Diabetes, Obesity, and Inflammation: Impact on Clinical and Radiographic Features of Breast Cancer
Abstract
:1. Introduction
2. Body Sections
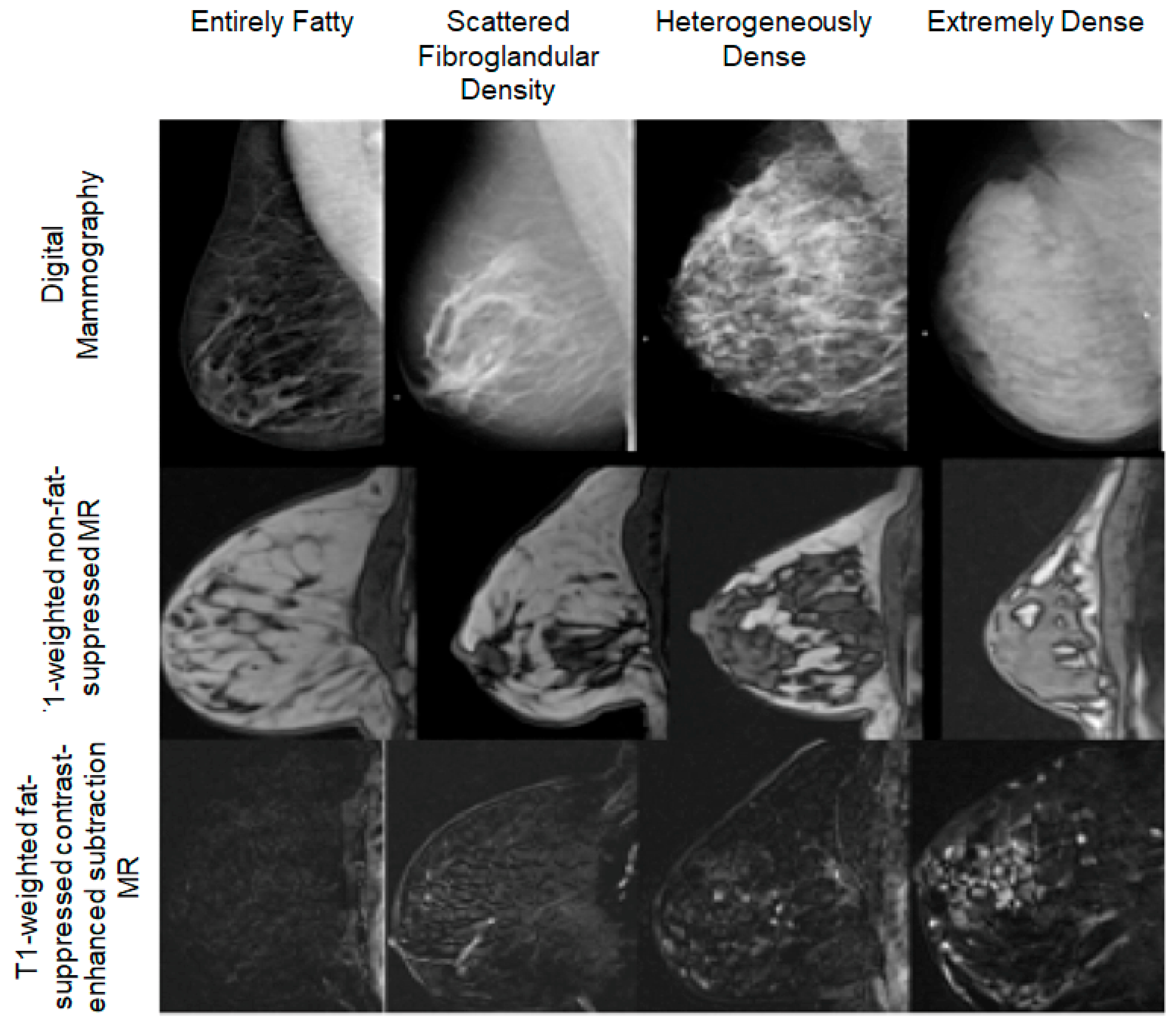
2.1. Obesity-Related Limitations to Conventional Imaging
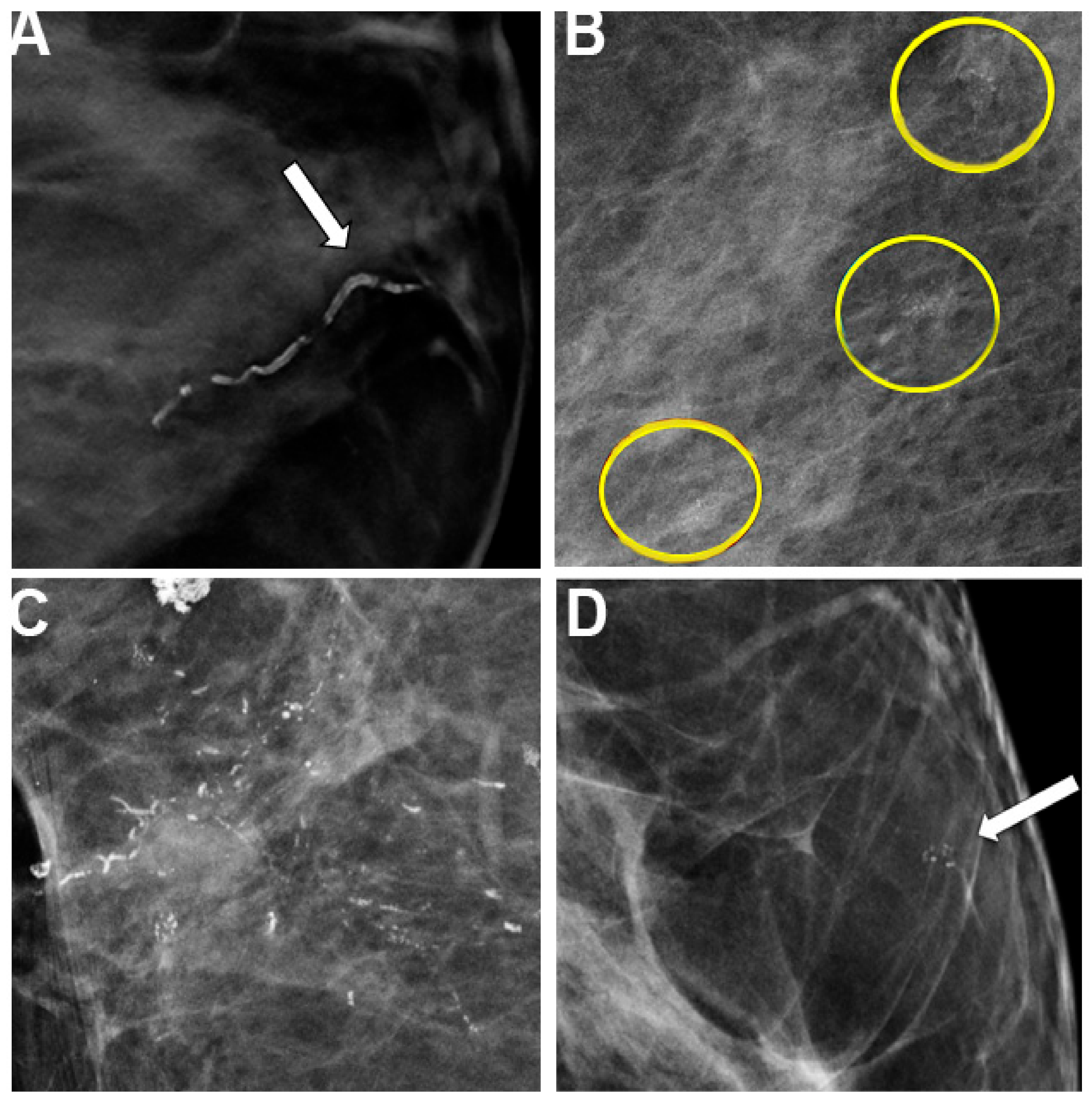
2.2. Diabetes-Related Changes in Imaging Quality
2.3. Influences of Inflammation and Anti-Inflammatory Agents in Breast Imaging
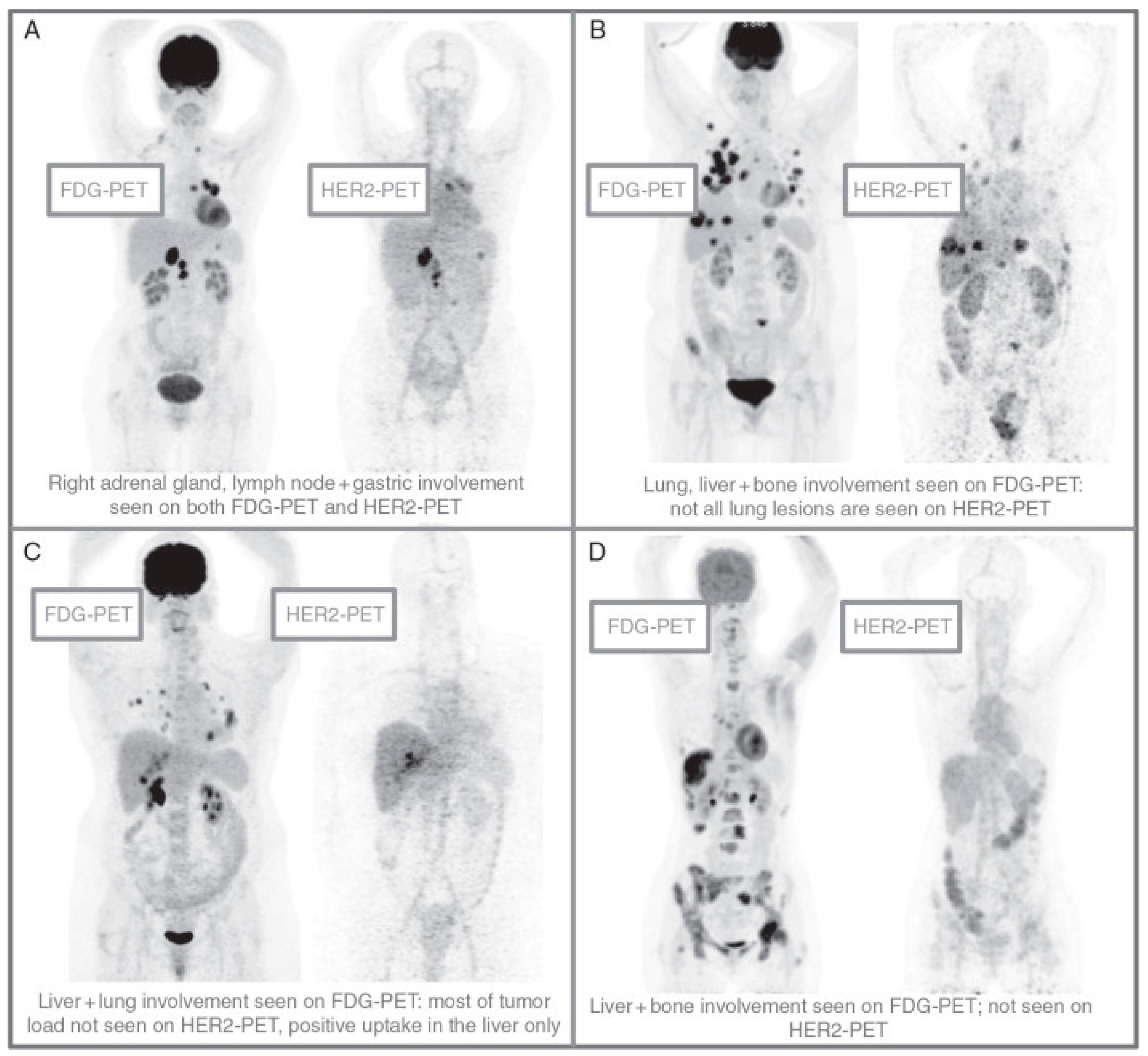
2.4. Novel Molecular Agents and Imaging Techniques to Detect Breast Cancer in Patients with Diabetes, Obesity, and Inflammation
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F.R. Cancer-related inflammation. Nat. Cell Biol. 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Rhoads, A.; Suhl, J.; Conway, K.M.; Hundley, W.G.; McNally, L.R.; Oleson, J.; Melin, S.A.; Lynch, C.F.; Romitti, P.A. Incidence and Survival by Human Epidermal Growth Factor Receptor 2 Status in Young Women with Stage I-III Breast Cancer: SEER, 2010-2016. Clin. Breast Cancer 2020, 20, e410–e422. [Google Scholar] [CrossRef]
- Thomas, A.; Rhoads, A.; Pinkerton, E.; Schroeder, M.C.; Conway, K.M.; Hundley, W.G.; McNally, L.R.; Oleson, J.; Lynch, C.F.; Romitti, P.A. Incidence and Survival Among Young Women with Stage I–III Breast Cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019, 3, pkz040. [Google Scholar] [CrossRef]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Tobias, D.K.; Akinkuolie, A.O.; Chandler, P.D.; Lawler, P.R.; Manson, J.E.; Buring, J.E.; Ridker, P.M.; Wang, L.; Lee, I.-M.; Mora, S. Markers of Inflammation and Incident Breast Cancer Risk in the Women’s Health Study. Am. J. Epidemiol. 2017, 187, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef]
- Kang, C.; Leroith, D.; Gallagher, E.J. Diabetes, Obesity, and Breast Cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- Kerlikowske, K.; Grady, D.; Rubin, S.M.; Sandrock, C.; Ernster, V.L. Efficacy of screening mammography. A meta-analysis. JAMA 1995, 273, 149–154. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Kricker, A.; Di Sipio, T.; Stone, J.; Goumas, C.; Armes, J.E.; Gertig, R.M.; Armstrong, B.K. Bodyweight and other correlates of symptom-detected breast cancers in a population offered screening. Cancer Causes Control. 2011, 23, 89–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deglise, C.; Bouchardy, C.; Burri, M.; Usel, M.; Neyroud-Caspar, I.; Vlastos, G.; Chappuis, P.O.; Ceschi, M.; Ess, S.; Castiglione, M.; et al. Impact of obesity on diagnosis and treatment of breast cancer. Breast Cancer Res. Treat. 2009, 120, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Yen, A.M.-F.; Wu, W.Y.-Y.; Tabar, L.; Duffy, S.W.; Smith, R.A.; Chen, H.-H. Initiators and promoters for the occurrence of screen-detected breast cancer and the progression to clinically-detected interval breast cancer. J. Epidemiol. 2017, 27, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Kerlikowske, K.; Walker, R.; Miglioretti, D.L.; Desai, A.; Ballard-Barbash, R.; Buist, D.S.M. Obesity, Mammography Use and Accuracy, and Advanced Breast Cancer Risk. J. Natl. Cancer Inst. 2008, 100, 1724–1733. [Google Scholar] [CrossRef] [Green Version]
