Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology
Abstract
:1. Introduction
2. Cholesterol, Oxysterols, LXRs and Breast Cancer
2.1. Cholesterol and Breast Cancer in Animal Studies
2.2. Cholesterol and Breast Cancer in Human Studies
3. 27OHC, Oxysterols, LXRs and Breast Cancer
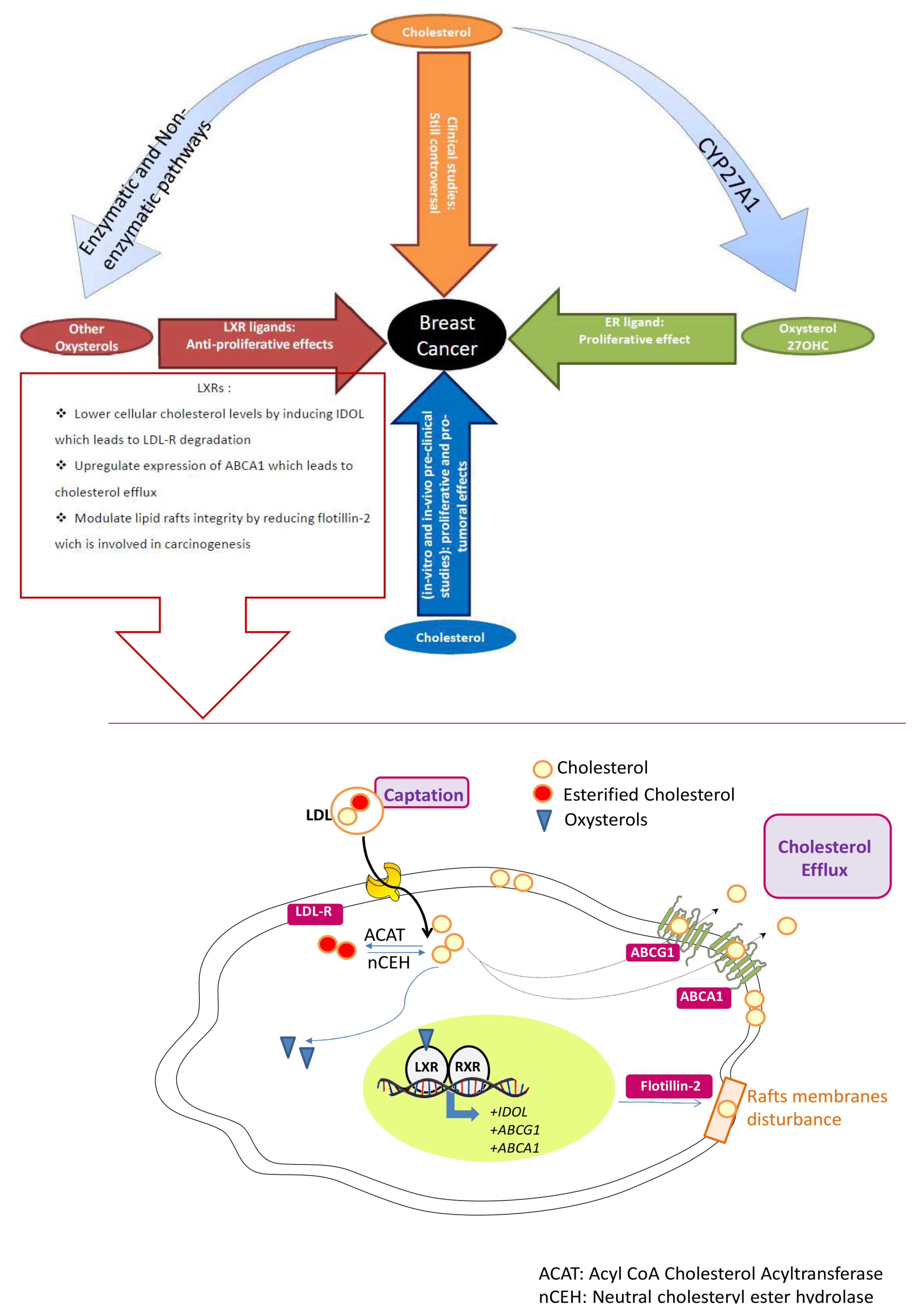
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Thompson, S.G. Low serum cholesterol and the risk of cancer: An analysis of the published prospective studies. Cancer Causes Control. 1991, 2, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Bjarnadottir, O.; Kimbung, S.; Ahern, T.P. Statins: A role in breast cancer therapy? J. Int. Med. 2018, 284, 346–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimbung, S.; Markholm, I.; Bjöhle, J.; Lekberg, T.; von Wachenfeldt, A.; Azavedo, E.; Saracco, A.; Hellström, M.; Veerla, S.; Paquet, E.; et al. Assessment of early response biomarkers in relation to long-term survival in patients with HER2-negative breast cancer receiving neoadjuvant chemotherapy plus bevacizumab: Results from the Phase II PROMIX trial. Int. J. Cancer 2018, 142, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019, 21. [Google Scholar] [CrossRef] [Green Version]
- Karuna, R.; Holleboom, A.G.; Motazacker, M.M.; Kuivenhoven, J.A.; Frikke-Schmidt, R.; Tybjaerg-Hansen, A.; Georgopoulos, S.; van Eck, M.; van Berkel, T.J.; von Eckardstein, A.; et al. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis 2011, 214, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Umetani, M.; Domoto, H.; Gormley, A.K.; Yuhanna, I.S.; Cummins, C.L.; Javitt, N.B.; Korach, K.S.; Shaul, P.W.; Mangelsdorf, D.J. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007, 13, 1185–1192. [Google Scholar] [CrossRef]
- El Roz, A.; Bard, J.M.; Huvelin, J.M.; Nazih, H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: Relation to proliferation and apoptosis. Anticancer Res. 2012, 32, 3007–3013. [Google Scholar]
- Carbonnelle, D.; Luu, T.H.; Chaillou, C.; Huvelin, J.M.; Bard, J.M.; Nazih, H. LXR Activation Down-regulates Lipid Raft Markers FLOT2 and DHHC5 in MCF-7 Breast Cancer Cells. Anticancer Res. 2017, 37, 4067–4073. [Google Scholar]
- Fukuchi, J.; Kokontis, J.M.; Hiipakka, R.A.; Chuu, C.P.; Liao, S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004, 64, 7686–7689. [Google Scholar] [CrossRef] [Green Version]
- Chuu, C.-P.; Lin, H.-P. Antiproliferative Effect of LXR Agonists T0901317 and 22(R)-Hydroxycholesterol on Multiple Human Cancer Cell Lines. Anticancer Res. 2010, 30, 3643–3648. [Google Scholar] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Schwaid, A.G.; Wang, X.; Wang, X.; Chen, S.; Chu, Q.; Saghatelian, A.; Wan, Y. Ligand Activation of ERRalpha by Cholesterol Mediates Statin and Bisphosphonate Effects. Cell Metab. 2016, 23, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; Sun, Q.; Patel, D.; Stommel, J.M. Cholesterol Metabolism: A Potential Therapeutic Target in Glioblastoma. Cancers 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef] [Green Version]
