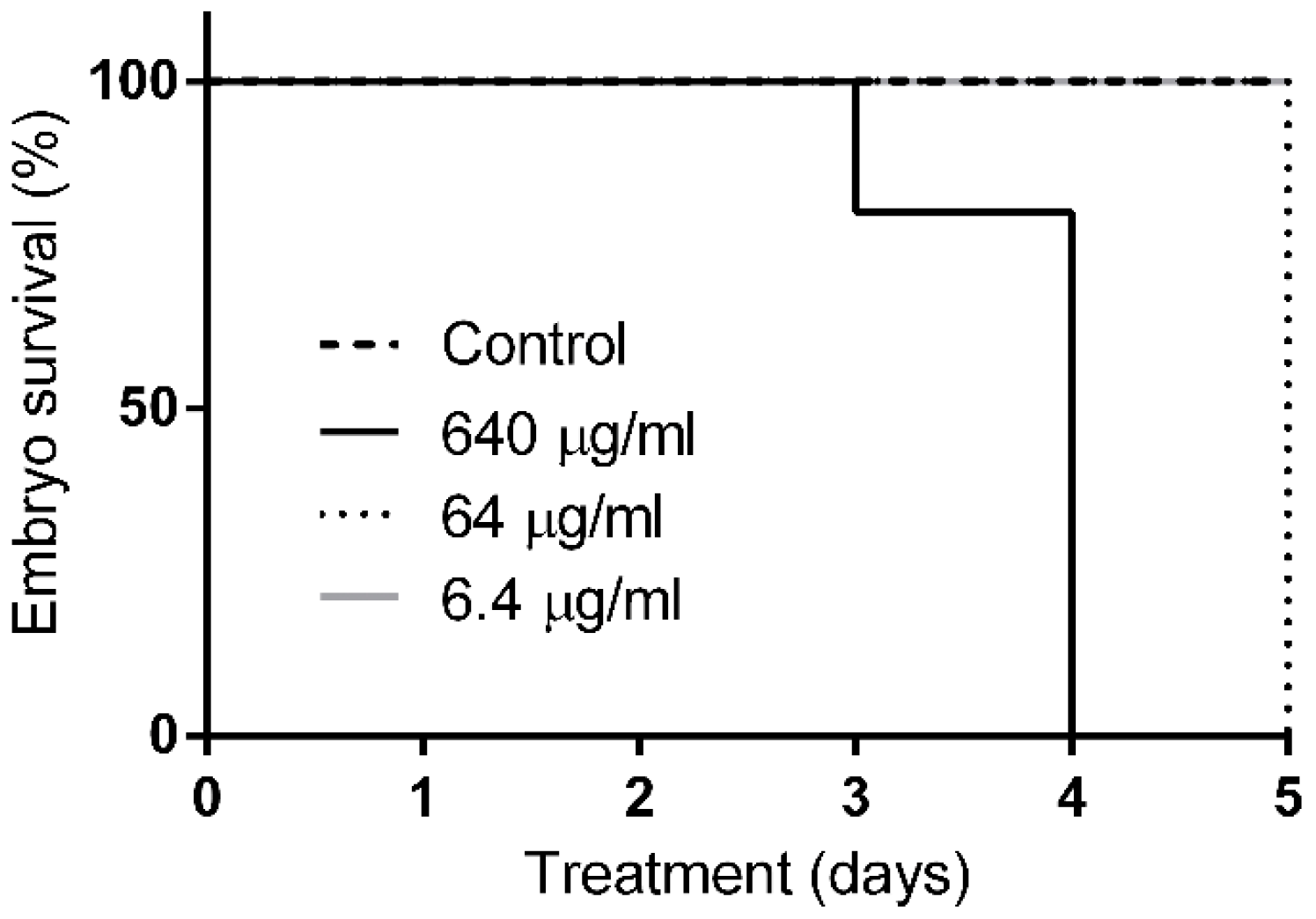
2.5. Q22 Toxicity In-Vivo Studies in Zebrafish and Mouse Models
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bacterial Strains and Growth Conditions
4.3. Q22 Treatment
4.4. Hydrogen Peroxide (H2O2) Protection Assay
4.5. Isolation of Bacterial Whole Cell Lysates (WCLs)
4.6. Purification of Lipopolysaccharide (LPS)
4.7. Isolation of Lipid A
4.8. Matrix Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF) Mass Spectrometry
4.9. Purification of Peptidoglycan (PG)
4.10. Cell Culture
4.11. Stimulation Assays and Cytokine Quantification
4.12. Zebrafish Model
4.13. Mouse Model
4.14. Statistical Methods
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chopra, I. Research and development of antibacterial agents. Curr. Opin. Microbiol. 1998, 1, 495–501. [Google Scholar] [CrossRef]
- Vollmer, W. The prokaryotic cytoskeleton: A putative target for inhibitors and antibiotics? Appl. Microbiol. Biotechnol. 2006, 73, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cowles, K.N.; Gitai, Z. Surface association and the MreB cytoskeleton regulate pilus production, localization and function in Pseudomonas aeruginosa. Mol. Microbiol. 2010, 76, 1411–1426. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, D.M.; Kharraz, L.; Grant, A.J.; Dean, P.; Morgan, F.J.; Karavolos, M.H.; Doble, A.C.; McGhie, E.J.; Koronakis, V.; Daniel, R.A.; et al. The bacterial cytoskeleton modulates motility, type 3 secretion, and colonization in Salmonella. PLoS Pathog. 2012, 8, e1002500. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Nagai, K.; Wachi, M. Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci. Biotechnol. Biochem. 2002, 66, 2658–2662. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Yanagimoto, K.; Nakaminami, H.; Wakabayashi, M.; Iwai, N.; Wachi, M.; Sasatsu, M. Anti-infectious effect of S-benzylisothiourea compound A22, which inhibits the actin-like protein, MreB, in Shigella flexneri. Biol. Pharm. Bull. 2008, 31, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.T.; Doyle, T.B.; Du, Q.; Duncan, L.; Mdluli, K.E.; Lynch, A.S. A Novel indole compound that inhibits Pseudomonas aeruginosa growth by targeting MreB is a substrate for MexAB-OprM. J. Bacteriol. 2007, 189, 6870–6881. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Perry, J.D.; James, A.L.; Stanforth, S.P.; Carnell, S.; Wilkinson, K.; Khan, C.A.; De Soyza, A.; Gould, F.K. In vitro activity of S-(3,4-dichlorobenzyl)isothiourea hydrochloride and novel structurally related compounds against multidrug-resistant bacteria, including Pseudomonas aeruginosa and Burkholderia cepacia complex. Int. J. Antimicrob. Agents 2012, 39, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.; Doherty, C.J.; Nelson, J.W.; Brown, P.H.; Greening, A.P.; Maddison, J.; Dodd, M.; Webb, A.K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 1993, 342, 15–19. [Google Scholar] [CrossRef]
- Lefebre, M.; Valvano, M. In vitro resistance of Burkholderia cepacia complex isolates to reactive oxygen species in relation to catalase and superoxide dismutase production. Microbiology 2001, 147, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Bylund, J.; Burgess, L.A.; Cescutti, P.; Ernst, R.K.; Speert, D.P. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 2006, 281, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Ellis, C.D.; Khan, C.M.; Corris, P.A.; Demarco de Hormaeche, R. Burkholderia cenocepacia lipopolysaccharide, lipid A, and proinflammatory activity. Am. J. Respir. Crit. Care Med. 2004, 170, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Molinaro, A.; Ierano, T.; De Soyza, A.; Sturiale, L.; Garozzo, D.; Aldridge, C.; Corris, P.A.; Khan, C.M.; Lanzetta, R.; et al. The complete structure and pro-inflammatory activity of the lipooligosaccharide of the highly epidemic and virulent gram-negative bacterium Burkholderia cenocepacia ET-12 (strain J2315). Chemistry 2007, 13, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Brodlie, M.; McKean, M.C.; Johnson, G.E.; Perry, J.D.; Nicholson, A.; Verdon, B.; Gray, M.A.; Dark, J.H.; Pearson, J.P.; Fisher, A.J.; et al. Primary bronchial epithelial cell culture from explanted cystic fibrosis lungs. Exp. Lung Res. 2010, 36, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Vergunst, A.C.; Meijer, A.H.; Renshaw, S.A.; O’Callaghan, D. Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 2010, 78, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Pirone, L.; Bragonzi, A.; Farcomeni, A.; Paroni, M.; Auriche, C.; Conese, M.; Chiarini, L.; Dalmastri, C.; Bevivino, A.; Ascenzioni, F. Burkholderia cenocepacia strains isolated from cystic fibrosis patients are apparently more invasive and more virulent than rhizosphere strains. Environ. Microbiol. 2008, 10, 2773–2784. [Google Scholar] [CrossRef] [PubMed]
- Health Do. UK Five Year Antimicrobial Resistance Strategy. 2013. Available online: https://wwwgovuk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategypdf (accessed on 10 January 2016).
