The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review
Abstract
:1. Introduction
1.1. Heart Failure
1.2. Nonconventional Therapies for HF
2. Methods
3. Resveratrol
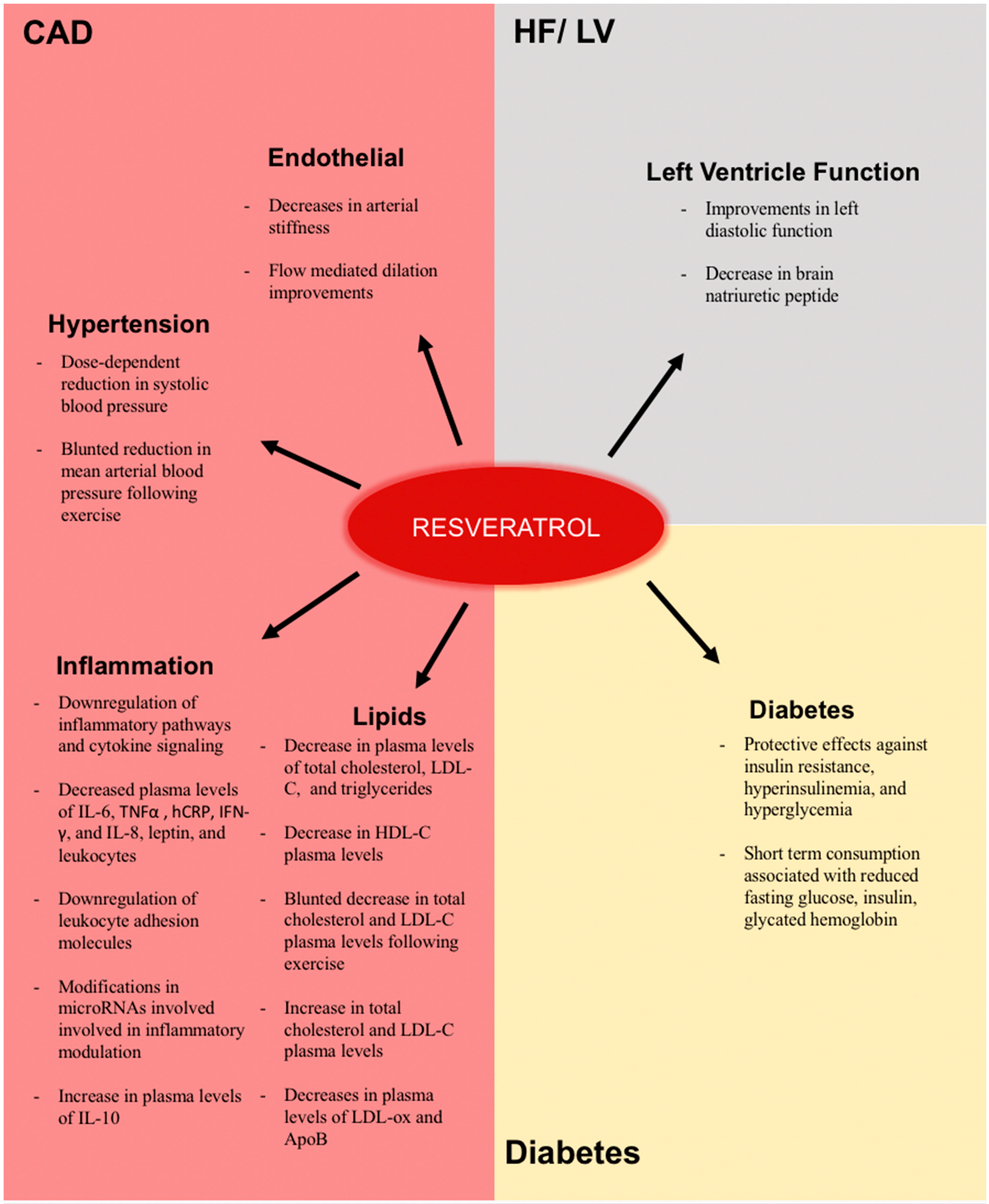
4. Clinical Evidence of the Effects of Resveratrol
4.1. Effects on Factors Related to the Pathogenesis of Atherosclerosis and Coronary Artery Disease
4.2. Effects in Inflammation
4.3. Endothelial Effects
4.4. Lipoprotein and Cholesterol Effects
4.5. Effects on Hypertension
4.6. Effects on Diabetes
4.7. Effects on Heart Failure and Left Ventricle Function
5. Conclusions
Funding
Conflicts of Interest
References
- Cardiovascular diseases (CVDs)—World Health Organization. Cardiovascular diseases (CVDs).
- Heart Disease in Canada. Heart disease—Heart health.
- Stern, C.S.; Lebowitz, J. Latest drug developments in the field of cardiovascular disease. Int. J. Angiol. 2010, 19, e100–e105. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Harris, A.D. Recent advances in managing septal defects: Ventricular septal defects and atrioventricular septal defects. F1000 Res. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Heart and Stroke Foundation. Foundation., H.a.S. Report on the Health of Canadians.
