Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo
Abstract
1. Introduction
2. Results
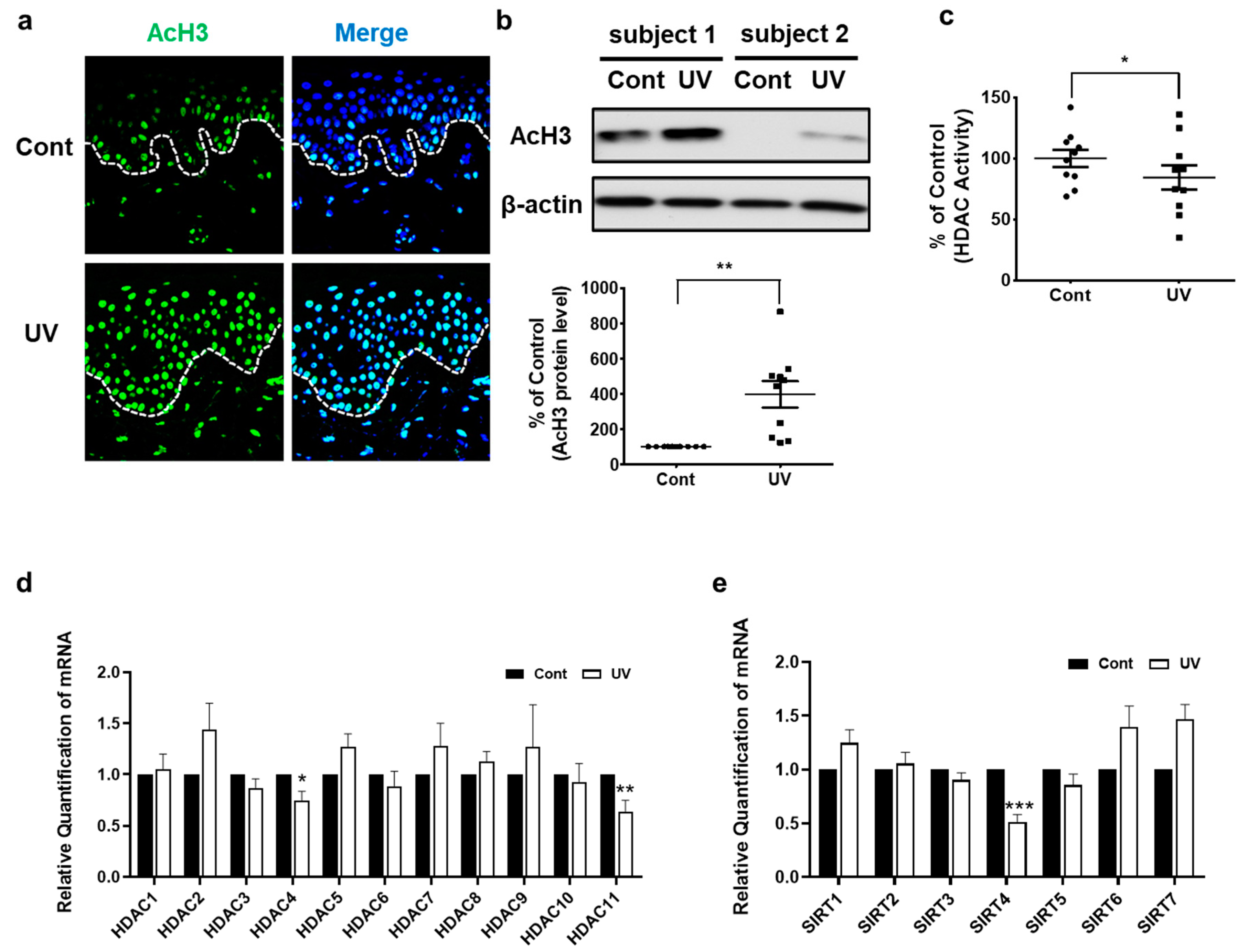
2.1. UV-Irradiated Human Skin Shows Increased AcH3 Levels but Decreased Global HDAC Activity and HDAC4, HDAC11, and SIRT4 Expression In Vivo
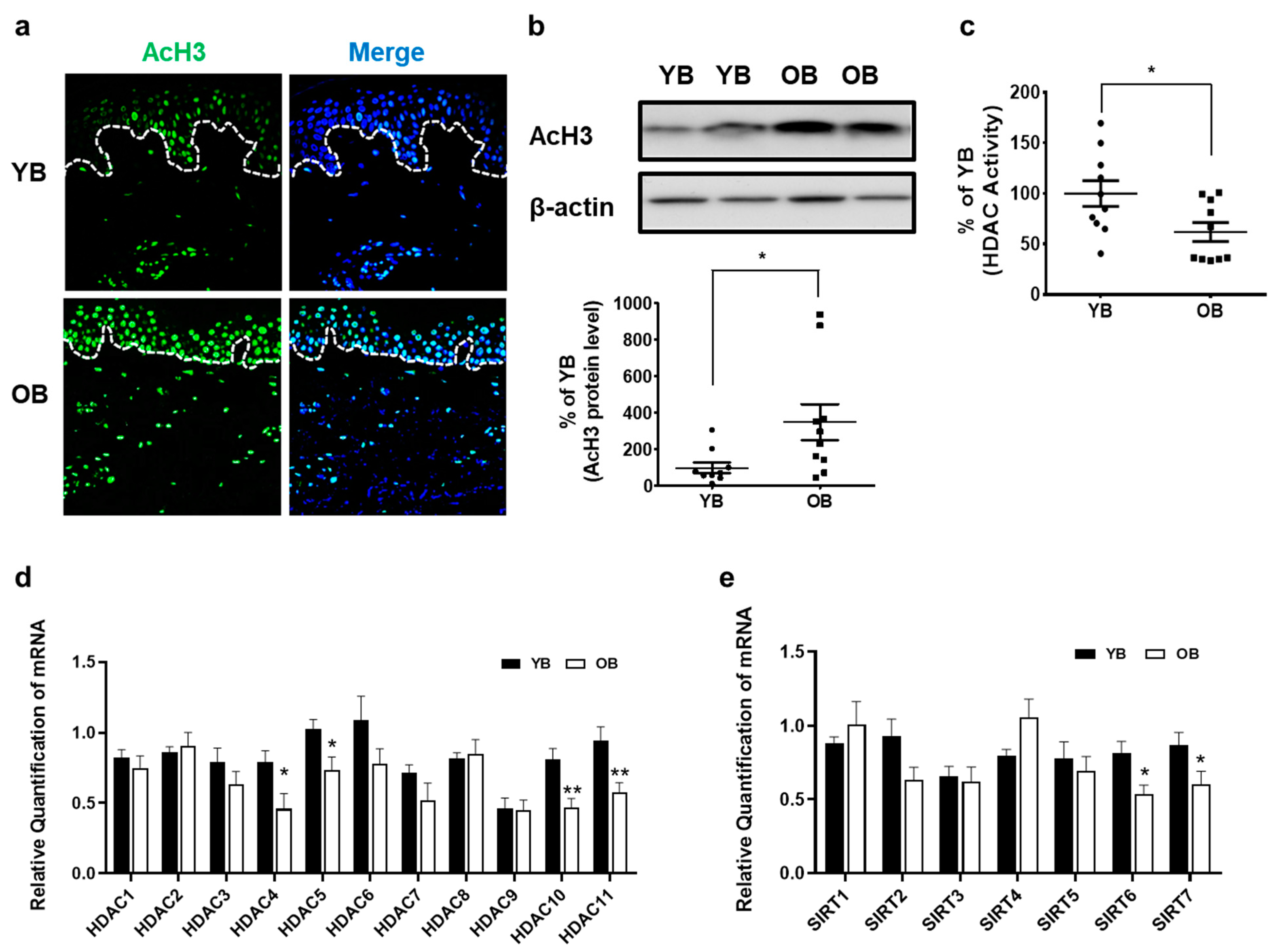
2.2. Intrinsically Aged Human Skin Shows Increased AcH3 Levels but Decreased Global HDAC Activity and HDAC4, HDAC5, HDAC10, HDAC11, SIRT6, and SIRT7 Expressions In Vivo
2.3. Acetylation of Histone 3, HDAC Activity, and Expression of HDACs and SIRTs Do Not Differ between Photoaged Forearm Skin and Intrinsically Aged Buttock Skin
3. Discussion
4. Materials and Methods
4.1. Human Skin Samples
4.2. Immunofluorescence Staining
4.3. Histone Deacetylase Activity Assay
4.4. Western Blot Analysis
4.5. Quantitative Real-Time RT-PCR
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcH3 | Acetylated histone H3 |
| HDAC | Histone deacetylase |
| SIRT | Sirtuin |
| UV | Ultraviolet |
References
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Chung, J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, s12–s16. [Google Scholar]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jo, S.; Choi, H.K.; Choi, S.; Byun, S.; Lim, T.G. Caffeic Acid Phenethyl Ester Inhibits UV-Induced MMP-1 Expression by Targeting Histone Acetyltransferases in Human Skin. Int. J. Mol. Sci. 2019, 20, 3055. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, E.J.; Cheng, Y.; Shin, M.H.; Oh, J.H.; Lee, D.H.; Chung, J.H. Inhibition of DNA Methylation in the COL1A2 Promoter by Anacardic Acid Prevents UV-Induced Decrease of Type I Procollagen Expression. J. Investig. Dermatol. 2017, 137, 1343–1352. