Insights into the Structure and Protein Composition of Moorella thermoacetica Spores Formed at Different Temperatures
Abstract
:1. Introduction
2. Results
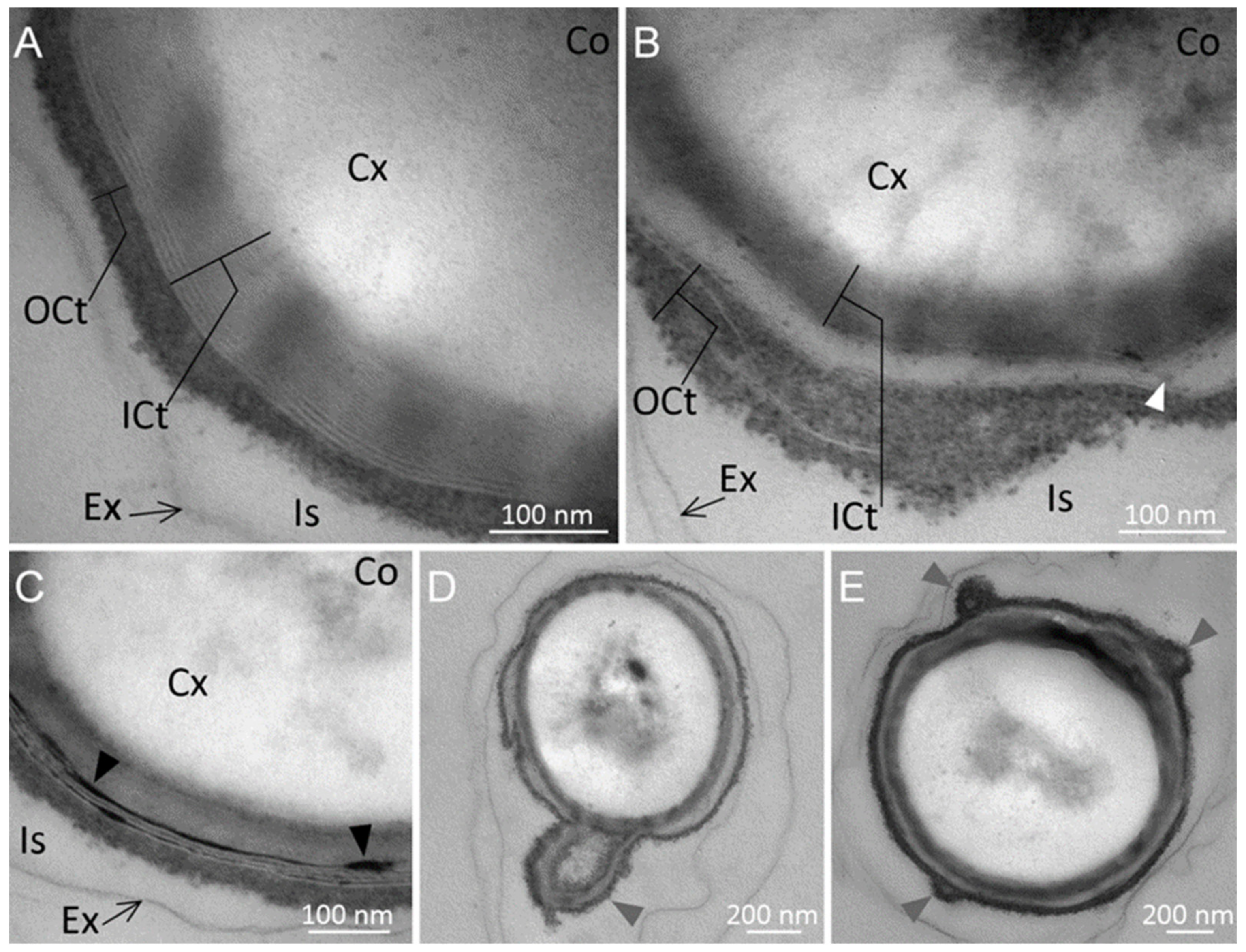
2.1. Spore Surface Structure
2.2. Spore Ultrastructure
2.3. Identification of Spore Proteins in Moorella thermoacetica ATCC 39073
2.4. Proteomic Analysis of Spores Formed at Different Temperatures
3. Discussion
3.1. Coat Layers of Moorella thermoacetica Differ According to Sporulation Temperature
3.2. Identification of Spore Protein Orthologs in Moorella thermoacetica ATCC 39073
3.3. Proteins Extracted from Moorella thermoacetica ATCC 39073 Spores Differ Based on Sporulation Temperature
4. Material and Methods
4.1. Strain and Spore Production
4.2. Spore Purification
4.3. Spore Structure Analysis
4.4. Spore Size Measurement
4.5. Spore Protein Extraction and Electrophoresis
4.6. LC-MS/MS Analysis
4.6.1. Protein In-Gel Digestion
4.6.2. Identification and Quantification of Proteins
4.7. Identification of Spore-Associated Proteins
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leggett, M.J.; McDonnell, G.; Denyer, S.; Setlow, S.; Maillard, J.-Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Popham, D.L.; Sengupta, S.; Setlow, P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl. Environ. Microbiol. 1995, 61, 3633–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Sabja, D.; Raju, D.; Torres, J.A.; Sarker, M.R. Role of small, acid-soluble spore proteins in the resistance of Clostridium perfringens spores to chemicals. Int. J. Food Microbiol. 2008, 122, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Christie, G. What’s new and notable in bacterial spore killing! World J. Microbiol. Biotechnol. 2021, 37, 144. [Google Scholar] [CrossRef] [PubMed]
- Tennen, R.; Setlow, B.; Davis, K.; Loshon, C.; Setlow, P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Appl. Microbiol. 2000, 89, 330–338. [Google Scholar] [CrossRef]
- Raju, D.; Setlow, P.; Sarker, M.R. Antisense-RNA-Mediated Decreased Synthesis of Small, Acid-Soluble Spore Proteins Leads to Decreased Resistance of Clostridium perfringens Spores to Moist Heat and UV Radiation. Appl. Environ. Microbiol. 2007, 73, 2048–2053. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007, 15, 172–180. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Bond, C.; Carman, R.J.; Setlow, P.; Sarker, M.R. Germination of spores of Clostridium difficile strains, including isolates from a hospital outbreak of Clostridium difficile-associated disease (CDAD). Microbiology 2008, 154, 2241–2250. [Google Scholar] [CrossRef] [Green Version]
- Riesenman, P.J.; Nicholson, W.L. Role of the Spore Coat Layers in Bacillus subtilis Spore Resistance to Hydrogen Peroxide, Artificial UV-C, UV-B, and Solar UV Radiation. Appl. Environ. Microbiol. 2000, 66, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Setlow, B.; Wahome, P.G.; Cowan, A.E.; Plomp, M.; Malkin, A.J.; Setlow, P. Characterization of Spores of Bacillus subtilis That Lack Most Coat Layers. J. Bacteriol. 2008, 190, 6741–6748. [Google Scholar] [CrossRef] [Green Version]
- Leggett, M.J.; Schwarz, J.S.; Burke, P.A.; McDonnell, G.; Denyer, S.P.; Maillard, J.-Y. Resistance to and killing by the sporicidal microbicide peracetic acid. J. Antimicrob. Chemother. 2014, 70, 773–779. [Google Scholar] [CrossRef]