- Maruthur, N.M.; Bolen, S.; Brancati, F.L.; Clark, J.M. Obesity and Mammography: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2009, 24, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Njor, S.H.; Von Euler-Chelpin, M.; Tjønneland, A.; Vejborg, I.; Lynge, E. Body weight and sensitivity of screening mammography. Eur. J. Cancer 2016, 60, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Berg, W.A.; D’Orsi, C.J.; Jackson, V.P.; Bassett, L.W.; Beam, C.A.; Lewis, R.S.; Crewson, P.E. Does Training in the Breast Imaging Reporting and Data System (BI-RADS) Improve Biopsy Recommendations or Feature Analysis Agreement with Experienced Breast Imagers at Mammography? Radiology 2002, 224, 871–880. [Google Scholar] [CrossRef]
- Gillman, J.; Chun, J.; Schwartz, S.; Schnabel, F.; Moy, L. The relationship of obesity, mammographic breast density, and magnetic resonance imaging in patients with breast cancer. Clin. Imaging 2016, 40, 1167–1172. [Google Scholar] [CrossRef]
- Banks, E.; Reeves, G.; Beral, V.; Bull, D.; Crossley, B.; Simmonds, M.; Hilton, E.; Bailey, S.; Barrett, N.; Briers, P.; et al. Influence of personal characteristics of individual women on sensitivity and specificity of mammography in the Million Women Study: Cohort study. BMJ 2004, 329, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, J.G.; Carney, P.A.; Abraham, L.A.; Barlow, W.E.; Egger, J.R.; Fosse, J.S.; Cutter, G.R.; Hendrick, R.E.; D’Orsi, C.J.; Paliwal, P.; et al. The Association Between Obesity and Screening Mammography Accuracy. Arch. Intern. Med. 2004, 164, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef] [Green Version]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of Digital Mammography Alone and Digital Mammography Plus Tomosynthesis in a Population-based Screening Program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Rose, S.L.; Tidwell, A.L.; Bujnoch, L.J.; Kushwaha, A.C.; Nordmann, A.S.; Sexton, R. Implementation of Breast Tomosynthesis in a Routine Screening Practice: An Observational Study. Am. J. Roentgenol. 2013, 200, 1401–1408. [Google Scholar] [CrossRef]