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, D.H.; Lee, S.; Kim, E.J.; Chung, J.H. UV-induced DNA damage and histone modification may involve MMP-1 gene transcription in human skin in vivo. J. Dermatol. Sci. 2014, 73, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Shin, J.M.; Eun, H.C.; Chung, J.H. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS ONE 2009, 4, e4864. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, D.H.; Shin, M.H.; Shin, H.S.; Kim, M.K.; Chung, J.H. UV-induced DNA methyltransferase 1 promotes hypermethylation of tissue inhibitor of metalloproteinase 2 in the human skin. J. Dermatol. Sci. 2018, 91, 19–27. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Shen, Y.; Stanislauskas, M.; Li, G.; Zheng, D.; Liu, L. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci. Rep. 2017, 7, 42646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hou, F.; Wang, X.; Kong, Q.; Han, X.; Bai, B. Aberrant Expression of Histone Deacetylases 4 in Cognitive Disorders: Molecular Mechanisms and a Potential Target. Front. Mol. Neurosci. 2016, 9, 114. [Google Scholar] [CrossRef]
- Han, X.; Niu, J.; Zhao, Y.; Kong, Q.; Tong, T.; Han, L. HDAC4 stabilizes SIRT1 via sumoylation SIRT1 to delay cellular senescence. Clin. Exp. Pharmacol. Physiol. 2016, 43, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Glenisson, W.; Castronovo, V.; Waltregny, D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim. Biophys. Acta 2007, 1773, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, M.H.; Kim, M.K.; Park, C.H.; Shin, H.S.; Lee, D.H.; Chung, J.H. Ultraviolet irradiation-induced inhibition of histone deacetylase 4 increases the expression of matrix metalloproteinase-1 but decreases that of type I procollagen via activating JNK in human dermal fibroblasts. J. Dermatol. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.K.; An, T.H.; Son, M.J.; Lee, D.S.; Kang, H.S.; Lee, E.-W.; Han, B.S.; Kim, W.K.; Bae, K.-H.; Oh, K.-J.; et al. HDAC11 Inhibits Myoblast Differentiation through Repression of MyoD-Dependent Transcription. Mol. Cells 2017, 40, 667–676. [Google Scholar] [PubMed]