- Leggett, M.J.; Schwarz, J.S.; Burke, P.A.; McDonnell, G.; Denyer, S.P.; Maillard, J.-Y. Mechanism of Sporicidal Activity for the Synergistic Combination of Peracetic Acid and Hydrogen Peroxide. Appl. Environ. Microbiol. 2016, 82, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Melly, E.; Genest, P.; Gilmore, M.; Little, S.; Popham, D.; Driks, A.; Setlow, P. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 2002, 92, 1105–1115. [Google Scholar] [CrossRef]
- Planchon, S.; Dargaignaratz, C.; Levy, C.; Ginies, C.; Broussolle, V.; Carlin, F. Spores of Bacillus cereus strain KBAB4 produced at 10°C and 30°C display variations in their properties. Food Microbiol. 2011, 28, 291–297. [Google Scholar] [CrossRef]
- Baweja, R.B.; Zaman, M.S.; Mattoo, A.R.; Sharma, K.; Tripathi, V.; Aggarwal, A.; Dubey, G.P.; Kurupati, R.K.; Ganguli, M.; Chaudhury, N.K.; et al. Properties of Bacillus anthracis spores prepared under various environmental conditions. Arch. Microbiol. 2007, 189, 71–79. [Google Scholar] [CrossRef]
- Garcia, D.; van der Voort, M.; Abee, T. Comparative analysis of Bacillus weihenstephanensis KBAB4 spores obtained at different temperatures. Int. J. Food Microbiol. 2010, 140, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Mtimet, N.; Trunet, C.; Mathot, A.-G.; Venaille, L.; Leguérinel, I.; Coroller, L.; Couvert, O. Modeling the behavior of Geobacillus stearothermophilus ATCC 12980 throughout its life cycle as vegetative cells or spores using growth boundaries. Food Microbiol. 2015, 48, 153–162. [Google Scholar] [CrossRef]
- Byrer, D.E.; Rainey, F.A.; Wiegel, J. Novel strains of Moorella thermoacetica form unusually heat-resistant spores. Arch. Microbiol. 2000, 174, 334–339. [Google Scholar] [CrossRef]
- Baril, E.; Coroller, L.; Couvert, O.; El Jabri, M.; Leguerinel, I.; Postollec, F.; Boulais, C.; Carlin, F.; Mafart, P. Sporulation boundaries and spore formation kinetics of Bacillus spp. as a function of temperature, pH and aw. Food Microbiol. 2012, 32, 79–86. [Google Scholar] [CrossRef]
- Rose, R.; Setlow, B.; Monroe, A.; Mallozzi, M.; Driks, A.; Setlow, P. Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J. Appl. Microbiol. 2007, 103, 691–699. [Google Scholar] [CrossRef]
- Abhyankar, W.R.; Kamphorst, K.; Swarge, B.N.; Van Veen, H.; van der Wel, N.; Brul, S.; de Koster, C.; De Koning, L.J. The Influence of Sporulation Conditions on the Spore Coat Protein Composition of Bacillus subtilis Spores. Front. Microbiol. 2016, 7, 1636. [Google Scholar] [CrossRef] [Green Version]
- Bressuire-Isoard, C.; Broussolle, V.; Carlin, F. Sporulation environment influences spore properties in Bacillus: Evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 2018, 42, 614–626. [Google Scholar] [CrossRef] [Green Version]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Córdoba, J.J.; Fernandez-Garayzabal, J.F.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A.E. The Phylogeny of the Genus Clostridium: Proposal of Five New Genera and Eleven New Species Combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Malleck, T.; Daufouy, G.; André, S.; Broussolle, V.; Planchon, S. Temperature impacts the sporulation capacities and spore resistance of Moorella thermoacetica. Food Microbiol. 2018, 73, 334–341. [Google Scholar] [CrossRef]