- Haas, B.M.; Kalra, V.; Geisel, J.; Raghu, M.; Durand, M.; Philpotts, L.E. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013, 269, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lowry, K.P.; Coley, R.Y.; Miglioretti, D.L.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Sprague, B.L.; Lee, J.M.; Herschorn, S.; Tosteson, A.N.A.; et al. Screening Performance of Digital Breast Tomosynthesis vs Digital Mammography in Community Practice by Patient Age, Screening Round, and Breast Density. JAMA Netw. Open 2020, 3, e2011792. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Lehman, C.D. Clinical indications: What is the evidence? Eur. J. Radiol. 2012, 81, S82–S84. [Google Scholar] [CrossRef]
- Hollingsworth, A.B.; Stough, R.G.; O’Dell, C.A.; Brekke, C.E. Breast magnetic resonance imaging for preoperative locoregional staging. Am. J. Surg. 2008, 196, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schelfout, K.; Van Goethem, M.; Kersschot, E.; Colpaert, C.; Schelfhout, A.; Leyman, P.; Verslegers, I.; Biltjes, I.; Haute, J.V.D.; Gillardin, J.; et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur. J. Surg. Oncol. (EJSO) 2004, 30, 501–507. [Google Scholar] [CrossRef]
- Parsyan, A.; Alqahtani, A.; Mesurolle, B.; Meterissian, S. Impact of Preoperative Breast MRI on Surgical Decision Making and Clinical Outcomes: A Systematic Review. World J. Surg. 2013, 37, 2134–2139. [Google Scholar] [CrossRef]
- Miller, B.T.; Abbott, A.M.; Tuttle, T.M. The influence of preoperative MRI on breast cancer treatment. Ann. Surg. Oncol. 2012, 19, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, R.J.; Ciocca, R.M.; Egleston, B.L.; Sesa, L.; Evers, K.; Sigurdson, E.R.; Morrow, M. Association of Routine Pretreatment Magnetic Resonance Imaging with Time to Surgery, Mastectomy Rate, and Margin Status. J. Am. Coll. Surg. 2009, 209, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Plana, M.N.; Carreira, C.; Muriel, A.; Chiva, M.; Abraira, V.; Emparanza, J.I.; Bonfill, X.; Zamora, J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: Systematic review of diagnostic accuracy and meta-analysis. Eur. Radiol. 2011, 22, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Destounis, S.; Newell, M.; Pinsky, R. Breast Imaging and Intervention in the Overweight and Obese Patient. Am. J. Roentgenol. 2011, 196, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Guest, A.R.; Helvie, M.A.; Chan, H.-P.; Hadjiiski, L.M.; Bailey, J.E.; Roubidoux, M.A. Adverse Effects of Increased Body Weight on Quantitative Measures of Mammographic Image Quality. Am. J. Roentgenol. 2000, 175, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Helvie, M.A.; Chan, H.P.; Adler, D.D.; Boyd, P.G. Breast thickness in routine mammograms: Effect on image quality and radiation dose. Am. J. Roentgenol. 1994, 163, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.; Chaudhry, S. The Need for a Multidisciplinary Approach to Cancer Care. J. Surg. Res. 2002, 105, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020.
- Liao, S.; Li, J.; Wei, W.; Wang, L.; Zhang, Y.; Li, J.; Wang, C.; Sun, S. Association between diabetes mellitus and breast cancer risk: A meta-analysis of the literature. Asian Pac. J. Cancer Prev. 2011, 12, 1061–1065. [Google Scholar]