- Iwai, N.; Fujii, T.; Nagura, H.; Wachi, M.; Kitazume, T. Structure-activity relationship study of the bacterial actin-like protein MreB inhibitors: Effects of substitution of benzyl group in S-benzylisothiourea. Biosci. Biotechnol. Biochem. 2007, 71, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Demarre, G.; Karpova, T.S.; McNally, J.; Chattoraj, D.K. Changes in nucleoid morphology and origin localization upon inhibition or alteration of the actin homolog, MreB, of Vibrio cholerae. J. Bacteriol. 2007, 189, 7450–7463. [Google Scholar] [CrossRef] [PubMed]
- Mahenthiralingam, E.; Coenye, T.; Chung, J.W.; Speert, D.P.; Govan, J.R.; Taylor, P.; Vandamme, P. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 2000, 38, 910–913. [Google Scholar] [PubMed]
- Van Acker, H.; Sass, A.; Bazzini, S.; De Roy, K.; Udine, C.; Messiaen, T.; Riccardi, G.; Boon, N.; Nelis, H.J.; Mahenthiralingam, E.; et al. Biofilm-grown Burkholderia cepacia complex cells survive antibiotic treatment by avoiding production of reactive oxygen species. PLoS ONE 2013, 8, e58943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takacs, C.N.; Poggio, S.; Charbon, G.; Pucheault, M.; Vollmer, W.; Jacobs-Wagner, C. MreB drives de novo rod morphogenesis in Caulobacter crescentus via remodeling of the cell wall. J. Bacteriol. 2010, 192, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Allison, S.E.; Zlitni, S.; Nguyen, N.D.; Das, R.; Melacini, G.; Capretta, A.A.; Brown, E.D. Degradation of MAC13243 and studies of the interaction of resulting thiourea compounds with the lipoprotein targeting chaperone LolA. Bioorg. Med. Chem. Lett. 2013, 23, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Sunny, S.S.; Oliphant, V.; Perry, J.; Brodlie, M.; Johnson, G.E.; Ward, C.; Gould, K.; Corris, P.A.; De Soyza, A.; et al. Pseudomonas aeruginosa accentuates epithelial-to-mesenchymal transition in the airway. Eur. Respir. J. 2011, 37, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Sturiale, L.; Garozzo, D.; Silipo, A.; Lanzetta, R.; Parrilli, M.; Molinaro, A. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun. Mass Spectrom. 2005, 19, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Sturiale, L.; Garozzo, D.; de Castro, C.; Lanzetta, R.; Parrilli, M.; Grant, W.D.; Molinaro, A. Structure elucidation of the highly heterogeneous lipid A from the lipopolysaccharide of the gram-negative extremophile bacterium Halomonas magadiensis strain 21 m1. Eur. J. Org. Chem. 2004, 2263–2271. [Google Scholar] [CrossRef]
- Bui, N.K.; Eberhardt, A.; Vollmer, D.; Kern, T.; Bougault, C.; Tomasz, A.; Simorre, J.P.; Vollmer, W. Isolation and analysis of cell wall components from Streptococcus pneumoniae. Anal. Biochem. 2012, 421, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal. Biochem. 1975, 67, 503–506. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]

