Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone
Abstract
:1. Introduction
2. Piezo Channels
2.1. The Genes, Members, and Structures of Piezo Channels
2.1.1. The Genes and Members of Piezo Channels
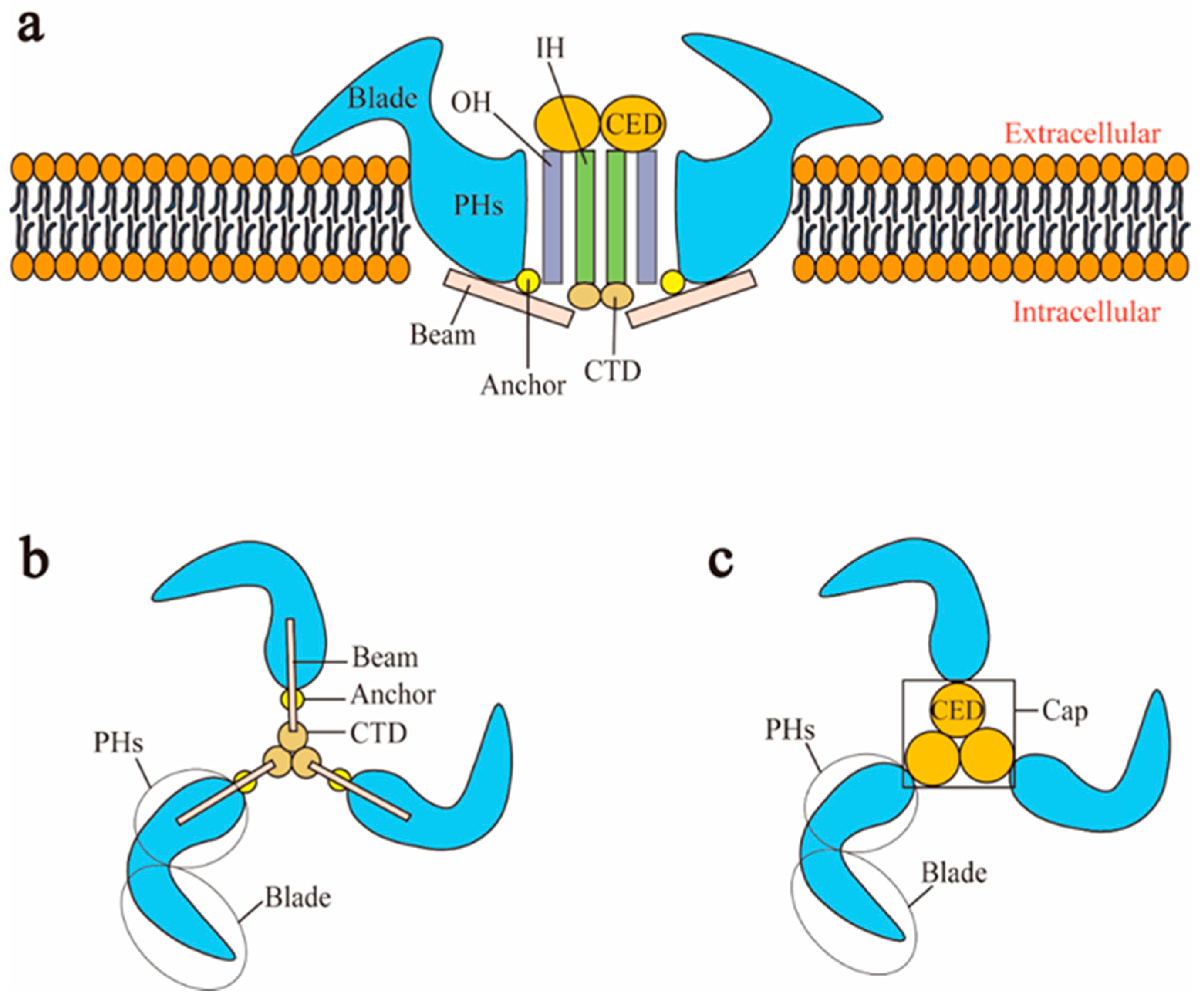
2.1.2. Structure
3. The Role of Piezo Channels in Cellular Mechanotransduction
4. Activators and Inhibitors of Piezo Channels
5. The Role of Piezo Channels in Bone
5.1. Piezo1 and Osteocytes
5.2. Piezo1 and Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
5.3. Piezo1 and Osteoblasts
5.4. Piezo1 and Osteoclasts
5.5. Piezo1 and Chondrocytes
6. Piezo Channels and Bone Disease
6.1. Osteoporosis (OP)
6.2. Osteoarthritis (OA)
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, N. Review of Cellular Mechanotransduction. J. Phys. D Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Muller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Khan, K.M.; Duronio, V.; Hart, D.A. Mechanotransduction in human bone: In vitro cellular physiology that underpins bone changes with exercise. Sports Med. 2008, 38, 139–160. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Xu, R.; Zhang, C.; Lv, Y. Responses of MSCs to 3D Scaffold Matrix Mechanical Properties under Oscillatory Perfusion Culture. ACS Appl. Mater. Interfaces 2017, 9, 1207–1218. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. Elife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Elliot, K.J.; Millward-Sadler, S.J.; Wright, M.O.; Robb, J.E.; Wallace, W.H.; Salter, D.M. Effects of methotrexate on human bone cell responses to mechanical stimulation. Rheumatology 2004, 43, 1226–1231. [Google Scholar] [CrossRef] [Green Version]
- Robling, A.G.; Turner, C.H. Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.Y.; Jin, Y.; Ma, X.H.; Wang, C.Y.; Guo, Y.; Zhou, D. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J. Orthop. Surg. 2020, 28, 2309499020950262. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Nicole, L.; Guido, A.; Pizzol, D. Spaceflight osteoporosis: Current state and future perspective. Endocr. Regul. 2015, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- DeFrate, L.E.; Kim-Wang, S.Y.; Englander, Z.A.; McNulty, A.L. Osteoarthritis year in review 2018: Mechanics. Osteoarthr. Cartil. 2019, 27, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinac, B.; Poole, K. Mechanically activated ion channels. Int. J. Biochem. Cell Biol. 2018, 97, 104–107. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo proteins: Regulators of mechanosensation and other cellular processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef] [Green Version]
- Li, X.F.; Zhang, Z.; Chen, Z.K.; Cui, Z.W.; Zhang, H.N. Piezo1 protein induces the apoptosis of human osteoarthritis-derived chondrocytes by activating caspase-12, the signaling marker of ER stress. Int. J. Mol. Med. 2017, 40, 845–853. [Google Scholar] [CrossRef]
- Martins, J.R.; Penton, D.; Peyronnet, R.; Arhatte, M.; Moro, C.; Picard, N.; Kurt, B.; Patel, A.; Honore, E.; Demolombe, S. Piezo1-dependent regulation of urinary osmolarity. Pflugers Arch. 2016, 468, 1197–1206. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, K.; Geng, J.; Chi, S.; Wang, Y.; Zhi, P.; Zhang, M.; Xiao, B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron 2016, 89, 1248–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, J.; Hwang, S.M.; Lee, S.H.; Lee, K.; Kim, Y.H.; Park, C.K. Functional Expression of Piezo1 in Dorsal Root Ganglion (DRG) Neurons. Int. J. Mol. Sci. 2020, 21, 3834. [Google Scholar] [CrossRef]