- Pierce, E.; Xie, G.; Barabote, R.D.; Saunders, E.; Han, C.S.; Detter, J.C.; Richardson, P.; Brettin, T.S.; Das, A.; Ljungdahl, L.G.; et al. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ. Microbiol. 2008, 10, 2550–2573. [Google Scholar] [CrossRef] [Green Version]
- Antunes, L.C.; Poppleton, D.; Klingl, A.; Criscuolo, A.; Dupuy, B.; Brochier-Armanet, C.; Beloin, C.; Gribaldo, S. Phylogenomic analysis supports the ancestral presence of LPS-outer membranes in the Firmicutes. eLife 2016, 5, e14589. [Google Scholar] [CrossRef]
- Das, A.; Coulter, E.D.; Kurtz, D.M.; Ljungdahl, L.G. Five-Gene Cluster in Clostridium thermoaceticum Consisting of Two Divergent Operons Encoding Rubredoxin Oxidoreductase- Rubredoxin and Rubrerythrin–Type A Flavoprotein–High-Molecular-Weight Rubredoxin. J. Bacteriol. 2001, 183, 1560–1567. [Google Scholar] [CrossRef] [Green Version]
- Gil, F.; Lagos-Moraga, S.; Calderón-Romero, P.; Pizarro-Guajardo, M.; Paredes-Sabja, D. Updates on Clostridium difficile spore biology. Anaerobe 2017, 45, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.; Imamura, A.; Kodama, T.; Asai, K.; Ogasawara, N.; Watabe, K. The yabG gene of Bacillus subtilis encodes a sporulation specific protease which is involved in the processing of several spore coat proteins. FEMS Microbiol. Lett. 2000, 192, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.; Schaffer, M.; Sousa, J.; Morawska, L.; Holsappel, S.; Hildebrandt, P.; Sappa, P.K.; Rath, H.; de Jong, A.; Lalk, M.; et al. Analyses of competent and non-competent subpopulations of Bacillus subtilis reveal yhfW, yhxC and ncRNAs as novel players in competence. Environ. Microbiol. 2020, 22, 2312–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhyankar, W.; Ter Beek, A.; Dekker, H.; Kort, R.; Brul, S.; de Koster, C.G. Gel-free proteomic identification of the Bacillus subtilis insoluble spore coat protein fraction. Proteomics 2011, 11, 4541–4550. [Google Scholar] [CrossRef]
- Abhyankar, W.; Hossain, A.H.; Djajasaputra, A.; Permpoonpattana, P.; Ter Beek, A.; Dekker, H.L.; Cutting, S.M.; Brul, S.; de Koning, L.J.; de Koster, C.G. In Pursuit of Protein Targets: Proteomic Characterization of Bacterial Spore Outer Layers. J. Proteome Res. 2013, 12, 4507–4521. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Shen, A.; Sorg, J. Clostridium difficile spore biology: Sporulation, germination, and spore structural proteins. Trends Microbiol. 2014, 22, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Díaz-González, F.; Milano, M.; Olguin-Araneda, V.; Pizarro-Cerda, J.; Castro-Córdova, P.; Tzeng, S.-C.; Maier, C.S.; Sarker, M.R.; Paredes-Sabja, D. Protein composition of the outermost exosporium-like layer of Clostridium difficile 630 spores. J. Proteom. 2015, 123, 1–13. [Google Scholar] [CrossRef]
- Stewart, G.C. The Exosporium Layer of Bacterial Spores: A Connection to the Environment and the Infected Host. Microbiol. Mol. Biol. Rev. 2015, 79, 437–457. [Google Scholar] [CrossRef] [Green Version]
- Popham, D.L.; Illades-Aguiar, B.; Setlow, P. The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5 *, is part of a three-gene operon required for proper spore cortex synthesis and spore core dehydration. J. Bacteriol. 1995, 177, 4721–4729. [Google Scholar] [CrossRef] [Green Version]
- Driks, A.; Eichenberger, P. The Spore Coat. Microbiol. Spectr. 2016, 4, 179–200. [Google Scholar] [CrossRef]
