Tobacco Hornworm (Manduca sexta) Oral Secretion Elicits Reactive Oxygen Species in Isolated Tomato Protoplasts
Abstract
:1. Introduction
2. Results
2.1. M. sexta OS Induces ROS Generation in Tomato Protoplast
2.2. Diet-Dependent M. sexta OS Effect on ROS Production in Tomato Protoplast
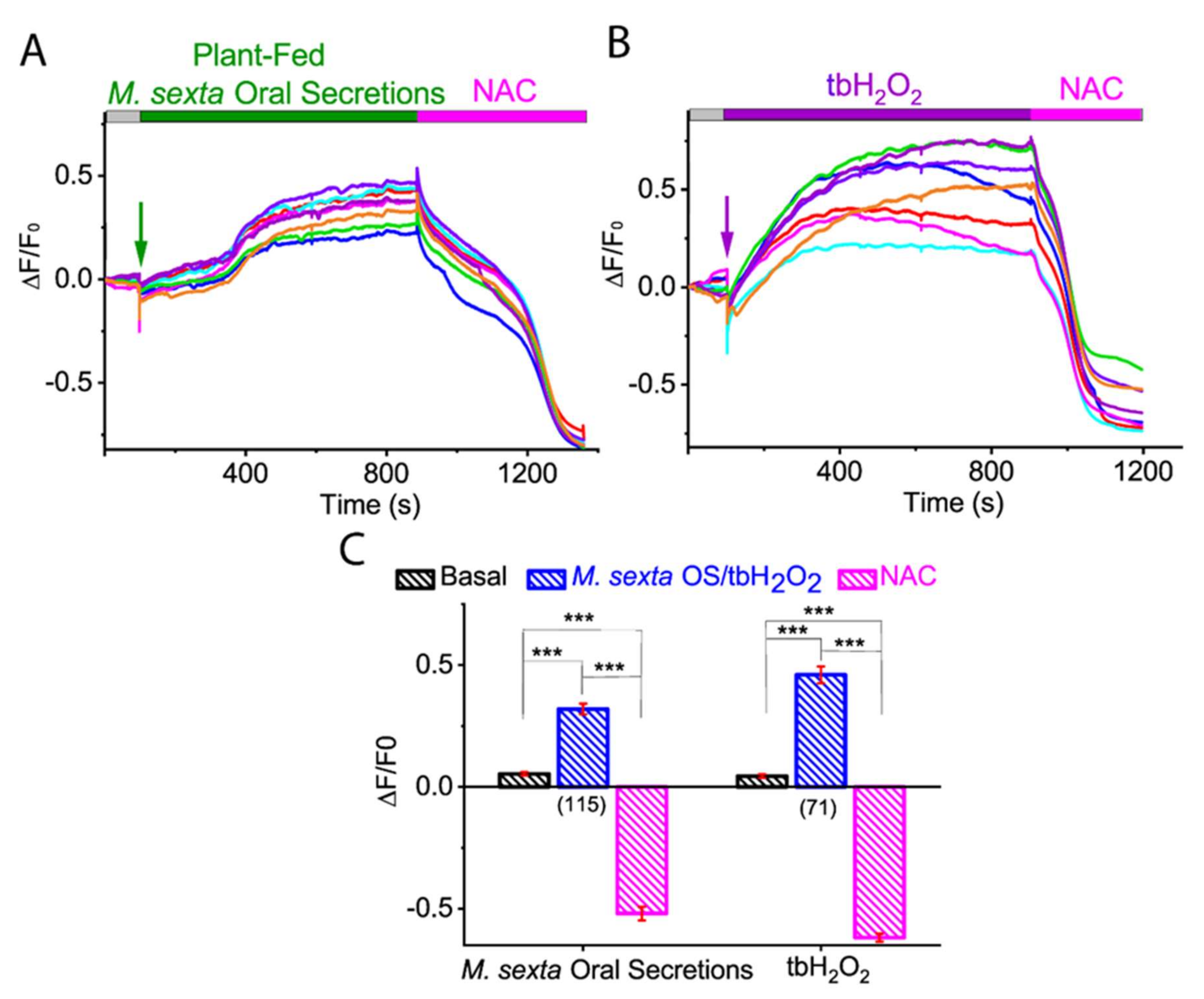
2.3. Membrane-Permeable Oxidant tbH2O2-Induced ROS in Tomato Protoplast
2.4. Antioxidant N-Acetylcysteine (NAC) Abolished M. sexta OS, and Oxidant tbH2O2-Induced ROS Generation in Tomato Protoplasts
2.5. Calcium Chelator BAPTA-AM Inhibited M. sexta OS-Induced ROS Generation in Tomato Protoplasts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Protoplast Isolation
4.3. Manduca sexta Rearing and Oral Secretion Collection
4.4. ROS Measurements
4.5. Data Analysis and Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schäfer, M.; Fischer, C.; Meldau, S.; Seebald, E.; Oelmüller, R.; Baldwin, I.T. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol. 2011, 156, 1520–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Sharma, S.; Kooner, R.; Arora, R. Insect pests and crop losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer: Singapore, 2017; p. 45. [Google Scholar]
- Zhao, L.; Chen, J.; Cheng, D.; Sun, J.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop Prot. 2009, 28, 435–442. [Google Scholar] [CrossRef]
- Karban, R. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 2010, 25, 339–347. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Mauck, K.E.; Moraes, C.M.D.; Stephenson, A.G.; Mescher, M.C. Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol. Lett. 2012, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kariyat, R.R.; Balogh, C.M.; Moraski, R.P.; Moraes, C.M.D.; Mescher, M.C.; Stephenson, A.G. Constitutive and herbivore-induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am. J. Bot. 2013, 100, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felton, G.W.; Chung, S.H.; Hernandez, M.G.E.; Louis, J.; Peiffer, M.; Tian, D. Herbivore oral secretions are the first line of protection against plant-induced defences. Annu. Plant Rev. Online 2018, 47, 37–76. [Google Scholar]
- Arimura, G.I.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- Arimura, G.I.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3723–3739. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007, 68, 2946–2959. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Maffei, M.E. Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem. Biophys. Res. Commun. 2010, 400, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.A.; Maffei, M.E. Signal transduction in plant–insect interactions: From membrane potential variations to metabolomics. In Plant Electrophysiology; Volkov, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 143–172. [Google Scholar]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in Lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Steffens, B.; Steffen-Heins, A.; Sauter, M. Reactive oxygen species mediate growth and death in submerged plants. Front. Plant Sci. 2013, 4, 179. [Google Scholar] [CrossRef] [Green Version]