- Mikhailov, N.; Leskinen, J.; Fagerlund, I.; Poguzhelskaya, E.; Giniatullina, R.; Gafurov, O.; Malm, T.; Karjalainen, T.; Grohn, O.; Giniatullin, R. Mechanosensitive meningeal nociception via Piezo channels: Implications for pulsatile pain in migraine? Neuropharmacology 2019, 149, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, K.; Woo, S.H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Begay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.H.; Ranade, S.; Weyer, A.D.; Dubin, A.E.; Baba, Y.; Qiu, Z.; Petrus, M.; Miyamoto, T.; Reddy, K.; Lumpkin, E.A.; et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014, 509, 622–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef] [PubMed]
- LaPaglia, D.M.; Sapio, M.R.; Burbelo, P.D.; Thierry-Mieg, J.; Thierry-Mieg, D.; Raithel, S.J.; Ramsden, C.E.; Iadarola, M.J.; Mannes, A.J. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 2018, 38, 912–932. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef] [Green Version]
- Copp, S.W.; Kim, J.S.; Ruiz-Velasco, V.; Kaufman, M.P. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J. Physiol. 2016, 594, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Drew, L.J.; Wood, J.N. FM1-43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol. Pain 2007, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, H.; Li, X.; Xiao, B. The mechanosensitive Piezo1 channel: A three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019, 286, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Liu, W.; Zhou, H.; Zhang, T.; Wang, L.; Zhang, M.; Li, Y.; Shen, B.; Li, X.; Xiao, B. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron 2020, 106, 438–451. [Google Scholar] [CrossRef]
- Zheng, W.; Gracheva, E.O.; Bagriantsev, S.N. A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels. Elife 2019, 8, e44003. [Google Scholar] [CrossRef]
- Burke, S.D.; Jordan, J.; Harrison, D.G.; Karumanchi, S.A. Solving Baroreceptor Mystery: Role of PIEZO Ion Channels. J. Am. Soc. Nephrol. 2019, 30, 911–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Zhao, Q.; Zhang, T.; Xiao, B. In Touch With the Mechanosensitive Piezo Channels: Structure, Ion Permeation, and Mechanotransduction. Curr. Top. Membr. 2017, 79, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, Q.; Xiao, S.; Sun, Z.; Ding, S.; Chen, Y.; Wang, L.; Yin, X.; Liao, F.; Jiang, L.H.; et al. Inhibition of Shear-Induced Platelet Aggregation by Xueshuantong via Targeting Piezo1 Channel-Mediated Ca(2+) Signaling Pathway. Front. Pharmacol. 2021, 12, 606245. [Google Scholar] [CrossRef] [PubMed]
- Suchyna, T.M. Piezo channels and GsMTx4: Two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Biol. 2017, 130, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, N.; Linley, J.E.; Torres, J.M.; Bee, L.; Dickenson, A.H.; Gringhuis, M.; Minett, M.S.; Hong, G.S.; Lee, E.; Oh, U.; et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 2013, 4, 1682. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, P.; Wang, Z.; Zhang, J.; Ding, C.; Shang, P. Biomechanical and biophysical environment of bone from the macroscopic to the pericellular and molecular level. J. Mech. Behav. Biomed. Mater. 2015, 50, 104–122. [Google Scholar] [CrossRef]