- Kannel, W.B.; D’Agostino, R.B.; Silbershatz, H.; Belanger, A.J.; Wilson, P.W.; Levy, D. Profile for estimating risk of heart failure. Arch. Int. Med. 1999, 159, 1197–1204. [Google Scholar] [CrossRef]
- Johansen, H.; Strauss, B.; Arnold, J.M.; Moe, G.; Liu, P. On the rise: The current and projected future burden of congestive heart failure hospitalization in Canada. Can. J. Cardiol. 2003, 19, 430–435. [Google Scholar] [PubMed]
- Jong, P.; Gong, Y.; Liu, P.P.; Austin, P.C.; Lee, D.S.; Tu, J.V. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation 2003, 108, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.M.; Liu, P.; Demers, C.; Dorian, P.; Giannetti, N.; Haddad, H.; Heckman, G.A.; Howlett, J.G.; Ignaszewski, A.; Johnstone, D.E.; et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: Diagnosis and management. Can. J. Cardiol. 2006, 22, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C., Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. Jama 2003, 289, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Lopatin, M.; Stevenson, L.W.; De Marco, T.; Fonarow, G.C. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am. Coll Cardiol. 2006, 47, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Tendera, M.; Adamus, J.; Freemantle, N.; Polonski, L.; Taylor, J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J. 2006, 27, 2338–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.T.; Ohinmaa, A.; Thanh, N.X.; Howlett, J.G.; Ezekowitz, J.A.; McAlister, F.A.; Kaul, P. The current and future financial burden of hospital admissions for heart failure in Canada: A cost analysis. Can. Med Assoc. J. 2016, 4, E365–E370. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Muller-Werdan, U.; Stockl, G.; Werdan, K. Advances in the management of heart failure: The role of ivabradine. Vasc. Health Risk Manag. 2016, 12, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Lang, C.C. Angiotensin receptor-neprilysin inhibitors: Clinical potential in heart failure and beyond. Vasc. Health Risk Manag. 2015, 11, 283–295. [Google Scholar] [PubMed]
- Yandrapalli, S.; Khan, M.H.; Rochlani, Y.; Aronow, W.S. Sacubitril/valsartan in cardiovascular disease: Evidence to date and place in therapy. Ther. Adv. Cardiovasc. Dis. 2018, 12, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Dyck, J.R. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.; Cook, J.; Koenig, R.A.; Sisson, E.M.; Dixon, D.L. Recent Developments in the Role of Coenzyme Q10 for Coronary Heart Disease: A Systematic Review. Curr. Atheroscler. Rep. 2018, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Romano, B.; Izzo, A.A.; Borrelli, F. The clinical efficacy of curcumin-containing nutraceuticals: An overview of systematic reviews. Pharmacol. Res. 2018, 134, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Ferguson, J.J.A.; Abbott, K.A.; Dias, C.B.; Garg, M.L. Science behind the cardio-metabolic benefits of omega-3 polyunsaturated fatty acids: Biochemical effects vs. clinical outcomes. Food Funct. 2018, 9, 3576–3596. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am. J. Hypertens 2010, 23, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; DiPette, D.J.; Supowit, S.C. Protective effect of resveratrol against pressure overload-induced heart failure. Food Sci. Nutr. 2014, 2, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Qian, L.H.; Deng, B.; Liu, Z.M.; Zhao, Y.; Le, Y.Y. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci. Ther. 2013, 19, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Wu, S.; Huang, S.S.; Lu, B.Y.; Lin, S.M.; Tsai, S.K. Resveratrol protects left ventricle by increasing adenylate kinase and isocitrate dehydrogenase activities in rats with myocardial infarction. Chin. J. Physiol. 2011, 54, 406–412. [Google Scholar] [PubMed]
- Szekeres, T.; Fritzer-Szekeres, M.; Saiko, P.; Jager, W. Resveratrol and resveratrol analogues--structure-activity relationship. Pharm. Res. 2010, 27, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Resveratrol. PubChem Compound Database; CID=445154. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445154 (accessed on 6 September 2018).