- Boyle, P.; Boniol, M.; Koechlin, A.; Robertson, C.R.; Valentini, F.; Coppens, K.; Fairley, L.L.; Zheng, T.; Zhang, Y.; Pasterk, M.; et al. Diabetes and breast cancer risk: A meta-analysis. Br. J. Cancer 2012, 107, 1608–1617. [Google Scholar] [CrossRef] [Green Version]
- Laudisio, D.; Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Obesity and breast cancer in premenopausal women: Current evidence and future perspectives. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 217–221. [Google Scholar] [CrossRef]
- Humpert, P.M.; Djuric, Z.; Zeuge, U.; Oikonomou, D.; Seregin, Y.; Laine, K.; Eckstein, V.; Nawroth, P.P.; Bierhaus, A. Insulin Stimulates the Clonogenic Potential of Angiogenic Endothelial Progenitor Cells by IGF-1 Receptor-Dependent Signaling. Mol. Med. 2008, 14, 301–308. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.F.; Xue, X.; Anderson, G.L.; et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. J. Natl. Cancer Inst. 2008, 101, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.; Ho, G.Y.F.; et al. A Prospective Evaluation of Insulin and Insulin-like Growth Factor-I as Risk Factors for Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Wanders, J.O.P.; Holland, K.; Veldhuis, W.B.; Mann, R.M.; Pijnappel, R.M.; Peeters, P.H.M.; Van Gils, C.H.; Karssemeijer, N. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res. Treat. 2017, 162, 95–103. [Google Scholar] [CrossRef] [Green Version]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgquist, S.; Rosendahl, A.H.; Czene, K.; Bhoo-Pathy, N.; Dorkhan, M.; Hall, P.; Brand, J.S. Long-term exposure to insulin and volumetric mammographic density: Observational and genetic associations in the Karma study. Breast Cancer Res. 2018, 20, 93. [Google Scholar] [CrossRef]
- Kim, B.-K.; Chang, Y.; Ahn, J.; Jung, H.-S.; Kim, C.-W.; Yun, K.E.; Kwon, M.-J.; Suh, B.-S.; Chung, E.C.; Shin, H.; et al. Metabolic syndrome, insulin resistance, and mammographic density in pre- and postmenopausal women. Breast Cancer Res. Treat. 2015, 153, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, Y.; Ursin, G.; Bjurstam, N.; Rinaldi, S.; Kaaks, R.; Gram, I.T. Insulin-like Growth Factor and Mammographic Density in Postmenopausal Norwegian Women. Cancer Epidemiol. Biomark. Prev. 2007, 16, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.; Colditz, G.A.; Willett, W.C.; Speizer, F.E.; Pollak, M.; Hankinson, S.E. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000, 60, 3744–3748. [Google Scholar] [PubMed]