- Henriques, A.O.; Moran, J.C.P. Structure, Assembly, and Function of the Spore Surface Layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef]
- Fazzini, M.M.; Schuch, R.; Fischetti, V.A. A Novel Spore Protein, ExsM, Regulates Formation of the Exosporium in Bacillus cereus and Bacillus anthracis and Affects Spore Size and Shape. J. Bacteriol. 2010, 192, 4012–4021. [Google Scholar] [CrossRef] [Green Version]
- Janganan, T.K.; Mullin, N.; Tzokov, S.B.; Stringer, S.; Fagan, R.P.; Hobbs, J.K.; Moir, A.; Bullough, P.A. Characterization of the spore surface and exosporium proteins of Clostridium sporogenes; implications for Clostridium botulinum group I strains. Food Microbiol. 2016, 59, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Isticato, R.; Lanzilli, M.; Petrillo, C.; Donadio, G.; Baccigalupi, L.; Ricca, E. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ. Microbiol. 2020, 22, 170–182. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.; Carlin, F.; Broussolle, V. Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.J.; Todd, S.J.; Ball, D.A.; Shepherd, A.M.; Sylvestre, P.; Moir, A. ExsY and CotY Are Required for the Correct Assembly of the Exosporium and Spore Coat of Bacillus cereus. J. Bacteriol. 2006, 188, 7905–7913. [Google Scholar] [CrossRef] [Green Version]
- De Hoon, M.J.; Eichenberger, P.; Vitkup, D. Hierarchical Evolution of the Bacterial Sporulation Network. Curr. Biol. 2010, 20, R735–R745. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y.; Mekhedov, S.L.; Puigbo, P.; Smirnov, S.; Wolf, Y.I.; Rigden, D.J. Genomic determinants of sporulation in Bacilli and Clostridia: Towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012, 14, 2870–2890. [Google Scholar] [CrossRef] [Green Version]
- Abecasis, A.B.; Serrano, M.; Alves, R.; Quintais, L.; Pereira-Leal, J.B.; Henriques, A. A Genomic Signature and the Identification of New Sporulation Genes. J. Bacteriol. 2013, 195, 2101–2115. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Silva, P.; Serrano, M.; O Henriques, A. From Root to Tips: Sporulation Evolution and Specialization in Bacillus subtilis and the Intestinal Pathogen Clostridioides difficile. Mol. Biol. Evol. 2019, 36, 2714–2736. [Google Scholar] [CrossRef] [Green Version]
- Putnam, E.; Nock, A.M.; Lawley, T.D.; Shen, A. SpoIVA and SipL Are Clostridium difficile Spore Morphogenetic Proteins. J. Bacteriol. 2013, 195, 1214–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touchette, M.H.; de la Puebla, H.B.; Ravichandran, P.; Shen, A. SpoIVA-SipL Complex Formation Is Essential for Clostridioides difficile Spore Assembly. J. Bacteriol. 2019, 201, e00042-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Puebla, H.B.; Giacalone, D.; Cooper, A.; Shen, A. Role of SpoIVA ATPase Motifs during Clostridioides difficile Sporulation. J. Bacteriol. 2020, 202, e00387-20. [Google Scholar] [CrossRef]