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta 2011, 1812, 1477–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, G.; Jensen, C.D.; Norkus, E.P.; Dalvi, T.B.; Wong, L.G.; McManus, J.F.; Hudes, M.L. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: A cross-sectional study. Nutr. J. 2007, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Iyer, A.; Chunduri, P.; Chan, V.; Brown, L. The cardiovascular nutrapharmacology of resveratrol: Pharmacokinetics, molecular mechanisms and therapeutic potential. Curr. Med. Chem. 2010, 17, 2442–2455. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, Y.; Luo, Y.; Niu, W.; Dong, M.; Liu, M.; Dong, H.; et al. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 2016, 13, 942–954. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y.J. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Tasatargil, A.; Tanriover, G.; Barutcigil, A.; Turkmen, E. Protective effect of resveratrol on methylglyoxal-induced endothelial dysfunction in aged rats. Aging Clin. Exp. Res. 2018, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Manganiello, V.; Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Das, S.K.; Levasseur, J.; Byrne, N.J.; Fung, D.; Kim, T.T.; Masson, G.; Boisvenue, J.; Soltys, C.L.; Oudit, G.Y.; et al. Resveratrol treatment of mice with pressure-overload-induced heart failure improves diastolic function and cardiac energy metabolism. Circ. Heart Fail. 2015, 8, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Colombo, E.S.; Campen, M.J. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging 2013, 5, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Lala, A.; Desai, A.S. The role of coronary artery disease in heart failure. Heart Fail. Clin. 2014, 10, 353–365. [Google Scholar] [CrossRef]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Bienso, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zortea, K.; Franco, V.C.; Guimaraes, P.; Belmonte-de-Abreu, P.S. Resveratrol Supplementation Did Not Improve Cognition in Patients with Schizophrenia: Results from a Randomized Clinical Trial. Front. Psychiatry 2016, 7, 159. [Google Scholar] [CrossRef]
- Heeboll, S.; Kreuzfeldt, M.; Hamilton-Dutoit, S.; Kjaer Poulsen, M.; Stodkilde-Jorgensen, H.; Moller, H.J.; Jessen, N.; Thorsen, K.; Kristina Hellberg, Y.; Bonlokke Pedersen, S.; et al. Placebo-controlled, randomised clinical trial: High-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2016, 51, 456–464. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Moller, N.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The Impact of Resveratrol Supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Olesen, J.; Gliemann, L.; Bienso, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Pu, R.; Xue, X. The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: A pooled analysis of randomized controlled trials. Obes. Rev. 2016, 17, 1329–1340. [Google Scholar] [CrossRef]
- Macedo, R.C.; Vieira, A.; Marin, D.P.; Otton, R. Effects of chronic resveratrol supplementation in military firefighters undergo a physical fitness test--a placebo-controlled, double blind study. Chem. -Biol. Interact. 2015, 227, 89–95. [Google Scholar] [CrossRef]
- Mendez-del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Related Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef] [PubMed]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Tome-Carneiro, J.; Larrosa, M.; Yanez-Gascon, M.J.; Davalos, A.; Gil-Zamorano, J.; Gonzalvez, M.; Garcia-Almagro, F.J.; Ruiz Ros, J.A.; Tomas-Barberan, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.; Schechtman, K.B.; Gu, C.; Kunz, I.; Rossi Fanelli, F.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Topper, J.N.; Cai, J.; Falb, D.; Gimbrone, M.A., Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl. Acad. Sci. USA 1996, 93, 10417–10422. [Google Scholar] [CrossRef]
- Lee, R.T.; Yamamoto, C.; Feng, Y.; Potter-Perigo, S.; Briggs, W.H.; Landschulz, K.T.; Turi, T.G.; Thompson, J.F.; Libby, P.; Wight, T.N. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J. Biol. Chem. 2001, 276, 13847–13851. [Google Scholar] [CrossRef]
- Mach, F.; Sauty, A.; Iarossi, A.S.; Sukhova, G.K.; Neote, K.; Libby, P.; Luster, A.D. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Investig. 1999, 104, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Smith, J.D.; Trogan, E.; Ginsberg, M.; Grigaux, C.; Tian, J.; Miyata, M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. USA 1995, 92, 8264–8268. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The role of the lymphocyte. In Atherosclerosis and Coronary Artery Disease; Fuster, V., Ross, R., Topol, E.J., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1996; Volume 1, pp. 557–568. [Google Scholar]
- Ross, R. Atherosclerosis—An inflammatory disease. New Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; de Oliveira, A.C.; Graf, S.; Bhatia, H.S.; Hull, M.; Munoz, E.; Fiebich, B.L. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflamm. 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999, 126, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Wahl, C.; Feldmann, M.; Jungck, D.; Strauch, J.; Stoelben, E.; Koch, A. Resveratrol attenuates the release of inflammatory cytokines from human bronchial smooth muscle cells exposed to lipoteichoic acid in chronic obstructive pulmonary disease. Basic Clin. Pharmacol. Toxicol. 2014, 114, 202–209. [Google Scholar] [CrossRef]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: Role of NF-kappaB inhibition. Am. J. Physiol. 2006, 291, H1694–H1699. [Google Scholar]