- Núñez-Álvarez, Y.; Hurtado, E.; Muñoz, M.; García-Tuñon, I.; Rech, G.E.; Pluvinet, R.; Sumoy, L.; Pendás, A.M.; Peinado, M.A.; Suelves, M. Loss of HDAC11 accelerates skeletal muscle regeneration. FEBS J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yanginlar, C.; Logie, C. HDAC11 is a regulator of diverse immune functions. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.; Zeng, Q.; Lu, J.; Tan, L.; Guo, A.; Kang, J.; Yang, S.; Xiang, Y.; Zuo, C.; et al. Chronic sun exposure is associated with distinct histone acetylation changes in human skin. Br. J. Dermatol. 2018, 179, 110–117. [Google Scholar] [CrossRef]
- Silva, M.B.D.; Melo, A.; Costa, L.A.; Barroso, H.; Oliveira, N.F.P. Global and gene-specific DNA methylation and hydroxymethylation in human skin exposed and not exposed to sun radiation. An. Bras. Dermatol. 2017, 92, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Epigenetic changes during aging and their reprogramming potential. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D(3) and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Tollefsbol, T.O. Epigenetic linkage of aging, cancer and nutrition. J. Exp. Biol. 2015, 218, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, J.; Liu, G.H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, S.; Kim, E.J.; Kong, K.H.; Lee, D.H.; Chung, J.H. Topical application of anacardic acid (6-nonadecyl salicylic acid) reduces UV-induced histone modification, MMP-13, MMP-9, COX-2 and TNF-α expressions in hairless mice skin. J. Dermatol. Sci. 2013, 70, 64–67. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers (5′->3′) | |
|---|---|---|
| Forward | Reverse | |