| Species | Description | Source |
|---|---|---|
| B. cenocepacia LMG16656 (J2315) | Clinical isolate, CF patient, ET-12 epidemic strain | BCCM |
| B. cenocepacia LMG18829 | Clinical isolate, CF patient, epidemic strain | BCCM |
| B. multivorans LMG13010 | Clinical isolate, CF patient | BCCM |
| B. vietnamiensis LMG16232 | Clinical isolate, CF patient | BCCM |
| B. cenocepacia LMG18829 Untreated | B. cenocepacia LMG18829 + 30 µg/mL Q22 | |||
|---|---|---|---|---|
| Lipid A Species | Intensity (%) | Mass | Intensity (%) | Mass |
| tetra-acylated lipid A | 58.8 | 1444.9 | ||
| tetra-acylated lipid A + Ara4N | 100 | 1576.4 | 100 | 1576.1 |
| tetra-acylated lipid A + 2 Ara4N | 87.8 | 1707.5 | 60 | 1707.0 |
| penta-acylated lipid A + Ara4N | 48.7 | 1800.9 | 38.2 | 1801.0 |
| penta-acylated lipid A + 2 Ara4N | 39 | 1934.0 | 29 | 1932.2 |
| Group | Dose Q22 (mg/kg) | Results |
|---|---|---|
| 1 | 100 | Mice died immediately after intra-tracheal administration of Q22 |
| 2 | 50 | Mice died immediately after intra-tracheal administration of Q22 |
| 3 | 25 | Mice showed reduced mobility compared with controls during first 2 days after treatment. Lungs were inflamed and damaged after 4 days |
| 4 | 0 | Mice were healthy after treatment |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnell, S.C.; Perry, J.D.; Borthwick, L.; Vollmer, D.; Biboy, J.; Facchini, M.; Bragonzi, A.; Silipo, A.; Vergunst, A.C.; Vollmer, W.; et al. Targeting the Bacterial Cytoskeleton of the Burkholderia cepacia Complex for Antimicrobial Development: A Cautionary Tale. Int. J. Mol. Sci. 2018, 19, 1604. https://doi.org/10.3390/ijms19061604
Carnell SC, Perry JD, Borthwick L, Vollmer D, Biboy J, Facchini M, Bragonzi A, Silipo A, Vergunst AC, Vollmer W, et al. Targeting the Bacterial Cytoskeleton of the Burkholderia cepacia Complex for Antimicrobial Development: A Cautionary Tale. International Journal of Molecular Sciences. 2018; 19(6):1604. https://doi.org/10.3390/ijms19061604
Chicago/Turabian StyleCarnell, Sonya C., John D. Perry, Lee Borthwick, Daniela Vollmer, Jacob Biboy, Marcella Facchini, Alessandra Bragonzi, Alba Silipo, Annette C. Vergunst, Waldemar Vollmer, and et al. 2018. "Targeting the Bacterial Cytoskeleton of the Burkholderia cepacia Complex for Antimicrobial Development: A Cautionary Tale" International Journal of Molecular Sciences 19, no. 6: 1604. https://doi.org/10.3390/ijms19061604
APA StyleCarnell, S. C., Perry, J. D., Borthwick, L., Vollmer, D., Biboy, J., Facchini, M., Bragonzi, A., Silipo, A., Vergunst, A. C., Vollmer, W., Khan, A. C. M., & De Soyza, A. (2018). Targeting the Bacterial Cytoskeleton of the Burkholderia cepacia Complex for Antimicrobial Development: A Cautionary Tale. International Journal of Molecular Sciences, 19(6), 1604. https://doi.org/10.3390/ijms19061604