- Wang, B.; Sun, J.; Li, X.; Zhou, Q.; Bai, J.; Shi, Y.; Le, G. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res. 2013, 33, 971–981. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjaer, T.N.; Nohr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Milne, J.C.; Imamura, T.; Schenk, S.; Sonoda, N.; Babendure, J.L.; Lu, J.C.; Smith, J.J.; Jirousek, M.R.; Olefsky, J.M. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell. Biol. 2009, 29, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Rieder, S.A.; Nagarkatti, P.; Nagarkatti, M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Br. J. Pharmacol. 2012, 167, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Abuaysheh, S.; Korzeniewski, K.; Patnaik, P.; Marumganti, A.; Chaudhuri, A.; Dandona, P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 2010, 95, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Bakker, G.C.; van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.; Kooistra, T.; van Ommen, B.; Hendriks, H.F. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef]
- Patti, G.; Melfi, R.; Di Sciascio, G. The role of endothelial dysfunction in the pathogenesis and in clinical practice of atherosclerosis. Current evidences. Recenti Progress. Med. 2005, 96, 499–507. [Google Scholar]
- Tousoulis, D.; Charakida, M.; Stefanadis, C. Inflammation and endothelial dysfunction as therapeutic targets in patients with heart failure. Int. J. Cardiol. 2005, 100, 347–353. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y. Acad. Sci. 2017, 1403, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [Green Version]
- Superko, H.R. Hypercholesterolemia and Dyslipidemia. Curr. Treat. Options Cardiovasc. Med. 2000, 2, 173–187. [Google Scholar] [CrossRef]
- Saeed, A.; Feofanova, E.V.; Yu, B.; Sun, W.; Virani, S.S.; Nambi, V.; Coresh, J.; Guild, C.S.; Boerwinkle, E.; Ballantyne, C.M.; et al. Remnant-Like Particle Cholesterol, Low-Density Lipoprotein Triglycerides, and Incident Cardiovascular Disease. J. Am. Coll Cardiol. 2018, 72, 156–169. [Google Scholar] [CrossRef]
- Indolfi, C.; De Rosa, S.; Colombo, A. Bioresorbable vascular scaffolds—basic concepts and clinical outcome. Nat. Rev. Cardiol. 2016, 13, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.G. Intensive reduction of low-density lipoprotein-cholesterol: Implications of recent trials. Am. J. Cardiovasc. Drugs 2006, 6, 367–371. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. New Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef]
- Perez-Mendez, O.; Pacheco, H.G.; Martinez-Sanchez, C.; Franco, M. HDL-cholesterol in coronary artery disease risk: Function or structure? Clin. Chim. Acta 2014, 429, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chyou, J.Y.; Mega, J.L.; Sabatine, M.S. Chapter 4—Pharmacogenetics. In Cardiovascular Therapeutics: A Companion to Braunwald’s Heart Disease, 4th ed.; Antman, E.M., Sabatine, M.S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 53–66. [Google Scholar]
- Stunkel, W.; Campbell, R.M. Sirtuin 1 (SIRT1): The misunderstood HDAC. J. Biomol. Screen. 2011, 16, 1153–1169. [Google Scholar] [CrossRef]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simon, E.; Zechner, R.; Portillo, M.P. Resveratrol regulates lipolysis via adipose triglyceride lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Davidson, M.H.; Pourfarzib, R. Underappreciated opportunities for low-density lipoprotein management in patients with cardiometabolic residual risk. Atherosclerosis 2010, 213, 1–7. [Google Scholar] [CrossRef]
- Georgiopoulou, V.V.; Kalogeropoulos, A.P.; Butler, J. Heart failure in hypertension: Prevention and treatment. Drugs 2012, 72, 1373–1398. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; He, J.; Muntner, P. Prevalence, awareness, treatment and control of hypertension in North America, North Africa and Asia. J. Human Hypertens. 2004, 18, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Vongpatanasin, W. Resistant hypertension: A review of diagnosis and management. Jama 2014, 311, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Song, Y.; Zhang, X.; Liu, Z.; Zhang, W.; Mao, W.; Wang, W.; Cui, W.; Zhang, X.; Jia, X.; et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin. Exp. Pharmacol. Physiol. 2005, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Sehirli, O.; Ersahin, M.; Suleymanoglu, S.; Yiginer, O.; Emekli-Alturfan, E.; Yarat, A.; Yegen, B.C.; Sener, G. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J. Pharm. Pharmacol. 2010, 62, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the eye: Activity and molecular mechanisms. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Mannari, C.; Bertelli, A.A.; Stiaccini, G.; Giovannini, L. Wine, sirtuins and nephroprotection: Not only resveratrol. Med. Hypotheses 2010, 75, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Porquet, D.; Vicente, A.; Sanfeliu, C. Resveratrol: New avenues for a natural compound in neuroprotection. Curr. Pharm. Des. 2013, 19, 6726–6731. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Hsieh, T.C. Resveratrol: A cardioprotective substance. Ann. N. Y. Acad. Sci. 2011, 1215, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysikou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2018, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef]
- Mentz, R.J.; Felker, G.M. Noncardiac comorbidities and acute heart failure patients. Heart Fail. Clin. 2013, 9, 359–367. [Google Scholar] [CrossRef]