- Llaverias, G.; Danilo, C.; Mercier, I.; Daumer, K.; Capozza, F.; Williams, T.M.; Sotgia, F.; Lisanti, M.P.; Frank, P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011, 178, 402–412. [Google Scholar] [CrossRef]
- Scheinman, E.J.; Rostoker, R.; Leroith, D. Cholesterol affects gene expression of the Jun family in colon carcinoma cells using different signaling pathways. Mol. Cell Endocrinol. 2013, 374, 101–107. [Google Scholar] [CrossRef]
- Hoque, M.; Rentero, C.; Conway, J.R.; Murray, R.Z.; Timpson, P.; Enrich, C.; Grewal, T. The cross-talk of LDL-cholesterol with cell motility: Insights fromhe Niemann Pick Type C1 mutation and altered integrin trafficking. Cell Adhes. Migr. 2015, 9, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Tatidis, L.; Masquelier, M.; Vitols, S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem. Pharmacol. 2002, 63, 2169–2180. [Google Scholar] [CrossRef]
- Antalis, C.J.; Uchida, A.; Buhman, K.K.; Siddiqui, R.A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Breast Cancer Res. Treat. 2011, 28, 733–741. [Google Scholar] [CrossRef]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilo, C.; Gutierrez-Pajares, J.L.; Mainieri, M.A.; Mercier, I.; Lisanti, M.P.; Frank, P.G. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 2013, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Clendening, J.W.; Penn, L.Z. Targeting tumor cell metabolism with statins. Oncogene 2012, 31, 4967–4978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krycer, J.R.; Phan, L. A key regulator of cholesterol homeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem. J. 2012, 446, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.W.; Chyuan, I.T.; Shiue, C.; Yu, M.C.; Hsu, Y.F.; Hsu, M.J. Lovastatin-mediated MCF-7 cancer cell death involves LKB1-AMPK-p38MAPK-p53-survivin signalling cascade. J. Cell Mol. Med. 2020, 24, 1822–1836. [Google Scholar] [CrossRef]
- Shibata, M.A.; Kavanaugh, C.; Shibata, E.; Abe, H.; Nguyen, P.; Otsuki, Y.; Trepel, J.B.; Green, J.E. Comparative effects of lovastatin on mammary and prostate oncogenesis in transgenic mouse models. Carcinogenesis 2003, 243, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Yu, Z.; Gao, X.; Gong, J.; Fan, L.; Liu, F. Simvastatin induces breast cancer cell death through oxidative stress up-regulating miR-140-5p. Aging 2019, 11, 3198–3219. [Google Scholar] [CrossRef]
- Clayman, R.V.; Gonzalez, R.; Elliott, A.Y.; Gleason, D.E.; Dempsey, M.E. Cholesterol accumulation in hetero transplanted renal cell cancer. J. Urol. 1983, 129, 621–624. [Google Scholar] [CrossRef]