- Kaur, I.; Kariyat, R. Eating barbed wire: Direct and indirect defensive roles of non-glandular trichomes. Plant Cell Environ. 2020, 43, 1–4. [Google Scholar] [CrossRef]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Bonaventure, G. Perception of insect feeding by plants. Plant Biol. 2012, 14, 872–880. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.A. Impacts of insect oral secretions on defoliation-induced plant defense. Curr. Opin. Insect Sci. 2015, 9, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fotopoulos, V. Oxidative and nitrosative signaling in plants. Plant Signal. Behav. 2011, 6, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Portman, S.L.; Kariyat, R.R.; Johnston, M.A.; Stephenson, A.G.; Marden, J.H. Inbreeding compromises host plant defense gene expression and improves herbivore survival. Plant Signal. Behav. 2015, 10, e998548. [Google Scholar] [CrossRef] [Green Version]
- Tayal, M.; Somavat, P.; Rodriguez, I.; Thomas, T.; Christoffersen, B.; Kariyat, R. Polyphenol-rich purple corn pericarp extract adversely impacts herbivore growth and development. Insects 2020, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Raya, C.E.; Chavana, J.; Cantu, J.; Guzman, G.; Sasidharan, L. Feeding on glandular and non-glandular leaf trichomes negatively affect growth and development in tobacco hornworm (Manduca sexta) caterpillars. Arthropod Plant Interact. 2019, 13, 321–333. [Google Scholar] [CrossRef]
- Portman, S.L.; Felton, G.W.; Kariyat, R.R.; Marden, J.H. Host plant defense produces species-specific alterations to flight muscle protein structure and flight-related fitness traits of two armyworms. J. Exp. Biol. 2020, 223, jeb224907. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Jensen, P.E.; Møller, I.M.; Schulz, A. Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol. Plant. 2009, 136, 369–383. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Bindschedler, L.V.; Blee, K.A.; Butt, V.S.; Davies, D.R.; Gardner, S.L.; Gerrish, C.; Minibayeva, F. The apoplastic oxidative burst in response to biotic stress in plants: A three-component system. J. Exp. Bot. 2002, 53, 1367–1376. [Google Scholar]
- Kwon, K.C.; Verma, D.; Jin, S.; Singh, N.D.; Daniell, H. Release of proteins from intact chloroplasts induced by reactive oxygen species during biotic and abiotic stress. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Arimura, G.I.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on Lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [Green Version]
- Woolley, J.; Stanicka, J.; Cotter, T. Recent advances in reactive oxygen species measurement in biological systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. In Methods in Enzymology. Conceptual Background and Bioenergetic/Mitochondrial Aspects of Oncometabolism; Galuzzi, L., Kroemer, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–262. [Google Scholar]
- Oparka, M.; Walczak, J.; Malinska, D.; Oppen, L.M.V.; Szczepanowska, J.; Koopman, W.J.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Swanson, S.J.; Choi, W.G.; Chanoca, A.; Gilroy, S. In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant Biol. 2011, 62, 273–297. [Google Scholar] [CrossRef]