- Yu, K.; Sellman, D.P.; Bahraini, A.; Hagan, M.L.; Elsherbini, A.; Vanpelt, K.T.; Marshall, P.L.; Hamrick, M.W.; McNeil, A.; McNeil, P.L.; et al. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J. Orthop. Res. 2018, 36, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Van Oers, R.F.; Wang, H.; Bacabac, R.G. Osteocyte shape and mechanical loading. Curr. Osteoporos. Rep. 2015, 13, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, L.I.; Bruzzaniti, A. Molecular signaling in bone cells: Regulation of cell differentiation and survival. Adv. Protein Chem. Struct. Biol. 2019, 116, 237–281. [Google Scholar] [CrossRef]
- Heino, T.J.; Hentunen, T.A. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.V.; Rackard, S.M.; Kennedy, O.D. In Vivo Osteocyte Mechanotransduction: Recent Developments and Future Directions. Curr. Osteoporos. Rep. 2018, 16, 746–753. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014, 2014, 648459. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife 2019, 8, e47454. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, F.; Hayashi, M.; Mouri, Y.; Nakamura, S.; Adachi, T.; Nakashima, T. Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem. Biophys. Res. Commun. 2020, 521, 806–813. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Z.; Li, X.D.; Wang, T.B.; Zhang, H.N. Mechanism of the Piezo1 protein-induced apoptosis of the chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi Xue Za Zhi 2016, 96, 2472–2477. [Google Scholar] [CrossRef]
- Moriishi, T.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Maeno, T.; Kawai, Y.; Komori, H.; Komori, T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS ONE 2012, 7, e40143. [Google Scholar] [CrossRef]
- Li, X.; Kordsmeier, J.; Xiong, J. New Advances in Osteocyte Mechanotransduction. Curr. Osteoporos. Rep. 2021, 19, 101–106. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Jia, X.Y.; Fang, J.Y.; Chai, H.; Huang, Q.; She, C.; Jia, P.; Geng, C.; Xu, W. Reduction of SOST gene promotes bone formation through the Wnt/beta-catenin signalling pathway and compensates particle-induced osteolysis. J. Cell Mol. Med. 2020, 24, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Oreffo, R.O.; Cooper, C.; Mason, C.; Clements, M. Mesenchymal stem cells: Lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005, 1, 169–178. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [Green Version]
- Kokabu, S.; Lowery, J.W.; Jimi, E. Cell Fate and Differentiation of Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3753581. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Jafari, A.; Zaher, W.; Qiu, W.; Kassem, M. Skeletal (stromal) stem cells: An update on intracellular signaling pathways controlling osteoblast differentiation. Bone 2015, 70, 28–36. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Y.; Hu, L.; Tian, Y.; Chen, Z.; Li, D.; Zhao, F.; Su, P.; Ma, X.; Zhang, G.; et al. Mechanical unloading reduces microtubule actin crosslinking factor 1 expression to inhibit beta-catenin signaling and osteoblast proliferation. J. Cell Physiol. 2018, 233, 5405–5419. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Jiang, J.; Ma, C.; Li, R.; Xia, Y. Effect of knocking down Piezo1 mechanically sensitive protein on migration of MC3T3-E1 osteoblast cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, L.; Lv, L.; Hu, S.; Tariq, A.; Wang, W.; Dang, X. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol. Int. 2020, 44, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- De Vries, T.J.; Schoenmaker, T.; Hooibrink, B.; Leenen, P.J.; Everts, V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J. Leukoc. Biol. 2009, 85, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Adapala, N.S.; Barbe, M.F.; Carpino, N.C.; Tsygankov, A.Y.; Sanjay, A. TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function. Cell Mol. Life Sci. 2013, 70, 1269–1284. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef]
- Kurata, K.; Uemura, T.; Nemoto, A.; Tateishi, T.; Murakami, T.; Higaki, H.; Miura, H.; Iwamoto, Y. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J. Bone Miner. Res. 2001, 16, 722–730. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. 1996, 81, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, J.H.; Yuan, S.; Shao, J.H.; Cai, Z.Y.; Chen, S.; Cao, J.; Wu, H.S.; Qian, Q.R. A New Insight of Kartogenin Induced the Mesenchymal Stem Cells (MSCs) Selectively Differentiate into Chondrocytes by Activating the Bone Morphogenetic Protein 7 (BMP-7)/Smad5 Pathway. Med. Sci. Monit. 2019, 25, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servin-Vences, M.R.; Moroni, M.; Lewin, G.R.; Poole, K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017, 6, e21074. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.M.; Jones, R.C.; Jackson, T.R.; Baylie, R.L.; Abbott, B.; Bruhn-Olszewska, B.; Board, T.N.; Locke, I.C.; Richardson, S.M.; Townsend, P.A. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci. Rep. 2017, 7, 5147. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp. Biol. Med. 2020, 245, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, G.; Fischer, V.; Liedert, A.; von Kroge, S.; Haffner-Luntzer, M.; Brylka, L.; Pawlus, E.; Schweizer, M.; Yorgan, T.; Baranowsky, A.; et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J. Bone Miner. Res. 2021, 36, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.; Abt, G.; Dobson, C.; Smith, T.; Evans, W.; Ditroilo, M. The mechanical loading and muscle activation of four common exercises used in osteoporosis prevention for early postmenopausal women. J. Electromyogr. Kinesiol. 2019, 44, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Li, J.; Yang, S.; Xu, J.; Yokota, H.; Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019, 33, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Braith, R.W.; Conner, J.A.; Fulton, M.N.; Lisor, C.F.; Casey, D.P.; Howe, K.S.; Baz, M.A. Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: A pilot study. J. Heart Lung Transplant. 2007, 26, 132–137. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2021, 1–13. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, S.; Mahlios, J.; Zhou, G.; Magenheimer, B.S.; Guo, D.; Dallas, S.L.; Maser, R.; Calvet, J.P.; Bonewald, L.; et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J. Biol. Chem. 2006, 281, 30884–30895. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Dallas, M.; Qiu, N.; Nicolella, D.; Cao, L.; Johnson, M.; Bonewald, L.; Quarles, L.D. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011, 25, 2418–2432. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730. [Google Scholar] [CrossRef]
- Dalagiorgou, G.; Piperi, C.; Georgopoulou, U.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol. Life Sci. 2013, 70, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Qiu, N.; Xiao, Z.; Cao, L.; David, V.; Quarles, L.D. Conditional mesenchymal disruption of pkd1 results in osteopenia and polycystic kidney disease. PLoS ONE 2012, 7, e46038. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, S.; Cao, L.; Qiu, N.; David, V.; Quarles, L.D. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J. Biol. Chem. 2010, 285, 1177–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Cao, L.; Liang, Y.; Huang, J.; Stern, A.R.; Dallas, M.; Johnson, M.; Quarles, L.D. Osteoblast-specific deletion of Pkd2 leads to low-turnover osteopenia and reduced bone marrow adiposity. PLoS ONE 2014, 9, e114198. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Quarles, L.D. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev. Endocr. Metab. Disord. 2015, 16, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesner, L.D.; Ray, B.; Hsu, Y.H.; Manichaikul, A.; Lum, E.; Bryda, E.C.; Rich, S.S.; Rosen, C.J.; Criqui, M.H.; Allison, M.; et al. Bicc1 is a genetic determinant of osteoblastogenesis and bone mineral density. J. Clin. Invest. 2014, 124, 2736–2749. [Google Scholar] [CrossRef] [Green Version]
- Leuenroth, S.J.; Okuhara, D.; Shotwell, J.D.; Markowitz, G.S.; Yu, Z.; Somlo, S.; Crews, C.M. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2007, 104, 4389–4394. [Google Scholar] [CrossRef] [Green Version]
- He, B.H.; Christin, M.; Mouchbahani-Constance, S.; Davidova, A.; Sharif-Naeini, R. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthr. Cartil. 2017, 25, 2091–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2001611118. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xing, R.; Huang, Z.; Yin, S.; Li, X.; Zhang, L.; Ding, L.; Wang, P. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019, 228, 158–166. [Google Scholar] [CrossRef] [PubMed]
| Items | Piezo1 | Piezo2 | Reference |
|---|---|---|---|
| Gene | Fam38A | Fam38B | [17] |
| Chromosomal localization | Human chromosome 16 | Human chromosome 18 | NCBI database (https://www.ncbi.nlm.nih.gov/gene/9780 (accessed on 06 June 2021); https://www.ncbi.nlm.nih.gov/gene/63895 (accessed on 06 June 2021)) |
| Amino acid size in humans | 2521 amino acids | 2752 amino acids | [34] |
| Amino acid size in mice | 2547 amino acids | 2822 amino acids | [35,36] |
| Structure | A homotrimer structure resembling a three-bladed propeller | A homotrimer structure resembling a three-bladed propeller | [36,37] |
| Transmembrane pore characteristics | Dilated | Closed | [36] |