- Swyer, G. The cholesterol content of normal and enlarged prostates. Cancer Res. 1942, 2, 372–375. [Google Scholar]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Dos Santo, C.R.; Domingues, G.; Matias, I.; Matos, J.; Fonseca, I.; de Almeida, J.M.; Dias, S. LDL-Cholesterol signling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014. [Google Scholar] [CrossRef] [Green Version]
- Jaworsky, K.; Jankowsky, P.; Kosior, D.A. PCSK9 inhibitors-From discovery of a single mutation to a groundbreaking therapy of lipid disorders in one decade. Arch. Med. Sci. 2017, 13, 914–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momtazi-Borojeni, A.A.; Nik, M.E.; Jaafari, M.R.; Banach, M.; Sahebkar, A. Effects of immunization against PCSK9 in an experimental model of breast cancer. Arch. Med. Sci. 2019, 15, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Hou, M.F.; Tsai, S.M.; Wu, S.H.; Hou, L.A.; Ma, H.; Shann, T.Y.; Wu, S.H.; Tsai, L.Y. The association between lipid profiles and breast cancer among Taiwanese women. Clin. Chem. Lab. Med. 2007, 45, 1219–1223. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.K.; Han, W.; Kim, D.H.; Hong, Y.C.; Ha, E.H.; Ahn, S.H.; Noh, D.Y.; Kang, D.; Yoo, K.Y. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol. Biomark. Prev. 2009, 18, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Aaron, R.; Folsom, A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef]
- Potluri, R.; Lavu, D.; Uppal, H.; Chandran, S. P740 Hyperlipidemia as a risk factor for breast cancer? Cardiovasc. Res. Suppl. 2014, 103, S135. [Google Scholar]
- Laisupasin, P.; Thompat, W.; Sukarayodhin, S.; Sornprom, A.; Sudjaroen, Y. Comparison of Serum lipid Profiles between normal controls and breast cancer patients. J. Lab. Physcians. 2013, 5, 38–41. [Google Scholar]
- Touvier, M.; Fassier, P.; His, M.; Norat, T.; Chan, D.S.; Blacher, J.; Hercberg, S.; Galan, P.; Druesne-Pecollo, N.; Latino-Martel, P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015, 114, 347–357. [Google Scholar] [CrossRef]
- Carter, P.R.; McGowan, J.; Uppal, H.; Chandran, S.; Sarma, J.; Potluri, R. Hyperlipidemia reduces mortality in breast, prostate, lung and bowel cancer. Heart 2016, 102, A57–A58. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; La Vecchia, C.; de Groh, M.; Negri, E.; Morrison, H.; Mery, L. Canadian Cancer registries epidemiology research G: Dietary cholesterol intake and cancer. Ann. Oncol. 2012, 23, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, L.; Zhang, D.; Jiang, W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr. Res. 2016, 36, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, H.C.; Nguyen, P.A.; Poly, T.N.; Huang, C.W.; Kekade, S.; Khalfan, A.M.; Debnath, T.; Li, Y.J.; Abdul, S.S. Exploring association between statin use and breast cancer risk: An updated meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Undela, K.; Srikanth, V.; Bansal, D. Statin use and risk of breast cancer: A meta-analysis of observational studies. Breast Cancer Res. Treat. 