Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover
Abstract
:1. Introduction
2. The Physiological Aspects of Lactation
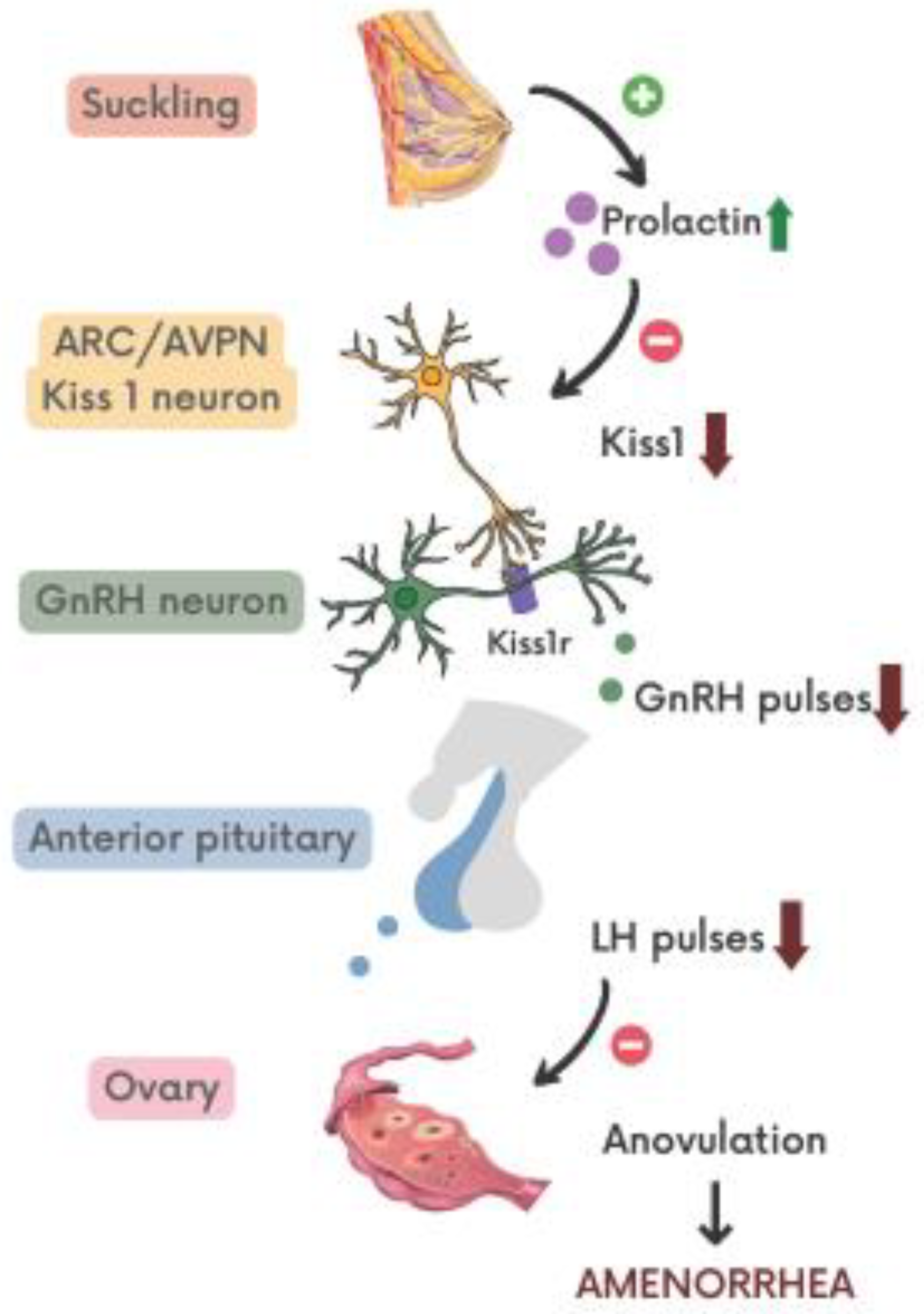
3. Endocrine Control of Lactational Amenorrhea
4. Lactational Amenorrhea as Natural Contraceptive Method
- A period of sixth months after delivery;
- “Full” or “nearly full” breastfeeding;
- Postpartum amenorrhea [70].
5. The Lactational Bone Loss
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Infant and Young Child Feeding. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 8 August 2021).