| Tissue distribution | Widely distributed in skin, bladder, kidney, lung, endothelial cells, erythrocytes, periodontal ligament cells, trigeminal sensory neurons, dorsal root ganglion, etc. | Trigeminal sensory neurons, dorsal root ganglion, Merkel cells, and somatic neuron cells, etc. | [19,27,28,34,38] |
| Function | Involving in mechanotransduction in a variety of cells | Sensing slight touch and proprioception | [23,25,26,27,28,29,30,31] |
| Activator | Yoda1, Jedi1/2 | Not found yet | [39,40] |
| Inhibitor | Ruthenium red, gadolinium, streptomycin, and GsMTx4 | Ruthenium red, gadolinium, streptomycin, GsMTx4, and FM1-43 | [17,19,41,42,43] |
| Cell Type | Piezo1’s Functions | Mechanism | Reference |
|---|---|---|---|
| Osteocytes | Promotes bone formation regulated by osteocytes | Activates downstream Wnt1 signaling and Akt signaling | [32,67] |
| Mesenchymal stem cells | Promotes the differentiation of MSC into osteoblasts | Upregulates BMP2 | [33] |
| Osteoblasts | Promotes bone formation by osteoblast; reduces cell proliferation but increases its migration ability; indirectly inhibits bone resorption of osteoclasts | Activates Piezo1-YAP1-collagen pathway; activates NFAT/YAP1/β-catenin transcription factor complex with Piezo2; activates AKT/GSK-3β/β-catenin pathway | [7,8,66,81,82,83] |
| Osteoclasts | No | No | [8] |
| Chondrocytes | Promotes chondrocyte apoptosis; promotes endochondral ossification in which chondrocytes are involved | Activates Ca2+ transient; activates MAPK/ERK1/2 pathway; activates caspase-12-dependent pathway; | [22,26,68,96] |
| Cell Type | Piezo1’s Functions | Mechanism | Reference |
|---|---|---|---|
| Osteoporosis | Downregulation in osteocyte and/or osteoblast; promotion of bone resorption leading to imbalance among bone formation and bone resorption under mechanical unloading | Unclear | [8,32,66] |
| Osteoarthritis | Upregulation in damaged chondrocytes; promotion of apoptosis of chondrocytes; rarefication of the F-actin cytoskeleton and amplification of mechanically induced deformation microtrauma | Activates caspase-12 signaling; promotes excessive Ca2+ influx, which in turn rarefies the F-actin cytoskeleton | [22,26,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z.; Liang, S.; Khan, Z.; Chen, Z.; Qian, A.; et al. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int. J. Mol. Sci. 2021, 22, 6429. https://doi.org/10.3390/ijms22126429
Xu X, Liu S, Liu H, Ru K, Jia Y, Wu Z, Liang S, Khan Z, Chen Z, Qian A, et al. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. International Journal of Molecular Sciences. 2021; 22(12):6429. https://doi.org/10.3390/ijms22126429
Chicago/Turabian StyleXu, Xia, Shuyu Liu, Hua Liu, Kang Ru, Yunxian Jia, Zixiang Wu, Shujing Liang, Zarnaz Khan, Zhihao Chen, Airong Qian, and et al. 2021. "Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone" International Journal of Molecular Sciences 22, no. 12: 6429. https://doi.org/10.3390/ijms22126429
APA StyleXu, X., Liu, S., Liu, H., Ru, K., Jia, Y., Wu, Z., Liang, S., Khan, Z., Chen, Z., Qian, A., & Hu, L. (2021). Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. International Journal of Molecular Sciences, 22(12), 6429. https://doi.org/10.3390/ijms22126429