| HDAC1 HDAC2 HDAC3 HDAC4 HDAC5 HDAC6 HDAC7 HDAC8 HDAC9 HDAC10 HDAC11 SIRT1 SIRT2 SIRT3 SIRT4 SIRT5 SIRT6 SIRT7 36B4 | ATCTATCGCCCTCACAAAGC TGCTACTACTACGACGGTGA GAGAGTCAGCCCCACCAATA GAGAGACTCACCCTTCCCG ACAGCATGACCCCTGACAAGG ATCTGGCGGAGTGGAAGAA CTCACTGTCAGCCCCAGAG AAACGGGCCAGTATGGTG CAACAAAACCCTAGCAGCCT CACTAGCGAGGGCGTTTG GGATGCTACACACAACCCA GCGATTGGGTACCGAGATAA GACCCCTCTCACCCTCTG TCCCAGTTTCTTCTTTTCGAGTA GACAGGGTCCTGTGCTTG GGGGCCCAAGTAAATGGAAA TCCCGGAGATCTTCGACC TGTGGACACTGCTTCAGAAAGGGA TGGGCTCCAAGCAGATGC | AATCTCTGCATCTGCTTGCT AGTGGCTTTATGGGGCCTA GTTGTTCAGCTGGGTTGCTC CCGGTCTGCACCAACCAAG GCTCCTGCTGCCGCTTGG AAGTGACACTGGAGTCCTGA CTGGTGCTTCAGCATGACC CTGACCTTCTGGAGATGCTG GCCCACAGGAACTTCTGACT GGGTCGTCCCAGAGCA CCCATTTTCCGGCATCAAAG TTGCATGTGAGGCTCTATCC CAGGAAGTCCATGTCTGCTT GAAAGCTTCCCCTTGTCACT GCTCCTCTGAGAGAAAGACG TGAAGATGGTTGTCTCCACG CCAGAGGCAGTGCTGATG CACAGTTCTGAGACACCACATGCT GGCTTCGCTGGCTCCCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Shin, M.H.; Kim, M.-K.; Kim, Y.K.; Shin, H.S.; Lee, D.H.; Chung, J.H. Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo. Int. J. Mol. Sci. 2021, 22, 2032. https://doi.org/10.3390/ijms22042032
Lee Y, Shin MH, Kim M-K, Kim YK, Shin HS, Lee DH, Chung JH. Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo. International Journal of Molecular Sciences. 2021; 22(4):2032. https://doi.org/10.3390/ijms22042032
Chicago/Turabian StyleLee, Yuri, Mi Hee Shin, Min-Kyoung Kim, Yeon Kyung Kim, Hye Sun Shin, Dong Hun Lee, and Jin Ho Chung. 2021. "Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo" International Journal of Molecular Sciences 22, no. 4: 2032. https://doi.org/10.3390/ijms22042032
APA StyleLee, Y., Shin, M. H., Kim, M.-K., Kim, Y. K., Shin, H. S., Lee, D. H., & Chung, J. H. (2021). Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo. International Journal of Molecular Sciences, 22(4), 2032. https://doi.org/10.3390/ijms22042032