- Swan, J.W.; Anker, S.D.; Walton, C.; Godsland, I.F.; Clark, A.L.; Leyva, F.; Stevenson, J.C.; Coats, A.J. Insulin resistance in chronic heart failure: Relation to severity and etiology of heart failure. J. Am. Coll Cardiol. 1997, 30, 527–532. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.A.; Gilbert, R.E.; Liu, P. Treatment of Diabetes in People With Heart Failure. Can. J. Diabetes 2018, 42, S196–S200. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Krim, S.; Ventura, H. The Bi-directional Impact of Two Chronic Illnesses: Heart Failure and Diabetes—A review of the Epidemiology and Outcomes. Card. Fail. Rev. 2015, 1, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Med. 2017, 130, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Connelly, K.A.; Gilbert, R.E.; Liu, P. Treatment of Diabetes in People With Heart Failure. Can. J. Diabetes 2018, 42, S196–S200. [Google Scholar]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.W.; Neal, B.; Perkovic, V.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Fabbrini, E.; Sun, T.; Li, Q.; et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018, 137, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Morgan, B.; Potter, B.J.; Ma, L.; Dellsperger, K.C.; Ungvari, Z.; Zhang, C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: In vivo demonstration with magnetic resonance imaging. Am. J. Physiol. 2010, 299, H985–H994. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bocchi, L.; Delucchi, F.; Savi, M.; Ray, S.; Brighenti, F.; Stilli, D.; Del Rio, D. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 408–415. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evid. -Based Complementary Altern. Med. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou Seif, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol Promotes Foot Ulcer Size Reduction in Type 2 Diabetes Patients. ISRN Endocrinol. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Ozturk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; Lakatta, E.G.; Talan, M. Long-term low dose dietary resveratrol supplement reduces cardiovascular structural and functional deterioration in chronic heart failure in rats. Can. J. Physiol. Pharmacol. 2017, 95, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciechowski, P.; Juric, D.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Taylor, C.; Netticadan, T. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J. Nutr. 2010, 140, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Takahara, S.; Maayah, Z.H.; Parajuli, N.; Byrne, N.J.; Shoieb, S.M.; Soltys, C.M.; Beker, D.L.; Masson, G.; El-Kadi, A.O.S.; et al. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J. Mol. Cell. Cardiol. 2018, 125, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, S.; Ruiz, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Veksler, V.; Garnier, A.; Ventura-Clapier, R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS ONE 2011, 6, e26391. [Google Scholar] [CrossRef]
- Sung, M.M.; Byrne, N.J.; Robertson, I.M.; Kim, T.T.; Samokhvalov, V.; Levasseur, J.; Soltys, C.L.; Fung, D.; Tyreman, N.; Denou, E.; et al. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am. J. Physiol. 2017, 312, H842–H853. [Google Scholar] [CrossRef]

| Study Done by | Study Design | Subjects | Dose and Treatment Period | Area of Interest | Primary or Key Exploratory Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|
| Atherosclerosis and Coronary Artery Disease | ||||||
| Endothelial Function | ||||||
| Fujitaka et al., 2011 [44] | Randomized | 34 patients with metabolic syndromes | 100 mg of resveratrol (Longvinex; contains reseveratrol but also vitamin D3, quercetin, and rice bran phytate) daily for 3–6 months | Effects of resveratrol on the endothelial function of metabolically unhealthy patients | Increase in flow mediated dilation (FMD; i.e., endothelial function improvements). | No effect on body composition, lipid profile, interleukin-6 (IL-6) and high-sensitive C-reactive protein (hsCRP). |
| Imamura et al., 2017 [45] | Double blind, randomized, placebo-controlled | 50 adults with type 2 diabetes mellitus | 100 mg of resveratrol (BHN Corporation (Tokyo) as resveratrol-ε) daily for 12 weeks | Effects of resveratrol on arterial stiffness | Decrease in arterial stiffness (measured by decrease in cardio-ankle vascular index; CAVI). | No effects on fasting plasma glucose, HbA1c, total cholesterol, triglycerides, high-density lipoproteins (HDL) cholesterol (HDL-C) and low-density lipoproteins (LDL) cholesterol (LDL-C). Improved systolic blood pressure but no effect on diastolic blood pressure. |
| Marques et al., 2018 [46] | Double blind, cross-over, randomized, placebo-controlled | 24 hypertensive adults | 300 mg of resveratrol (Bioderm Pharmacy (Rio de Janeiro, Brazil) once daily | Cardiovascular effects of acute resveratrol dose | Improved endothelial function (FMD improvements); no effect on peripheral blood pressure (BP), Augmentation Index, and aortic systolic BP (SBP). | |
| Wong et al., 2013 [47] | Randomized, placebo-controlled, double-blind crossover | 28 obese, otherwise healthy, adults | 75 mg daily of resveratrol for 6 weeks | Effects of resveratrol on endothelial functioning of obese patients | Increase in endothelial function (FMD improvements). No effect on BP or arterial compliance. | |
| Lipoprotein and Cholesterol | ||||||