- Permpoonpattana, P.; Phetcharaburanin, J.; Mikelsone, A.; Dembek, M.; Tan, S.; Brisson, M.-C.; La Ragione, R.; Brisson, A.R.; Fairweather, N.; Hong, H.A.; et al. Functional Characterization of Clostridium difficile Spore Coat Proteins. J. Bacteriol. 2013, 195, 1492–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, A.; Beall, B.W.; Roland, K.; Moran, C.P. Characterization of cotJ, a sigma E-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J. Bacteriol. 1995, 177, 3394–3406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saggese, A.; Isticato, R.; Cangiano, G.; Ricca, E.; Baccigalupi, L. CotG-Like Modular Proteins Are Common among Spore-Forming Bacilli. J. Bacteriol. 2016, 198, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayer, C.V.; Popham, D.L. YpeB dimerization may be required to stabilize SleB for effective germination of Bacillus anthracis spores. BMC Microbiol. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Thomas, S.; Li, Y.-Q.; Setlow, P. Effects of Cortex Peptidoglycan Structure and Cortex Hydrolysis on the Kinetics of Ca2+-Dipicolinic Acid Release during Bacillus subtilis Spore Germination. J. Bacteriol. 2012, 194, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Paredes-Sabja, D.; Sarker, N.; Setlow, B.; Setlow, P.; Sarker, M.R. Roles of DacB and Spm Proteins in Clostridium perfringens Spore Resistance to Moist Heat, Chemicals, and UV Radiation. Appl. Environ. Microbiol. 2008, 74, 3730–3738. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, Y. Germination Behavior of Moorella Thermoacetica Spores; Toyo Food Research Institute: Tokyo, Japan, 2014; pp. 49–54. [Google Scholar]
- Weaver, J.; Kang, T.J.; Raines, K.W.; Cao, G.-L.; Hibbs, S.; Tsai, P.; Baillie, L.; Rosen, G.M.; Cross, A.S. Protective Role of Bacillus anthracis Exosporium in Macrophage-Mediated Killing by Nitric Oxide. Infect. Immun. 2007, 75, 3894–3901. [Google Scholar] [CrossRef] [Green Version]
- Jamroskovic, J.; Chromikova, Z.; List, C.; Bartova, B.; Barak, I.; Bernier-Latmani, R. Variability in DPA and Calcium Content in the Spores of Clostridium Species. Front. Microbiol. 2016, 7, 1791. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef] [Green Version]
- Craig, R.; Beavis, R.C. TANDEM: Matching proteins with tandem mass spectra. Bioinformatics 2004, 20, 1466–1467. [Google Scholar] [CrossRef]
- Langella, O.; Valot, B.; Balliau, T.; Blein-Nicolas, M.; Bonhomme, L.; Zivy, M. X!TandemPipeline: A Tool to Manage Sequence Redundancy for Protein Inference and Phosphosite Identification. J. Proteome Res. 2017, 16, 494–503. [Google Scholar] [CrossRef]
- Carvalho, P.; Hewel, J.; Barbosa, V.; Iii, J.Y. Identifying differences in protein expression levels by spectral counting and feature selection. Genet. Mol. Res. 2008, 7, 342–356. [Google Scholar] [CrossRef]
- Lundgren, D.H.; Hwang, S.; Wu, L.; Han, D.K. Role of spectral counting in quantitative proteomics. Expert Rev. Proteom. 2010, 7, 39–53. [Google Scholar] [CrossRef]
- Usaite, R.; Wohlschlegel, J.; Venable, J.D.; Park, S.K.; Nielsen, J.; Olsson, L.; Iii, J.R.Y. Characterization of Global Yeast Quantitative Proteome Data Generated from the Wild-Type and Glucose RepressionSaccharomyces cerevisiaeStrains: The Comparison of Two Quantitative Methods. J. Proteome Res. 2008, 7, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Xiao, Y.; van Hijum, S.; Abee, T.; Wells-Bennik, M.H.J. Genome-Wide Transcriptional Profiling of Clostridium perfringens SM101 during Sporulation Extends the Core of Putative Sporulation Genes and Genes Determining Spore Properties and Germination Characteristics. PLoS ONE 2015, 10, e0127036. [Google Scholar] [CrossRef] [Green Version]