- Buschard, K.; Thomassen, K.; Lynge, E.; Vejborg, I.; Tjønneland, A.; Von Euler-Chelpin, M.; Andersen, Z.J. Diabetes, diabetes treatment, and mammographic density in Danish Diet, Cancer, and Health cohort. Cancer Causes Control. 2016, 28, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, S.W.; Elmore, J.G. Mammographic Screening for Breast Cancer. N. Engl. J. Med. 2003, 348, 1672–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors that Influence Them: An Analysis of 27,825 Patient Evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conant, E.F.; Barlow, W.E.; Herschorn, S.D.; Weaver, D.L.; Beaber, E.F.; Tosteson, A.N.A.; Haas, J.S.; Lowry, K.P.; Stout, N.K.; Trentham-Dietz, A.; et al. Association of Digital Breast Tomosynthesis vs Digital Mammography with Cancer Detection and Recall Rates by Age and Breast Density. JAMA Oncol. 2019, 5, 635–642. [Google Scholar] [CrossRef]
- McCarthy, A.M.; Kontos, D.; Synnestvedt, M.; Tan, K.S.; Heitjan, D.F.; Schnall, M.; Conant, E.F. Screening Outcomes Following Implementation of Digital Breast Tomosynthesis in a General-Population Screening Program. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.A.; Czene, K.; Hall, P.; Humphreys, K. Association of Microcalcification Clusters with Short-term Invasive Breast Cancer Risk and Breast Cancer Risk Factors. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Horvat, J.V.; Keating, D.M.; Rodrigues-Duarte, H.; Morris, E.A.; Mango, V.L. Calcifications at Digital Breast Tomosynthesis: Imaging Features and Biopsy Techniques. Radiographics 2019, 39, 307–318. [Google Scholar] [CrossRef]
- Okello, J.; Kisembo, H.; Bugeza, S.; Galukande, M. Breast cancer detection using sonography in women with mammographically dense breasts. BMC Med. Imaging 2014, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.-H.; Hsu, H.-C.; Chen, Y.-Y.; Wu, C.-H. Supplemental breast cancer-screening ultrasonography in women with dense breasts: A systematic review and meta-analysis. Br. J. Cancer 2020, 123, 673–688. [Google Scholar] [CrossRef]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women with Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women with Dense Breasts: A Systematic Review for the U.S. Preventive Service Task Force. Prev. Serv. Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef]
- Simpson, E.R.; Brown, K.A. Obesity and breast cancer: Role of inflammation and aromatase. J. Mol. Endocrinol. 2013, 51, T51–T59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, V.; D’Avenia, M.; Argentiero, A.; Felici, C.; Rizzo, F.M.; De Pergola, G.; Silvestris, F. Obesity and Breast Cancer: Molecular Interconnections and Potential Clinical Applications. Oncology 2016, 21, 404–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, P.G.; Hudis, C.A.; Giri, D.; Morrow, M.; Falcone, D.J.; Zhou, X.K.; Du, B.; Brogi, E.; Crawford, C.B.; Kopelovich, L.; et al. Inflammation and Increased Aromatase Expression Occur in the Breast Tissue of Obese Women with Breast Cancer. Cancer Prev. Res. 2011, 4, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Subbaramaiah, K.; Morris, P.G.; Zhou, X.K.; Morrow, M.; Du, B.; Giri, D.; Kopelovich, L.; Hudis, C.A.; Dannenberg, A.J. Increased Levels of COX-2 and Prostaglandin E2 Contribute to Elevated Aromatase Expression in Inflamed Breast Tissue of Obese Women. Cancer Discov. 