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; Devine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Groër, M.W. Differences Between Exclusive Breastfeeders, Formula-Feeders, and Controls: A Study of Stress, Mood, and Endocrine Variables. Biol. Res. Nurs. 2005, 7, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Tamim, H. The Relationship between Postpartum Depression and Breastfeeding. Int. J. Psychiatry Med. 2012, 43, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Gila-Díaz, A.; Díaz-Rullo Alcántara, N.; Herranz Carrillo, G.; Singh, P.; Arribas, S.M.; Ramiro-Cortijo, D. Healthy Habits and Emotional Balance in Women during the Postpartum Period: Differences between Term and Preterm Delivery. Children 2021, 8, 937. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J.C. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Norat, T.; Romundstad, P.R.; Vatten, L.J. Breastfeeding and the maternal risk of type 2 diabetes: A systematic review and dose–response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 107–115. [Google Scholar] [CrossRef]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martín-Cabrejas, M.A.; De Pablo, Á.L.L.; De Pipaón, M.S.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [Green Version]
- Gila-Díaz, A.; Alcántara, N.D.-R.; Carrillo, G.H.; Singh, P.; Arribas, S.; Ramiro-Cortijo, D. Multidimensional Approach to Assess Nutrition and Lifestyle in Breastfeeding Women during the First Month of Lactation. Nutrients 2021, 13, 1766. [Google Scholar] [CrossRef]
- Lord, M.; Sahni, M. Secondary Amenorrhea; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431055/ (accessed on 19 July 2021).
- Hovey, R.C.; Trott, J.F.; Vonderhaar, B.K. Establishing a framework for the functional mammary gland: From endocrinology to morphology. J. Mammary Gland Biol. Neoplasia 2002, 7, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Tucker, H.A. Hormones, Mammary Growth, and Lactation: A 41-Year Perspective. J. Dairy Sci. 2000, 83, 874–884. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Q.; Cai, J.; Xiao, L.; Zhang, J. Three lactation-related hormones: Regulation of hypothalamus-pituitary axis and function on lactation. Mol. Cell. Endocrinol. 2020, 520, 111084. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C. Physiology of Lactation. Clin. Perinatol. 1999, 26, 251–279. [Google Scholar] [CrossRef]
- Froemke, R.C.; Carcea, I. Oxytocin and Brain Plasticity. In Principles of Gender-Specific Medicine; Academic Press: Cambridge, MA, USA, 2017; pp. 161–182. [Google Scholar]
- Crowley, W.R.; Armstrong, W.E. Neurochemical Regulation of Oxytocin Secretion in Lactation. Endocr. Rev. 1992, 13, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Crowley, W.R. Neuroendocrine Regulation of Lactation and Milk Production. Compr. Physiol. 2015, 5, 255–291. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; McFadden, T.B.; Forsyth, I. Hormonal Regulation of Mammary Differentiation and Milk Secretion. J. Mammary Gland Biol. Neoplasia 2002, 7, 49–66. [Google Scholar] [CrossRef]
- Hernandez, L.L.; Stiening, C.M.; Wheelock, J.B.; Baumgard, L.H.; Parkhurst, A.M.; Collier, R.J. Evaluation of Serotonin as a Feedback Inhibitor of Lactation in the Bovine. J. Dairy Sci. 2008, 91, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Flint, D.J.; Tonner, E.; Beattie, J.; Panton, D. Investigation of the mechanism of action of growth hormone in stimulating lactation in the rat. J. Endocrinol. 1992, 134, 377–383. [Google Scholar] [CrossRef]
- Gunn, A.J.; Gunn, T.R.; Rabone, D.L.; Breier, B.H.; Blum, W.F.; Gluckman, P.D. Growth Hormone Increases Breast Milk Volumes in Mothers of Preterm Infants. Pediatrics 1996, 98, 279–282. [Google Scholar] [CrossRef]