| Gilemann et al., 2013 [48] | Randomized double-blind placebo-controlled | 27 physically inactive aged (mean age = 65 ± 1 year) men | 250 mg of resveratrol (Fluxome Inc., Stenlose, Denmark) daily for 8 weeks | Effect of resveratrol with exercise on cardiovascular health | Blunted decreases in total cholesterol, and ratio of total cholesterol/HDL levels following exercise. Blunted the phosphorylation of endothelial nitric oxide (NO) synthase (eNOS) following exercise. Blunted increase of eNOS following exercise. Decreased maximum oxygen uptake after exercise. | Blunted mean arterial pressure decreases following exercise. No effect on blood glucose, body mass index (BMI), protein expression in skeletal muscle, including for silent information regulator factor 2 related enzyme 1 (SIRT1). Blunted effects on increases in prostacyclin (PGI2) after exercise. |
| Haghighatdoost et al., 2018 [49] | Systematic review and meta-analysis | 763 adults included in total cholesterol analysis, 728 adults included in LDL-C analysis, 777 adults included in HDL-C analysis, and 921 in serum triglyceride analysis. | Resveratrol doses ranged from 10 mg/day to 1500 mg/day with treatment periods ranging from 4 to 24 weeks | Effects of resveratrol on lipid profile | Decreased total cholesterol in subjects with normal BMI, but not those overweight or obese; No effect on LDL-C or HDL-C. | Increase in plasma triglyceride levels, this effect became insignificant when one study (Zortea et al. [50]) was removed from the meta-analysis). |
| Heebøll et al., 2016 [51] | Double blind, randomized, placebo-controlled | 28 adults with non-alcoholic fatty liver disease | 1500 mg of resveratrol (Evolva SA (Basel, Switzerland) daily for 6 months | Effects of resveratrol on symptoms associated with non-alcoholic fatty liver disease | No changes in plasma glucose, insulin, lipid profile or homeostatic model assessment (HOMA) index. | No effect of BMI, weight, waist-hip ratio, SIRT1 or AMP-activated protein kinase (AMPK) activity. |
| Poulsen et al., 2013 [52] | Randomized, placebo-controlled, double blinded | 24 obese, otherwise healthy, males | 500 mg of resveratrol (Fluxome Inc., Stenlose, Denmark) daily for 4 weeks | Effects of high dose of resveratrol | No effects on lipid oxidation, adiponectin or insulin, body composition. | No effect on BP, lipid profile, liver function, SIRT1, AMPK pathways or inflammatory biomarkers. |
| Tomé-Carneiro J et al., 2012 [53] | Triple-blind, randomized, placebo-controlled | 75 adult patients given primary prevention of CVD | 370 mg capsule with 350 mg Stilvid® (23 mg resveratrol/gram and other minor grape stilbenes) daily and 20 mg magnesium stearate and SiO2 (inactive) for 6 months | Cardiovascular effects of resveratrol | Decrease in apolipoprotein B-100 (ApoB) and oxidized LDL (LDL-ox) plasma levels, cannot be ruled out if resveratrol had a synergistic effect with other grape polyphenols in the capsule. | |
| Zare Javid et al., 2017 [54] | Randomized double-blind, placebo-controlled | 43 adults with type 2 diabetes | 480 mg of resveratrol [(ingredients: Polygonum cuspidatum extract (72%) with at least 60% trans-resveratrol, gelatin, microcrystalline cellulose (filler), and magnesium stearate) from Herbafit] for 4 weeks | Metabolic effects of resveratrol | Increased insulin resistance. No effect on plasma levels of fasting glucose or triglycerides. | |
| Zortea et al., 2016 [50] | Randomized double-blind, placebo-controlled | 19 adult men with schizophrenia | 200 mg of resveratrol (trans-resveratrol, 98% purified) daily for 30 days | Cardiovascular effects of resveratrol | Decrease in triglyceride plasma levels. No effects on serum glucose or body weight, BMI, and waist circumference. | |
| Inflammatory Effects | ||||||
| Olesen et al., 2014 [55] | Randomized, double-blinded, placebo-controlled | 43 healthy, physically inactive, elderly, men | 250 mg of resveratrol (Fluxome Inc., Stenlose, Denmark) daily with and without exercise for 8 weeks | Effects of resveratrol on skeletal muscle inflammation both alone and with exercise | No anti-inflammatory effect without exercise, including no plasma level changes of c-reactive protein (CRP), IL-6, or tumor necrosis factor (TNF)α. A blunting on anti-inflammatory effect with exercise training. | No endurance effects, effects on SIRTI or AMPK pathways but an overall decrease in acetylation level. No effect on protein content of skeletal muscle, or protein carbonylation. |
| Tomé-Carneiro J et al., 2013 [56] | Triple-blind, randomized, placebo-controlled | 75 stable CAD patients | 370 mg capsule with 350 mg Stilvid® (23 mg resveratrol/gram) daily and 20 mg magnesium stearate and SiO2 (inactive) for 1 year | Cardiovascular effects of resveratrol | Increase in serum adiponectin levels. Decrease in plasminogen activator inhibitor-1 (PAI-1) plasma levels. General suppression of peripheral blood mononuclear cell (PBMC) -mediated inflammatory pathway, however no changes in levels of TNFα, IL-6, or IL-10. | |
| Tomé-Carneiro J et al., 2012 [57] | Triple-blind, randomized, placebo-controlled | 75 adults undergoing primary prevention for CVD | 8 mg of resveratrol daily for 1 year | Inflammatory effects of resveratrol | Decrease in hsCRP, TNFα, plasminogen activator inhibitor type 1, or IL-6/IL-10 ratio. Increase in IL-10 and adiponectin plasma levels. Decrease in soluble intercellular adhesion molecule plasma levels. | |
| Various Measures Relating to Atherosclerosis | ||||||