| Moorella thermoacetica ATCC 39073 LocusS a | Protein ID b | M. thermoacetica Protein Identification b | Putative Protein Name c | Hypothetical Function d | Putative Localization d | ProteinsS of Bs/Bc/Ba/Cd/Cp c,d |
|---|---|---|---|---|---|---|
| moth_1518 | Q2RIB2 | Ferredoxin-NADP(+) reductase subunit alpha | - | Nd e | Exosporium | CD1536 |
| moth_1286 moth_1879 | Q2RIY9 Q2RHB0 | Rubrerythrin | Rbr | Likely to play a role in spore resistance | Exosporium | CD0825 CD1524 |
| moth_0602 | Q2RKV7 | Uncharacterized protein | - | nd | Exosporium | CD2434 |
| moth_1747 moth_1815 | Q2RHP1 Q2RHH4 | Uncharacterized protein/Agmatinase | Arginase | nd | Exosporium | BAS0155 BAS2260 |
| moth_2167 | Q2RGI3 | Alanine racemase | Alr | Spore protection | Exosporium | BSU17640 BAS0238 |
| moth_1405 | Q2RIM1 | Nucleoside recognition | FeoB | nd | Exosporium | CD1517 |
| moth_1683 | Q2RHV4 | Cation diffusion facilitator family transporter | - | nd | Exosporium | CD0902 |
| moth_0034 | Q2RMG5 | 2-oxoglutarate synthase | - | nd | Exosporium | CD0117 |
| moth_0033 | Q2RMG6 | Pyruvate flavodoxin/ferredoxin oxidoreductase-like protein | - | nd | Exosporium | CD0116 |
| moth_0035 | Q2RMG4 | 2-oxoacid: acceptor oxidoreductase, gamma subunit, pyruvate/2-ketoisovalerate | - | nd | Exosporium | CD0118 |
| moth_0266 | Q2RLT8 | Enolase | Eno | nd | Coat/exosporium | CD3170 |
| moth_0837 | Q2RK85 | Stage V sporulation protein D | SpoVD | mother-cell specific penicillin-binding protein (spore cortex) | Coat/exosporium | BC3915 |
| moth_2301 | Q2RG51 | Kynurenine formamidase | - | Metal-dependent hydrolase | Coat/exosporium | BC0395 |
| moth_0739 | Q2RKI2 | Polysaccharide deacetylase | YlxY or Pda or PdaB | PdaA/PdaB:spore cortex peptidoglycan synthesis | Coat/exosporium | CD2598 |
| moth_1414 | Y1414_MOOTA | UPF0597 protein moth_1414 | - | nd | Coat/exosporium | CD630 |
| moth_0738 | Q2RKI3 | Peptidase M1, membrane alanine aminopeptidase | - | nd | Coat/exosporium | CD3652 |
| moth_1693 | Q2RHU4 | Protein translocase subunit YajC | YajC | nd | Coat/exosporium | BC4410 |
| moth_1319 | Q2RIV7 | Stage IV sporulation protein A | SpoIVA | Anchors the spore coat to the spore surface via SpoVM | Spore coat basement | BSU22800 BC1509 CD2629 |
| moth_1782 f | Q2RHK6 | CoatF | - | nd | Coat | - |
| moth_1059 | Q2RJL6 | Peptidase M16-like protein | YmxG | nd | Coat | BC3786 |
| moth_1391 | Q2RIN5 | Spore coat peptide assembly protein CotJB | CotJB | nd | Inner coat | BSU06900 BC0822 CD630 |
| moth_1392 | Q2RIN4 | Manganese containing catalase | CotJC | May protect against oxydative stress | Inner coat | BSU06910 BC0821 CD0598 CD2401 CPR0934 |
| moth_0257 | Q2RLU7 | HAD-superfamily hydrolase subfamily IIB | YhaX | Protection of the spore | Spore coat basement | BSU09830 |
| moth_1069 | Q2RJK6 | Ribonuclease J | RnjA | RNA processing | Coat | BC3977 |
| moth_2016 | Q2RGX7 | CoatF-like protein | YhcQ | nd | Coat | BSU30910 |
| moth_1365 | Q2RIR1 | Coat protein SA | CotSA | Spore resistance | Coat | BSU30910 |
| moth_1126 | Q2RJE9 | Amino acid ABC transporter substrate-binding protein, PAAT family | TcyA (YckK) | Cystine uptake | Coat | BSU03610 |
| moth_1016 | Q2RJQ9 | Spore coat protein manganese catalase | CotG | nd | Coat | CD1567 |
| moth_1693 | Q2RHU4 | Protein translocase subunit YajC | YajC/YrbF | nd | Inner membrane/coat | BSU27700 |