- Owens, M.B.; Hill, A.D.; Hopkins, A.M. Ductal barriers in mammary epithelium. Tissue Barriers 2013, 1, e25933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, T.M.; Boecker, A.; Chiu, J.; Plaut, K. Glucocorticoids Maintain the Extracellular Matrix of Differentiated Mammary Tissue during Explant and Whole Organ Culture. In Proceedings of the Society for Experimental Biology and Medicine; Society for Experimental Biology and Medicine: New York, NY, USA, 2000; Volume 224, pp. 76–86. [Google Scholar] [CrossRef]
- Menzies, K.K.; Lee, H.J.; Lefèvre, C.; Ormandy, C.J.; Macmillan, K.L.; Nicholas, K.R. Insulin, a key regulator of hormone responsive milk protein synthesis during lactogenesis in murine mammary explants. Funct. Integr. Genom. 2009, 10, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.S.C.; Aquino, N.S.S.; Gusmao, D.O.; Henriques, P.C.; Reis, A.M.; Szawka, R.E. Reduced dopaminergic tone during lactation is permissive to the hypothalamic stimulus for suckling-induced prolactin release. J. Neuroendocr. 2020, 32, e12880. [Google Scholar] [CrossRef] [PubMed]
- Giglia, R.; Binns, C. Alcohol and lactation: A systematic review. Nutr. Diet. 2006, 63, 103–116. [Google Scholar] [CrossRef]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.M. Association of Maternal Obesity Before Conception with Poor Lactation Performance. Annu. Rev. Nutr. 2007, 27, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant Overweight and Obesity Diminish the Prolactin Response to Suckling in the First Week Postpartum. Pediatrics 2004, 113, e465–e471. [Google Scholar] [CrossRef] [Green Version]
- Buonfiglio, D.C.; Ramos-Lobo, A.M.; Freitas, V.M.; Zampieri, T.T.; Nagaishi, V.S.; Magalhães, M.; Cipolla-Neto, J.; Cella, N.; Donato, J., Jr. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 2016, 6, 22421. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Shushanov, S.; LeRoith, D.; Wood, T.L. Decreased IGF Type 1 Receptor Signaling in Mammary Epithelium during Pregnancy Leads to Reduced Proliferation, Alveolar Differentiation, and Expression of Insulin Receptor Substrate (IRS)-1 and IRS-2. Endocrinology 2011, 152, 3233–3245. [Google Scholar] [CrossRef] [Green Version]
- Cobo, E. Effect of different doses of ethanol on the milk-ejecting reflex in lactating women. Am. J. Obstet. Gynecol. 1973, 115, 817–821. [Google Scholar] [CrossRef]
- Newton, M.; Newton, N.R. The let-down reflex in human lactation. J. Pediatr. 1948, 33, 698–704. [Google Scholar] [CrossRef]
- Figaroa, M.N.; Bellizzi, S.; Delvaux, T.; Benova, L. Lactational amenorrhoea among adolescent girls in low-income and middle-income countries: A systematic scoping review. BMJ Global Health 2020, 5, e002492. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, L.E.; Wlodek, M.E.; Geddes, D.T. What Evidence Do We Have for Pharmaceutical Galactagogues in the Treatment of Lactation Insufficiency?—A Narrative Review. Nutrients 2019, 11, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, G.M.; Stevenson, R.; Zizzo, G.; Rumbold, A.R.; Amir, L.H.; Keir, A.K.; Grzeskowiak, L.E. Use and experiences of galactagogues while breastfeeding among Australian women. PLoS ONE 2021, 16, e0254049. [Google Scholar] [CrossRef]
- Grattan, D.R. 60 Years of Neuroendocrinology: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef] [Green Version]
- De La Lastra, M.; Llados, C. Luteinizing Hormone Content of the Pituitary Gland in Pregnant and Non-Pregnant Women. J. Clin. Endocrinol. Metab. 1977, 44, 921–923. [Google Scholar] [CrossRef]
- Glasier, A.; McNEILLY, A.S.; Howie, P.W. Pulsatile secretion of LH in relation to the resumption of ovarian activity post partum. Clin. Endocrinol. 1984, 20, 415–426. [Google Scholar] [CrossRef]
- Flynn, A.M.; Docker, M.; Brown, J.B.; Kennedy, K.I. Ultrasonographic patterns of ovarian activity during breastfeeding. Am. J. Obstet. Gynecol. 1991, 165, 2027–2031. [Google Scholar] [CrossRef]