| Agarwal et al., 2013 [58] | Double blind, randomized, placebo-controlled | 41 healthy adult subjects | 400mg trans-resveratrol (98% pure, sourced from Polygonum Cuspidatum), 400mg grape-skin extract, and 100mg quercetin daily for 4 weeks | Effects of resveratrol on endothelial function and atherosclerosis | Reduction in mRNA expression of vascular cell adhesion molecule (VCAM), intercellular adhesion molecule 1 (ICAM-1) and IL-8. Reduction in plasma interferon (IFN)-γ. No effect on IL-1β, IL-6, and TNFα plasma levels, however overall endothelial cell cytokine activation decreased. | Reduction in fasting insulin concentrations. |
| Bhatt et al., 2012 [59] | Open-label, randomized, controlled | 57 male adults with type 2 diabetes mellitus | 250 mg of resveratrol (Biofort; Biotivia Bioceuticals International, New York, NY, USA) daily for 3 months | Cardiovascular and metabolic effects of resveratrol | Decreases in hemoglobin A1c (HbA1c), SBP, total cholesterol, and total protein. | No significant change in LDL plasma levels or body weight. |
| Chen et al., 2015 [60] | Double blind, randomized, placebo-controlled | 60 adults with non-alcoholic fatty liver disease | 300 mg of resveratrol (brand not provided) for 3 months | Metabolic effects of resveratrol | Decreased LDL-C and total cholesterol, glucose, or inflammatory cytokines. Improved insulin resistance. Increased adiponectin levels. | |
| Huang et al., 2016 [61] | Systematic review and meta-analysis | 681 adults | Resveratrol doses ranging from 8 mg/day to 3000 mg/day With a duration of treatment ranging from 2 weeks to 6 months | Effects of resveratrol on cardiovascular disease risk factors in overweight and obese adults | Decreases in blood plasma total cholesterol levels (no change in LDL-C and HDL-C levels were observed). Decreases in SBP and no effect on DBP. No effect on fasting glucose levels, except when stratified for patients with metabolic syndrome. No effect in inflammatory biomarkers IL-6 and TNFα plasma levels. | No effect on body weight. In higher resveratrol doses (more than or exactly 300 mg per day) significant decreases in SBP, fasting insulin, fasting glucose, and total cholesterol was seen. In lower doses (less than 300 mg daily) reductions in HbA1c were observed. Decreases in total cholesterol, glucose, and HbA1c were more significant for participants who took resveratrol for more or equal to 3 months. Decreases in fasting insulin plasma levels were more significant for patients who took resveratrol for less than 3 months. |
| Macedo et al., 2015 [62] | Double-blind, placebo-controlled study | 60 healthy adults | 100 mg of resveratrol (Polygonum cuspidatum provided by Farmel Pharmacy (São Paulo, SP, Brazil)) daily for 3 months with routine fitness tests | Effects of resveratrol of participants undergoing a fitness test | No effect on total lipid profile. Reduction in IL-6 and TNFα plasma levels. No effect on IL-8 plasma levels. No antioxidant effects observed. | |
| Mendez-del Villar et al., 2012 [63] | Double blind, randomized, placebo-controlled | 24 adults with metabolic syndromes | 1500 mg of resveratrol daily for 90 days | Cardiovascular and metabolic effects of resveratrol | Decreases in total weight, BMI, fat mass, and waist circumference. Decreases in total insulin secretion and area under the curve (AUC) of insulin. | |
| Millatru et al., 2013 [64] | Randomized, double-blinded, active-controlled, parallel | 87 adults with stable angina pectoris | 20 mg of resveratrol daily or 20 mg of resveratrol daily and 112 mg of calcium fructoborate (CF) daily (shown to slow down the breakdown of resveratrol in the digestive system) | Cardiovascular effects of resveratrol alone and in combination with CF | In combination with CF, decreased N-terminal pro b-type natriuretic peptide (NT-proBNP) plasma levels. Decreased plasma levels of total cholesterol and triglycerides. Decreased number of angina episodes. | Less effective than CF alone in decreasing LDL plasma levels and increasing HDL plasma levels. |
| S. Bo et al., 2016 [65] | Double blind, randomized, placebo-controlled | 179 adults with type 2 diabetes | Either 500 mg or 40 mg of resveratrol (provided by Biotivia Bioceuticals (International SrL, Italy) daily for 6 months | Cardiovascular effects of resveratrol | No changes in CRP levels. | Slight increase in plasma levels of total cholesterol and triglycerides. No changes in BMI, waist circumference, arterial blood pressure, IL-6, fasting glucose, HbA1c, and insulin. |
| S. Bo et al., 2013 [66] | Double blind, randomized, placebo-controlled | 49 healthy adult smokers | 500 mg of resveratrol (provided by Biotivia Bioceuticals (International SrL, Italy)) daily for 30 days | Anti-inflammatory and antioxidant effects of resveratrol | Reduction in CRP plasma levels. | Reduction in triglyceride plasma levels. Increase in Total Antioxidant Status. |
| Sahebkar et al., 2013 [67] | Systematic review Meta-analysis | 600 adults | Resveratrol doses ranged from 8 mg/day to 1500 mg/day. Treatment periods ranged from 60 days to one year. | Effects of resveratrol on CRP plasma levels and other cardiovascular risk factors | No effect on total cholesterol plasma levels. No effect on plasma triglyceride or glucose concentrations. Slightly reduced HDL-C plasma concentrations. | No effect on CRP plasma levels. No effect on BP. |