| moth_1916 | Q2RH73 | Superoxide dismutase | SodF/SodA | Detoxication of oxygen radicals | Coat | BSU19330 BC1468 CD1631 |
| moth_0056 | Q2RME4 | Sporulation-specific protease | YabG | Modification of spore coat proteins | Coat | BSU00430 BC0047 CD3569 CPR2191 |
| moth_0373 | Q2RLI2 | Putative transcriptional regulator | YkvN | MarR/DUF24 family transcription regulator | Coat | BSU13760 |
| moth_0426 | Q2RLD2 | Short-chain dehydrogenase/reductase | YhxC | Similar to alcohol dehydrogenase | Coat | BSU10400 |
| moth_0517 | Q2RL42 | N-acetylmuramoyl-L-alanine amidase | - | Involved in germination | Coat | BC2207 |
| moth_0527 | Q2RL32 | Trigger Factor | - | nd | Coat | BC4480 |
| moth_0063 | Q2RMD7 | Glycoside hydrolase, family 18 | YaaH ot YdhD | YaaH: spore germination cortex lytic enzyme YdhD: spore coat peptidoglycan hydrolase | Inner coat (YaaH) Coat (YdhD) | BSU01160/BSU05710 BC3607 |
| moth_0088 | Q2RMB2 | Uncharacterized protein | YabP | Required for sporulation at a late stage | Outer membrane | BSU00600 BC0063 CPR2486 |
| moth_1357 g moth_1058 |
Q2RIR9 Q2RJL7 | Serine-type D-Ala-D-Ala carboxypeptidase | DacB | Sporulation-specific carboxypeptidase involved in spore cortex peptidoglycane cross-linking | Cortex | BSU23190 CPR1770 |
| moth_0201 | Q2RM03 | Propeptide, PepSY amd peptidase M4 | YpeB | Germination protein, essential for SleB assembly in spores | Inner membrane | BSU22920 BC2752 |
| moth_1499 | Q2RID1 | Serine-type D-Ala-D-Ala carboxypeptidase | DacF | Penicillin-binding protein I | Inner membrane | BSU23480 BC4075 CD1291 CPR1775 |
| moth_0887 | Q2RK35 | ATPase, E1-E2 type | AtcL | Similar to the E. coli magnesium transporter | Inner membrane | BSU15650 |
| moth_0734 moth_0054 moth_0202 | Q2RKI7 Q2RME6 Q2RM02 | Cell wall hydrolase | SleB | Spore cortex-lytic enzyme involved in germination | Outer surface of the inner spore membrane | BSU13930 BC2753 |
| moth_1358 | Q2RIR8 | Uncharacterized protein | GerW/YtfJ | Germination protein | Inner membrane | BSU29500 BC4640/BC2095 |
| moth_0926 | Q2RJZ6 | Germination protease | Gpr | Degradation of SASPs | Inner membrane/core | BSU25540 BC4319 CPR2013 |
| moth_2417 moth_0736 | Q2RFU0 Q2RKI5 | Peptidase S1 and S6, chymotrypsin/Hap | YyxA | Similar to quality control membrane serine protease HtrA | Inner membrane | BSU40360 |
| moth_0925 | Q2RJZ7 | Small acid-soluble spore protein, alpha/beta type | SspA | Protection of spore DNA | Core | BSU29750 CD2688 |
| moth_0806 | Q2RKB5 | Small, acid-soluble spore protein, alpha/beta family | SspF | Protection of the spore DNA | Core | BSU24210 |
| moth_1875 | Q2RHB4 | Small, acid-soluble spore protein | SASP | Small, acid-soluble spore protein | Core | CPR1870 |
| moth_2056 | Q2RGT7 | NADH:flavin oxidoreductase/NADH oxidase | YqiG | nd | nd | BSU24210 |
| moth_1272 | Q2RJ03 | N-acetylmuramoyl-L-alanine amidase | CwlC | Sporulation-specific N-acetylmuramoyl_L-alanine amidase | nd | BSU17410 |
| moth_1828 | Q2RHG1 | Uncharacterized protein | YckD | nd | nd | BSU03400 |
| moth_1059 | Q2RJL6 | Peptidase M16-like protein | YmxG | Control of proteolytic activity | nd | BSU16710 |
| moth_1356 | Q2RIS0 | Nucleoside recognition | SpmA | Spore maturation protein, spore core dehydration, involved in germination | nd | BSU23180 BC1470 |
| moth_1355 | Q2RIS1 | Nucleoside recognition | SpmB | Spore maturation protein, spore core dehydration, involved in germination | nd | BSU23170 BC1471 |