- Burger, H.G.; Hee, J.P.C.; Mamers, P.; Bangah, M.; Zissimos, M.; McCloud, P.I. Serum inhibin during lactation: Relation to the gonadotrophins and gonadal steroids. Clin. Endocrinol. 1994, 41, 771–777. [Google Scholar] [CrossRef]
- McNeilly, A.S.; Tay, C.C.K.; Glasier, A. Physiological Mechanisms Underlying Lactational Amenorrhea. Ann. N. Y. Acad. Sci. 1994, 709, 145–155. [Google Scholar] [CrossRef]
- Tay, C.C.; Glasier, A.F.; McNeilly, A.S. The 24 h pattern of pulsatile luteinizing hormone, follicle stimulating hormone and prolactin release during the first 8 weeks of lactational amenorrhoea in breastfeeding women. Hum. Reprod. 1992, 7, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Schiøler, V. Influence of breast-feeding pattern on pituitary-ovarian axis of women in an industrialized community. Am. J. Obstet. Gynecol. 1982, 143, 673–677. [Google Scholar] [CrossRef]
- Christian-Hinman, C.A.; Moenter, S.M. The Neurobiology of Preovulatory and Estradiol-Induced Gonadotropin-Releasing Hormone Surges. Endocr. Rev. 2010, 31, 544–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsumi, R.; Webster, N.J. GnRH Pulsatility, the Pituitary Response and Reproductive Dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 Gene as a Regulator of Puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.C.; Griffin, M.L. The role of changing pulse frequency in the regulation of ovulation. Hum. Reprod. 1993, 8 (Suppl. 2), 57–61. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Clifton, D.K.; Steiner, R.A.; Herbison, A.E. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J. Neurosci. 2005, 25, 11349–11356. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- De Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.T.; Popa, S.M.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A. Kiss1 Neurons in the Forebrain as Central Processors for Generating the Preovulatory Luteinizing Hormone Surge. J. Neurosci. 2006, 26, 6687–6694. [Google Scholar] [CrossRef] [Green Version]
- Spergel, D.J. Neuropeptidergic modulation of GnRH neuronal activity and GnRH secretion controlling reproduction: Insights from recent mouse studies. Cell Tissue Res. 2019, 375, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; True, C.; Grove, K.L. The neuroendocrine basis of lactation-induced suppression of GnRH: Role of kisspeptin and leptin. Brain Res. 2010, 1364, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cells of the Arcuate Nucleus: A Central Node in the Control of Gonadotropin-Releasing Hormone Secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Uenoyama, Y.; Kinoshita, M.; Iwata, K.; Takase, K.; Matsui, H.; Adachi, S.; Inoue, K.; Maeda, K.-I.; Tsukamura, H. Inhibition of Metastin (Kisspeptin-54)-GPR54 Signaling in the Arcuate Nucleus-Median Eminence Region during Lactation in Rats. Endocrinology 2007, 148, 2226–2232. [Google Scholar] [CrossRef] [Green Version]
- True, C.; Kirigiti, M.; Ciofi, P.; Grove, K.L.; Smith, M.S. Characterisation of Arcuate Nucleus Kisspeptin/Neurokinin B Neuronal Projections and Regulation during Lactation in the Rat. J. Neuroendocr. 2010, 23, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-F.; Kinsey-Jones, J.S.; Cheng, Y.; Knox, A.M.I.; Lin, Y.; Petrou, N.A.; Roseweir, A.K.; Lightman, S.L.; Milligan, S.R.; Millar, R.P.; et al. Kisspeptin Signalling in the Hypothalamic Arcuate Nucleus Regulates GnRH Pulse Generator Frequency in the Rat. PLoS ONE 2009, 4, e8334. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Becker, I.R.; Selmanoff, M.; Wise, P.M. Hyperprolactinemia Alters the Frequency and Amplitude of Pulsatile Luteinizing Hormone Secretion in the Ovariectomized Rat. Neuroendocrinology 1986, 42, 328–333. [Google Scholar] [CrossRef]