- Fichman, Y.; Miller, G.; Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transducation in plants. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2011, 35, 441–453. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Occhipinti, A.; Atsbaha Zebelo, S.; Foti, M.; Fliegmann, J.; Bossi, S.; Maffei, M.E.; Bertea, C.M. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE 2012, 7, e32822. [Google Scholar] [CrossRef]
- Rae, M.G.; Martin, D.J.; Collingridge, G.L.; Irving, A.J. Role of Ca2+ stores in metabotropicl-glutamate receptor-mediated supralinear Ca2+ signaling in Rat Hippocampal neurons. J. Neurosci. 2000, 20, 8628–8636. [Google Scholar] [CrossRef] [Green Version]
- Chen, C. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef]
- Davies, D.R. Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: Implications for basal resistance. J. Exp. Bot. 2006, 57, 1817–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeworutzki, E.; Roelfsema, M.R.G.; Anschütz, U.; Krol, E.; Elzenga, J.T.M.; Felix, G.; Boller, T.; Hedrich, R.; Becker, D. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. Plant J. 2010, 62, 367–378. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2014, 66, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wei, C.L.; Zhang, W.R.; Cheng, H.P.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]
- Kimura, S.; Kaya, H.; Kawarazaki, T.; Hiraoka, G.; Senzaki, E.; Michikawa, M.; Kuchitsu, K. Protein phosphorylation is a prerequisite for the Ca2+–dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2 and reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drerup, M.M.; Schlücking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein Kinase CIPK26 regulate the Arabidopsis NADPH Oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; Jung, H.I.; Vatamaniuk, O.K. Isolation of protoplasts from tissues of 14-day-old seedlings of Arabidopsis thaliana. J. Vis. Exp. 2009, 30–1149. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Shan, L.; Sheen, J. The use of protoplasts to study innate immune responses. Methods Mol. Biol. 2007, 354, 1–9. [Google Scholar]
- Maischak, H.; Grigoriev, P.A.; Vogel, H.; Boland, W.; Mithöfer, A. Oral secretions from herbivorous lepidopteran larvae exhibit ion channel-forming activities. FEBS Lett. 2007, 581, 898–904. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, A.; Kariyat, R.R.; Chappa, C.; Tayal, M.; Sahoo, N. Tobacco Hornworm (Manduca sexta) Oral Secretion Elicits Reactive Oxygen Species in Isolated Tomato Protoplasts. Int. J. Mol. Sci. 2020, 21, 8297. https://doi.org/10.3390/ijms21218297
Gandhi A, Kariyat RR, Chappa C, Tayal M, Sahoo N. Tobacco Hornworm (Manduca sexta) Oral Secretion Elicits Reactive Oxygen Species in Isolated Tomato Protoplasts. International Journal of Molecular Sciences. 2020; 21(21):8297. https://doi.org/10.3390/ijms21218297
Chicago/Turabian StyleGandhi, Akanksha, Rupesh R. Kariyat, Cruz Chappa, Mandeep Tayal, and Nirakar Sahoo. 2020. "Tobacco Hornworm (Manduca sexta) Oral Secretion Elicits Reactive Oxygen Species in Isolated Tomato Protoplasts" International Journal of Molecular Sciences 21, no. 21: 8297. https://doi.org/10.3390/ijms21218297