2012, 135, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, M.; Haghjooy-Javanmard, S.; Eshraghi, A.; Vaseghi, G.; Hayatshahi, A.; Thomas, J. Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2016, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bovenga, F.; Sabbà, C.; Moschetta, A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015, 21, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, M.V.; van Gilst, W.H.; de Boer, R.A. Emerging role of liver X receptors in cardiac pathophysiology and heart failure. Basic Res. Cardiol. 2016, 111, 3. [Google Scholar] [CrossRef]
- Prunet, C.; Petit, J.M.; Ecarnot-Laubriet, A.; Athias, A.; Miguet-Alfonsi, C.; Rohmer, J.F.; Steinmetz, E.; Neel, D.; Gambert, P.; Lizard, G. High circulating levels of 7beta- and 7alpha-hydroxycholesterol and presence of apoptotic and oxidative markers in arterial lesions of normocholesterolemic atherosclerotic patients undergoing endarterectomy. Pathol. Biol. (Paris) 2006, 54, 22–32. [Google Scholar] [CrossRef]
- Nelson, E.R. The significance of cholesterol and its metabolite, 27-hydroxycholesterol in breast cancer. Mol. Cell Endocrinol. 2018, 466, 73–80. [Google Scholar] [CrossRef]
- DuSell, C.D.; Umetani, M.; Shaul, W.; Mangelsdorf, D.J.; McDonnell, D.P. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 2008, 22, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, E.R.; DuSell, C.D.; Wang, X.; Howe, M.K.; Evans, G.; Michalek, R.D.; Umetani, M.; Rathmell, J.C.; Khosla, S.; Gesty-Palmer, D.; et al. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinology 2011, 152, 4691–4705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ishikawa, T.; Sirianni, R.; Tang, H.; McDonald, J.G.; Yuhanna, I.S.; Thompson, B.; Girard, L.; Mineo, C.; Brekken, R.A.; et al. 27-hydroxycholesterol promotes cell autonomous, ER-positive breast cancer growth. Cell Rep. 2013, 5, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solheim, S.; Hutchinsonb, S.A.; Lundanesa, E.; Wilsona, S.R.; James, L.; Thorneb, J.L.; Roberg-Larsena, H. Fast liquid chromatography-mass spectrometry reveals side chain oxysterol heterogeneity in breast cancer tumour samples. J. Steroid. Biochem. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Roberg-Larsen, H.; Lund, K.; Seterdal, K.E.; Solheim, S.; Vehus, T.; Solberg, N.; Krauss, S.; Lundanes, E.; Wilson, S.R. Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J. Steroid. Biochem. Mol. Biol. 2017, 169, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Le Cornet, C.; Sookthai, D.; Johnson, T.S.; Kaaks, R.; Fortner, R.T. Circulating 27-hydroxycholesterol and breast cancer risk: Results from the EPICHeidelberg Cohort. J. Natl. Cancer Inst. 2019, 111, 365–371. [Google Scholar] [CrossRef]
- Dalenc, F.; Iuliano, L.; Filleron, T.; Zerbinati, C.; Voisin, M.; Arellano, C.; Chatelut, E.; Marquet, P.; Samadi, M.; Roche, H.; et al. Circulating oxysterol metabolites as potential new surrogate markers in patients with hormone receptor-positive breast cancer: Results of the OXYTAM study. J. Steroid. Biochem. Mol. Biol. 2016, 169, 210–218. [Google Scholar] [CrossRef]