- Fox, S.R.; Hoefer, M.T.; Bartke, A.; Smith, M.S. Suppression of Pulsatile LH Secretion, Pituitary GnRH Receptor Content and Pituitary Responsiveness to GnRH by Hyperprolactinemia in the Male Rat. Neuroendocrinology 1987, 46, 350–359. [Google Scholar] [CrossRef]
- Kotani, M.; Katagiri, F.; Hirai, T.; Kagawa, J.; Tanaka, I. Plasma kisspeptin levels in lactational amenorrhea. Gynecol. Endocrinol. 2017, 33, 819–821. [Google Scholar] [CrossRef]
- Sonigo, C.; Bouilly, J.; Carré, N.; Tolle, V.; Caraty, A.; Tello, J.A.; Simony-Conesa, F.-J.; Millar, R.P.; Young, J.; Binart, N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Investig. 2012, 122, 3791–3795. [Google Scholar] [CrossRef]
- Araujo-Lopes, R.; Crampton, J.R.; Aquino, N.S.; Miranda, R.M.; Kokay, I.C.; Reis, A.M.; Franci, C.R.; Grattan, D.R.; Szawka, R.E. Prolactin Regulates Kisspeptin Neurons in the Arcuate Nucleus to Suppress LH Secretion in Female Rats. Endocrinology 2014, 155, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P.; Sonigo, C.; Anderson, R.A.; George, J.; Maione, L.; Brailly-Tabard, S.; Chanson, P.; Binart, N.; Young, J. Hypothalamic-Pituitary-Ovarian Axis Reactivation by Kisspeptin-10 in Hyperprolactinemic Women with Chronic Amenorrhea. J. Endocr. Soc. 2017, 1, 1362–1371. [Google Scholar] [CrossRef]
- Van Der Wijden, C.; Manion, C. Lactational amenorrhoea method for family planning. Cochrane Database Syst. Rev. 2015, 10, CD001329. [Google Scholar] [CrossRef] [PubMed]
- Hight-Laukaran, V.; Rutstein, S.O.; Labbok, M.H.; Ballard, E. Contraceptive use during lactational amenorrhea. Int. J. Gynecol. Obstet. 1996, 54, 101–108. [Google Scholar] [CrossRef]
- Breastfeeding as a family planning method. Lancet 1988, 2, 1204–1205.
- Perez, A.; Vela, P.; Masnick, G.S.; Potter, R.G. First ovulation after childbirth: The effect of breast-feeding. Am. J. Obstet. Gynecol. 1972, 114, 1041–1047. [Google Scholar] [CrossRef]
- Campbell, O.M.; Gray, R.H. Characteristics and determinants of postpartum ovarian function in women in the United States. Am. J. Obstet. Gynecol. 1993, 169, 55–60. [Google Scholar] [CrossRef]
- Gray, R.H.; Campbell, O.M.; Apelo, R.; Eslami, S.S.; Zacur, H.; Ramos, R.M.; Gehret, J.C.; Labbok, M.H. Risk of ovulation during lactation. Lancet 1990, 335, 25–29. [Google Scholar] [CrossRef]
- Romero-Gutiérrez, G.; Vaca-Ortiz, N.; Ponce-Ponce De León, A.L.; López-Martínez, M.G. Actual use of the lactational amenorrhoea method. Eur. J. Contracept. Reprod. Health Care 2007, 12, 340–344. [Google Scholar] [CrossRef]
- Hoi, A.G.; Daiy, K.; Altman, R.M.; Venners, S.; Valeggia, C.; Nepomnaschy, P. Postpartum amenorrhea duration by sex of the newborn in two natural fertility populations. Am. J. Phys. Anthropol. 2020, 174, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Labbok, M.H. Postpartum Sexuality and the Lactational Amenorrhea Method for Contraception. Clin. Obstet. Gynecol. 2015, 58, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, M. Postpartum contraception: The lactational amenorrhea method. Eur. J. Contracept. Reprod. Health Care 1997, 2, 105–111. [Google Scholar] [CrossRef]
- Howie, P.W.; McNeilly, A.S. Effect of breast-feeding patterns on human birth intervals. Reproduction 1982, 65, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.I.; Visness, C.M. Contraceptive efficacy of lactational amenorrhoea. Lancet 1992, 339, 227–230. [Google Scholar] [CrossRef]
- Cleland, J.; Shah, I.H.; Daniele, M. Interventions to Improve Postpartum Family Planning in Low- and Middle-Income Countries: Program Implications and Research Priorities. Stud. Fam. Plan. 2015, 46, 423–441. [Google Scholar] [CrossRef]
- The World Health Organization multinational study of breast-feeding and lactational amenorrhea。 I. Description of infant feeding patterns and of the return of menses. Fertil. Steril. 1998, 70, 448–460. [CrossRef]