2012, 2, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Bulun, S.E.; Price, T.M.; Aitken, J.; Mahendroo, M.S.; Simpson, E.R. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 1993, 77, 1622–1628. [Google Scholar] [CrossRef]
- Vachon, C.M.; Sasano, H.; Ghosh, K.; Brandt, K.R.; Watson, D.A.; Reynolds, C.; Lingle, W.L.; Goss, P.E.; Li, R.; Aiyar, S.E.; et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res. Treat. 2011, 125, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellberg, E.A.; Kabos, P.; Gillen, A.E.; Jacobsen, B.M.; Brechbuhl, H.M.; Johnson, S.J.; Rudolph, M.C.; Edgerton, S.M.; Thor, A.D.; Anderson, S.M.; et al. FGFR1 underlies obesity-associated progression of estrogen receptor–positive breast cancer after estrogen deprivation. JCI Insight 2018, 3, 14. [Google Scholar] [CrossRef]
- Giles, E.D.; Jindal, S.; Wellberg, E.A.; Schedin, T.; Anderson, S.M.; Thor, A.D.; Edwards, D.P.; MacLean, P.S.; Schedin, P. Metformin inhibits stromal aromatase expression and tumor progression in a rodent model of postmenopausal breast cancer. Breast Cancer Res. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Irahara, N.; Miyoshi, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. Int. J. Cancer 2006, 118, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.; Lu, Q.; Long, B.; Fulton, A.; Chen, T.; MacPherson, N.; DeJong, P.; Blankenstein, M.; Nortier, J.; Slee, P.; et al. Aromatase and COX-2 expression in human breast cancers. J. Steroid Biochem. Mol. Biol. 2001, 79, 41–47. [Google Scholar] [CrossRef]
- Karuppu, D.; Kalus, A.; Simpson, E.R.; Clyne, C. Aromatase and prostaglandin inter-relationships in breast adipose tissue: Significance for breast cancer development. Breast Cancer Res. Treat. 2002, 76, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity Is Associated with Inflammation and Elevated Aromatase Expression in the Mouse Mammary Gland. Cancer Prev. Res. 2011, 4, 329–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nes, J.G.H.; De Kruijf, E.M.; Faratian, D.; Van De Velde, C.J.H.; Putter, H.; Falconer, C.; Smit, V.T.H.B.M.; Kay, C.; Van De Vijver, M.J.; Kuppen, P.J.K.; et al. COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Breast Cancer Res. Treat. 2010, 125, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Kehm, R.D.; Hopper, J.L.; John, E.M.; Phillips, K.-A.; MacInnis, R.J.; Dite, G.S.; Milne, R.L.; Liao, Y.; Zeinomar, N.; Knight, J.A.; et al. Regular use of aspirin and other non-steroidal anti-inflammatory drugs and breast cancer risk for women at familial or genetic risk: A cohort study. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Buist, D.S.; Trentham-Dietz, A.; James-Todd, T.M.; Liao, Y. Nonsteroidal Anti-inflammatory Drugs and Change in Mammographic Density: A Cohort Study Using Pharmacy Records on Over 29,000 Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Smith-Warner, S.A.; Collins, L.C.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Use of Aspirin, Other Nonsteroidal Anti-Inflammatory Drugs, and Acetaminophen and Postmenopausal Breast Cancer Incidence. J. Clin. Oncol. 2012, 30, 3468–3477. [Google Scholar] [CrossRef] [Green Version]
- De Pedro, M.; Baeza, S.; Escudero, M.-T.; Dierssen-Sotos, T.; Gómez-Acebo, I.; Pollán, M.; Llorca, J. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2015, 149, 525–536. [Google Scholar] [CrossRef]
- Sree, S.V.; Ng, E.Y.; Acharya, R.U.; Faust, O. Breast imaging: A survey. World J. Clin. Oncol 2011, 2, 171–178. [Google Scholar] [CrossRef]
- Basu, S.; Hess, S.; Braad, P.-E.N.; Olsen, B.B.; Inglev, S.; Høilund-Carlsen, P.F. The Basic Principles of FDG-PET/CT Imaging. PET Clin. 2014, 9, 355–370. [Google Scholar] [CrossRef]
- Bénard, F.; Turcotte, É. Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography. Breast Cancer Res. 2005, 7, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerman, H.; Lievshitz, G.; Zak, O.; Metser, U.; Schneebaum, S.; Even-Sapir, E. Improved sentinel node identification by SPECT/CT in overweight patients with breast cancer. J. Nucl. Med. 2007, 48, 201. [Google Scholar]
- Kostakoglu, L.; Agress, H.; Goldsmith, S.J. Clinical Role of FDG PET in Evaluation of Cancer Patients. Radiographics 2003, 23, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Benchekroun, M.T.; Abraham, T. FDG-PET/CT in the Postoperative Period: Utility, Expected Findings, Complications, and Pitfalls. Semin. Nucl. Med. 2017, 47, 579–594. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tasaki, Y.; Kuwada, Y.; Ozawa, Y.; Inoue, T. A preliminary report of breast cancer screening by positron emission mammography. Ann. Nucl. Med. 2015, 30, 130–137. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, L.; He, Z. Performance of Whole-Body PET/CT for the Detection of Distant Malignancies in Various Cancers: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2012, 53, 1847–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, W.A.; Madsen, K.S.; Schilling, K.; Tartar, M.; Pisano, E.D.; Larsen, L.H.; Narayanan, D.; Ozonoff, A.; Miller, J.P.; Kalinyak, J.E. Breast Cancer: Comparative Effectiveness of Positron Emission Mammography and MR Imaging in Presurgical Planning for the Ipsilateral Breast. Radiology 2011, 258, 59–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, H.; Shimizu, C.; Miyakita, Y.; Yoshida, M.; Hamada, A.; Kanayama, Y.; Yonemori, K.; Hashimoto, J.; Tani, H.; Kodaira, M.; et al. Molecular imaging using PET for breast cancer. Breast Cancer 2015, 23, 24–32. [Google Scholar] [CrossRef]