- Hutchinson, S.A.; Lianto, P.; Roberg-Larsen, H.; Battaglia, S.; Hughes, T.A.; Thorne, J.L. ER-Negative breast cancer is highly responsive to cholesterol metabolite signalling. Nutrients 2019, 11, 2618. [Google Scholar] [CrossRef] [Green Version]
- Doig, C.L.; Singh, P.K.; Dhiman, V.K.; Thorne, J.L.; Battaglia, S.; Sobolewski, M.; Maguire, O.; O’Neill, L.P.; Turner, B.M.; McCabe, C.J.; et al. Recruitment of NCOR1 to VDR target genes is enhanced in prostate cancer cells and associates with altered DNA methylation patterns. Carcinogenesis 2012, 34, 248–256. [Google Scholar] [CrossRef]
- Jalaguier, S.; Teyssier, C.; Nait Achour, T.; Lucas, A.; Bonnet, S.; Rodriguez, C.; Elarouci, N.; Lapierre, M.; Cavailles, V. Complex regulation of LCoR signaling in breast cancer cells. Oncogene 2017, 36, 4790–4801. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Wu, J.; Xia, C.; Lala, D.S. Liver X receptors interact with corepressors to regulate gene expression. Mol. Endocrinol. 2003, 17, 1019–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Zheng, Y.; Pan, Q.; Chen, H.; Chen, F.; Wu, J.; Di, D. Expression of LXR-β, ABCA1 and ABCG1 in human triple-negative breast cancer tissues. Oncol. Rep. 2019, 42, 1869–1877. [Google Scholar] [CrossRef]
- Gomig, T.H.B.; Cavalli, I.J.; Souza, R.L.R.; Vieira, E.; Lucena, A.C.R.; Batista, M.; Machado, K.C.; Marchini, F.K.; Marchi, F.A.; Lima, R.S.; et al. Quantitative label-free mass spectrometry using contralateral and adjacent breast tissues reveal differentially expressed proteins and their predicted impacts on pathways and cellular functions in breast cancer. J. Proteomics. 2019, 199, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luquis, O.; Madden, K.; N’dri, N.M.; Berg, R.; Olopade, O.; Ngwa, W.; Abuidris, D.; Mittal, S.; Lyn-Cook, B.; Mohammed, S. LXR/RXR pathway signaling associated with triple-negative breast cancer in African American Women. Breast Cancer 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazih, H.; Bard, J.M. Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology. Int. J. Mol. Sci. 2020, 21, 1356. https://doi.org/10.3390/ijms21041356
Nazih H, Bard JM. Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology. International Journal of Molecular Sciences. 2020; 21(4):1356. https://doi.org/10.3390/ijms21041356
Chicago/Turabian StyleNazih, Hassan, and Jean Marie Bard. 2020. "Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology" International Journal of Molecular Sciences 21, no. 4: 1356. https://doi.org/10.3390/ijms21041356
APA StyleNazih, H., & Bard, J. M. (2020). Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology. International Journal of Molecular Sciences, 21(4), 1356. https://doi.org/10.3390/ijms21041356