| moth_1064 | Q2RJL1 | Alanine dehydrogenase/PNT-like protein | SpoVFA | Dipicolinate synthase (subunit A) | nd | BSU16730 BC3801 |
| moth_1065 | Q2RJL0 | Flavoprotein | SpoVFB | Dipicolinate synthase (subunit B) | nd | BSU16740 BC3800 |
| Protein ID a | Moorella thermoacetica Protein Identification a | Mean Spectral Counts | p Adjust Value | |
|---|---|---|---|---|
| 55 °C b | 45 °C b | |||
| Q2RIR1 | Glycosyl transferase (CotSA) | 5.3 ± 2.9 | 0.0 ± 0.0 | 0.0002 |
| Q2RIV7 | Stage IV sporulation protein A (SpoIVA) | 41.0 ± 6.6 | 21.3 ± 11.5 | 0.0007 |
| Q2RGT7 | NADH:flavin oxidoreductase/NADH oxidase (YqiG) | 11.3 ± 7.6 c | 2.7 ± 3.1 | 0.0016 |
| Q2RIB2 | FAD/NAD(P)-binding oxidoreductase | 53.0 ± 4.6 | 32.0 ± 4.6 | 0.0027 |
| Q2RHK6 | Coat protein F d | 9.7 ± 3.2 | 2.3 ± 2.1 | 0.0045 |
| Q2RM03 | Propeptide, PepSY amd peptidase M4 (YpeB) | 3.3 ± 3.1 e | 0.0 ± 0.0 | 0.0057 |
| Q2RJL6 | Peptidase M16-like protein (YmxG) | 8.3 ± 1.2 | 2.3 ± 0.6 | 0.0190 |
| Q2RH73 | Superoxide dismutase (SodF) | 2.3 ± 2.1 e | 0.0 ± 0.0 | 0.0275 |
| Q2RIN4 | Manganese containing catalase (CotJC) | 8.3 ±± 4.7 | 1.7 ± 1.5 | 0.0043 |
| Q2RGX7 | Coat protein YhcQ | 6.0 ± 1.7 | 2.0 ± 2.0 | 0.0949 |
| Q2RIY9 | Rubrerythrin (Rbr) | 30.0 ± 4.5 | 41.0 ± 3.0 | 0.1141 |
| Q2RMG5 | 2-oxoglutarate synthase | 1.0 ± 1.0 | 0.0 ± 0.0 | 0.1841 |
| Q2RGI3 | Alanine racemase (Alr) | 8.0 ± 1.0 | 4.0 ± 1.0 | 0.1907 |
| Q2RJQ9 | Catalase (CotG) | 2.3 ± 0.6 | 0.7 ± 1.2 | 0.2825 |
| Q2RJE9 | Amino acid ABC transporter substrate-binding protein (TcyA/YckK) | 4.0 ± 1.0 | 1.7 ± 1.5 | 0.2825 |
| Q2RHU4 | Protein translocase subunit (YajC) | 0.7 ±0.6 | 2.3 ± 2.3 | 0.2825 |
| Q2RIN5 | Spore coat protein (CotJB) | 0.7 ± 1.2 | 0.0 ± 0.0 | 0.2931 |
| Q2RJZ7 | Small acid-soluble spore protein, alpha/beta type (SspA) | 5.0 ± 1.0 | 2.7 ± 1.2 | 0.3763 |
| Q2RLT8 | Enolase (Eno) | 46.0 ± 6.5 | 53.0 ± 7.2 | 0.4941 |
| Q2RHH4 | Agmatinase | 1.3 ± 1.2 | 2.7 ± 1.2 | 0.5072 |
| Q2RKV7 | Hypothetical protein | 2.7 ± 1.2 | 3.3 ± 1.5 | 0.8454 |
| Q2RLU7 | HAD-superfamily hydrolase subfamily IIB (YhaX) | 5.7 ± 1.2 | 6.3 ± 1.2 | 0.8787 |
| Q2RHB0 | Rubrerythrin (Rbr) | 20.0 ± 3.2 | 21.3 ± 2.3 | 0.9068 |
| Q2RJK6 | Ribonuclease J (RnjA) | 3.0 ± 0.0 | 3.3 ± 0.6 | 0.9207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malleck, T.; Fekraoui, F.; Bornard, I.; Henry, C.; Haudebourg, E.; Planchon, S.; Broussolle, V. Insights into the Structure and Protein Composition of Moorella thermoacetica Spores Formed at Different Temperatures. Int. J. Mol. Sci. 2022, 23, 550. https://doi.org/10.3390/ijms23010550
Malleck T, Fekraoui F, Bornard I, Henry C, Haudebourg E, Planchon S, Broussolle V. Insights into the Structure and Protein Composition of Moorella thermoacetica Spores Formed at Different Temperatures. International Journal of Molecular Sciences. 2022; 23(1):550. https://doi.org/10.3390/ijms23010550
Chicago/Turabian StyleMalleck, Tiffany, Fatima Fekraoui, Isabelle Bornard, Céline Henry, Eloi Haudebourg, Stella Planchon, and Véronique Broussolle. 2022. "Insights into the Structure and Protein Composition of Moorella thermoacetica Spores Formed at Different Temperatures" International Journal of Molecular Sciences 23, no. 1: 550. https://doi.org/10.3390/ijms23010550