- Miladinova, D. Molecular Imaging in Breast Cancer. Nucl. Med. Mol. Imaging 2019, 53, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rainone, P.; Riva, B.; Belloli, S.; Sudati, F.; Ripamonti, M.; Verderio, P.; Colombo, M.; Colzani, B.; Gilardi, M.C.; Moresco, R.M.; et al. Development of 99mTc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int. J. Nanomed. 2017, 12, 3447–3461. [Google Scholar] [CrossRef] [Green Version]
- Kenny, L.; Coombes, R.C.; Vigushin, D.M.; Al-Nahhas, A.; Shousha, S.; Aboagye, E.O. Imaging early changes in proliferation at 1 week post chemotherapy: A pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Munnink, T.O.; Nagengast, W.; Brouwers, A.; Schröder, C.; Hospers, G.; Hooge, M.L.-D.; Van Der Wall, E.; Van Diest, P.; De Vries, E. Molecular imaging of breast cancer. Breast 2009, 18, S66–S73. [Google Scholar] [CrossRef]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Samykutty, A.; Thomas, A.; McNally, M.; Chiba, A.; McNally, L.R. Osteopontin-targeted probe detects orthotopic breast cancers using optoacoustic imaging. Biotech. Histochem. 2018, 93, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, Z. Molecular Imaging Probes for Diagnosis and Therapy Evaluation of Breast Cancer. Int. J. Biomed. Imaging 2013, 2013, 230487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosley, M.; Knight, J.; Neesse, A.; Michl, P.; Iezzi, M.; Kersemans, V.; Cornelissen, B. Claudin-4 SPECT Imaging Allows Detection of Aplastic Lesions in a Mouse Model of Breast Cancer. J. Nucl. Med. 2015, 56, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, B.; Chalfant, H.; Thomas, A.; Wellberg, E.; Henson, C.; McNally, M.W.; Grizzle, W.E.; Jain, A.; McNally, L.R. Diabetes, Obesity, and Inflammation: Impact on Clinical and Radiographic Features of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 2757. https://doi.org/10.3390/ijms22052757
Miller B, Chalfant H, Thomas A, Wellberg E, Henson C, McNally MW, Grizzle WE, Jain A, McNally LR. Diabetes, Obesity, and Inflammation: Impact on Clinical and Radiographic Features of Breast Cancer. International Journal of Molecular Sciences. 2021; 22(5):2757. https://doi.org/10.3390/ijms22052757
Chicago/Turabian StyleMiller, Braden, Hunter Chalfant, Alexandra Thomas, Elizabeth Wellberg, Christina Henson, Molly W. McNally, William E. Grizzle, Ajay Jain, and Lacey R. McNally. 2021. "Diabetes, Obesity, and Inflammation: Impact on Clinical and Radiographic Features of Breast Cancer" International Journal of Molecular Sciences 22, no. 5: 2757. https://doi.org/10.3390/ijms22052757