- Eliason, S.K.; Bockarie, A.S.; Eliason, C. Postpartum fertility behaviours and contraceptive use among women in rural Ghana. Contracept. Reprod. Med. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Pieh Holder, K. Contraception and Breastfeeding. Clin. Obstet. Gynecol. 2015, 58, 928–935. [Google Scholar] [CrossRef]
- Berens, P.; Labbok, M. The Academy of Breastfeeding Medicine ABM Clinical Protocol #13: Contraception during Breastfeeding, Revised 2015. Breastfeed. Med. 2015, 10, 3–12. [Google Scholar] [CrossRef]
- Peterson, A.E.; Peŕez-Escamilla, R.; Labbok, M.H.; Hight, V.; Von Hertzen, H.; Van Look, P. Multicenter study of the lactational amenorrhea method (LAM) III: Effectiveness, duration, and satisfaction with reduced client–provider contact. Contraception 2000, 62, 221–230. [Google Scholar] [CrossRef]
- Connolly, A.; Thorp, J.; Pahel, L. Effects of pregnancy and childbirth on postpartum sexual function: A longitudinal prospective study. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2005, 16, 263–267. [Google Scholar] [CrossRef]
- Vanya, M.; Devosa, I.; Barabás, K.; Bartfai, G.; Kozinszky, Z. Choice of contraception at 6–8 weeks postpartum in south-eastern Hungary. Eur. J. Contracept. Reprod. Health Care 2018, 23, 52–57. [Google Scholar] [CrossRef]
- Valdés, V.; Labbok, M.H.; Pugin, E.; Perez, A. The efficacy of the lactational amenorrhea method (LAM) among working women. Contraception 2000, 62, 217–219. [Google Scholar] [CrossRef]
- Gross, B.A.; Burger, H.; WHO Task Force on Methods for the Natural Regulation of Fertility. Breastfeeding patterns and return to fertility in Australian women. Aust. N. Z. J. Obstet. Gynaecol. 2002, 42, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Ravera, C.; Reggiori, A.; Cocozza, E.; Cianta, F.; Riccioni, G.; Kleimayr, R. A study of breastfeeding and the return of menses in Hoima District, Uganda. East Afr. Med. J. 1995, 72, 147–149. [Google Scholar] [PubMed]
- Türk, R.; Terzioǧlu, F.; Eroǧlu, K. The Use of Lactational Amenorrhea as A Method of Family Planning in Eastern Turkey and Influential Factors. J. Midwifery Women’s Health 2010, 55, e1–e7. [Google Scholar] [CrossRef]
- Audu, B.M.; Yahya, S.J.; Bassi, A. Knowledge, attitude and practice of natural family planning methods in a population with poor utilisation of modern contraceptives. J. Obstet. Gynaecol. 2006, 26, 555–560. [Google Scholar] [CrossRef]
- Sipsma, H.L.; Bradley, E.H.; Chen, P.G. Lactational Amenorrhea Method as a Contraceptive Strategy in Niger. Matern. Child Health J. 2012, 17, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Abraha, T.H.; Teferra, A.S.; Gelagay, A.A.; Welesamuel, T.G.; Fisseha, G.K.; Aregawi, B.G.; Belay, D.S. Knowledge and associated factors of lactational amenorrhea as a contraception method among postpartum women in Aksum town, Tigray Region, Ethiopia. BMC Res. Notes 2018, 11, 641. [Google Scholar] [CrossRef]
- Cooper, C.M.; Kavle, J.A.; Nyoni, J.; Drake, M.; Lemwayi, R.; Mabuga, L.; Pfitzer, A. Perspectives on maternal, infant, and young child nutrition and family planning: Considerations for rollout of integrated services in Mara and Kagera, Tanzania. Matern. Child Nutr. 2019, 15 (Suppl. 1), e12735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Report of a WHO Technical Consultation on Birth Spacing, Geneva, Switzerland, 13–15 June 2005; World Health Organization, Department of Making Pregnancy Safer (MPS), Department of Reproductive Health and Research, (RHR): Geneva, Switzerland, 2007. [Google Scholar]