| Van der Made et al., 2015 [68] | Double blind, randomized, placebo-controlled, cross over | 45 overweight or slightly obese adults | 150 mg of resveratrol (resVida) daily for 4 weeks, followed by 4 weeks wash out, and another 4 weeks of supplementation | Cardiovascular and metabolic effects of resveratrol | No differences in serum apolipoprotein A-I (apoA-I) or apoB-100 concentrations. | No effect on the levels of metabolic risk factors in plasma (including LDL and HDL). Increase in diastolic BP and heart rate. No effect on mean arterial pressure, SBP, or insulin concentrations. No effect on biomarkers of inflammation (hsCRP, IL-6, E-selectin, thromobomodulin, P-selectin or TNFα). No effect on ICAM-3, soluble ICAM-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1) plasma levels. |
| Timmers et al., 2011 [69] | Randomized double-blind crossover design | 11 obese, but otherwise healthy, patients | 150 mg of 99% pure trans-resveratrol (resVida™) daily for 30 days | Effects of resveratrol on metabolism | Decrease in alanine transaminase plasma levels. Lower leptin and leukocyte plasma levels. Decrease in IL-6 and TNFα plasma levels. Lower HOMA index. Lower plasma levels of triglycerides. No changes in plasma non-esterified fatty acids. Higher respiratory quotient. Lower mean arterial pressure and SBP and no effect on DBP. Lower non- esterified fatty acids and free glycerol in the late postprandial phase, however no effect on postprandial triglycerides and lactate response. No difference on ethanol in/out ratios or blood flow in adipose tissue and skeletal muscle. No effect on interstitial glucose, pyruvate and lactate responses in adipose tissue. No effect on interstitial glucose, pyruvate, lactate and glycerol concentrations in skeletal muscle. In the postprandial phase energy expenditure was lower and fat oxidation decreased. Upregulation of mitochondrial oxidative phosphorylation in vastus lateralis muscle cells. Down regulation of cytokine signaling in vastus lateralis muscle cells. Increased phosphorylated AMPK in muscle cells. No effect on mitochondrial DNA copy number. No effect on mitochondrial density. Overall mitochondrial activity increased. No effect on mitochondrial recovery following moderate exercise. Lower storage of lipids within the liver and higher storage in type 1 muscle fibres. | |
| Tomé-Carneiro J et al., 2013 [70] | Triple-blind, randomized, placebo-controlled | 35 adult males with type 2 diabetes or hypertension | 370 mg capsule with 350 mg Stilvid® (23 mg resveratrol/gram and other minor grape stillbenes) daily and 20 mg magnesium stearate and SiO2 (inactive) for 1 year | Cardiovascular effects of resveratrol | A downregulation of inflammatory cytokines. No effect on SBP, DBP, weight, lipid profile, glucose plasma levels, HbA1C, hsCRP, adiponectin, PAI-1, TNFα, and IL-10. Decrease in IL-6 plasma levels. Modifies microRNA (miR)s involved in inflammatory modulation. | |
| Yoshino et al., 2012 [71] | Randomized, double-blind, placebo-controlled | 29 non-obese, normal glucose tolerant, woman | 75 mg of resveratrol 99% pure trans-resveratrol [(resVida™ from DSM Nutritional Products, Ltd.)] a day for 12 weeks | Effects of resveratrol on metabolically healthy individuals | No effect on body composition, insulin sensitivity, AMPK or SIRT1 pathways. | |
| Study Done by | Study Design | Subjects | Dose and Treatment Period | Area of Interest | Primary or Key Exploratory Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|
| Hypertension | ||||||
| Fogacci et al., 2018 [124] | Meta-analysis | 681 adults | Several doses for a time period ranging from 30 days to six months | Effects of resveratrol on SBP and DBP and mean arterial pressure | No significant effect on SBP and DBP or mean arterial pressure. | Lower DBP in higher doses (more or exactly 300 mg/day) and with diabetic patients. |
| Liu et al., 2015 [125] | Meta-analysis | 274 adults | Doses ranging from 16 mg daily to 1000 mg daily with supplementation periods ranging from 30 days to 12 months | Effect of resveratrol on SBP and DBP | No significant reduction of SBP or DBP. | Resveratrol was more effective at reducing SBP in higher doses ( ≥150 mg daily). |
| Theodotou et al., 2016 [123] | Double blind, randomized, placebo-controlled | 97 patients with hypertension | 50 mg of resveratrol (Elevlor) daily for six months | 97 patients with hypertension | Resveratrol supplementation with Dapril reduces BP to normal levels. | Resveratrol prevents liver damage. |
| Study Done by | Study Design | Subjects | Dose and Treatment Period | Area of Interest | Primary or Key Exploratory Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|
| Heart Failure/LV Function | ||||||
| Maygar et al., 2012 [95] | Double blind, randomized, placebo-controlled | 40 adults who had a previous myocardial infarction | 10 mg of resveratrol daily for 3 months | Cardio-protective effects of resveratrol | Improvement in left ventricular diastolic function, endothelial functioning (FMD improvements). Decrease in plasma LDL levels. No effect on HbA1c, TNF-alpha, or CRP. | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. https://doi.org/10.3390/ijms20040904
Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. International Journal of Molecular Sciences. 2019; 20(4):904. https://doi.org/10.3390/ijms20040904
Chicago/Turabian StyleDyck, Garrison J. B., Pema Raj, Shelley Zieroth, Jason R. B. Dyck, and Justin A. Ezekowitz. 2019. "The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review" International Journal of Molecular Sciences 20, no. 4: 904. https://doi.org/10.3390/ijms20040904
APA StyleDyck, G. J. B., Raj, P., Zieroth, S., Dyck, J. R. B., & Ezekowitz, J. A. (2019). The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. International Journal of Molecular Sciences, 20(4), 904. https://doi.org/10.3390/ijms20040904