- Conde-Agudelo, A.; Belizán, J.M. Maternal morbidity and mortality associated with interpregnancy interval: Cross sectional study. BMJ 2000, 321, 1255–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prentice, A. Calcium in Pregnancy and Lactation. Annu. Rev. Nutr. 2000, 20, 249–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillman, L.; Sateesha, S.; Haussler, M.; Wiest, W.; Slatopolsky, E.; Haddad, J. Control of mineral homeostasis during lactation: Interrelationships of 25-hydroxyvitamin D, 24,25-dihydroxyvitamin D, 1,25-dihydroxyvitamin D, parathyroid hormone, calcitonin, prolactin, and estradiol. Am. J. Obstet. Gynecol. 1981, 139, 471–476. [Google Scholar] [CrossRef]
- Mehta, S. Bone loss, contraception and lactation. Acta Obstet. Gynecol. Scand. 1993, 72, 148–156. [Google Scholar] [CrossRef]
- Cross, N.A.; Hillman, L.S.; Allen, S.H.; Krause, G.F. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J. Bone Miner. Res. 1995, 10, 1312–1320. [Google Scholar] [CrossRef]
- Koppelman, M.C.S.; Kurtz, D.W.; Morrish, K.A.; Bou, E.; Susser, J.K.; Shapiro, J.R.; Loriaux, D.L. Vertebral Body Bone Mineral Content in Hyperprolactinemic Women. J. Clin. Endocrinol. Metab. 1984, 59, 1050–1053. [Google Scholar] [CrossRef]
- Winter, E.M.; Ireland, A.; Butterfield, N.C.; Haffner-Luntzer, M.; Horcajada, M.-N.; Veldhuis-Vlug, A.G.; Oei, L.; Colaianni, G.; Bonnet, N. Pregnancy and lactation, a challenge for the skeleton. Endocr. Connect. 2020, 9, R143–R157. [Google Scholar] [CrossRef]
- Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V.T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P.; et al. Cathepsin K–deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J. Clin. Investig. 2019, 129, 3058–3071. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [Green Version]
- VanHouten, J.N.; Dann, P.; Stewart, A.F.; Watson, C.J.; Pollak, M.; Karaplis, A.C.; Wysolmerski, J.J. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J. Clin. Investig. 2004, 113, 492. [Google Scholar] [CrossRef]
- Kolthoff, N.; Eiken, P.; Kristensen, B.; Nielsen, S.P. Bone Mineral Changes during Pregnancy and Lactation: A Longitudinal Cohort Study. Clin. Sci. 1998, 94, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskey, M.A.; Prentice, A.; Hanratty, L.A.; Jarjou, L.M.; Dibba, B.; Beavan, S.R.; Cole, T.J. Bone changes after 3 mo of lactation: Influence of calcium intake, breast-milk output, and vitamin D-receptor genotype. Am. J. Clin. Nutr. 1998, 67, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affinito, P.; Tommaselli, G.A.; Di Carlo, C.; Guida, F.; Nappi, C. Changes in bone mineral density and calcium metabolism in breastfeeding women: A one year follow-up study. J. Clin. Endocrinol. Metab. 1996, 81, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Hayslip, C.C.; Klein, T.A.; Wray, H.L.; Duncan, W.E. The effects of lactation on bone mineral content in healthy postpartum women. Obstet. Gynecol. 1989, 73, 588–592. [Google Scholar] [PubMed]
- Pepe, J.; Body, J.-J.; Hadji, P.; McCloskey, E.; Meier, C.; Obermayer-Pietsch, B.; Palermo, A.; Tsourdi, E.; Zillikens, M.C.; Langdahl, B.; et al. Osteoporosis in Premenopausal Women: A Clinical Narrative Review by the ECTS and the IOF. J. Clin. Endocrinol. Metab. 2020, 105, dgaa306. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, G.M.; Pike, A.M. The effect of lactation on peak adult shaft and ultra-distal forearm bone mass in women. Am. J. Clin. Nutr. 1986, 44, 283–286. [Google Scholar] [CrossRef]
- Atkinson, P.J.; West, R.R. Loss of skeletal calcium in lactating women. Obstet. Gynaecol. Br. Commonw. 1970, 77, 555–560. [Google Scholar] [CrossRef]
- Sowers, M.; Eyre, D.; Hollis, B.W.; Randolph, J.F.; Shapiro, B.; Jannausch, M.L.; Crutchfield, M. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J. Clin. Endocrinol. Metab. 1995, 80, 2210–2216. [Google Scholar] [CrossRef]
- Lamke, B.; Brundin, J.; Moberg, P. Changes of Bone Mineral Content During Pregnancy and Lactation. Acta Obstet. Gynecol. Scand. 1977, 56, 217–219. [Google Scholar] [CrossRef]
- Brembeck, P.; Lorentzon, M.; Ohlsson, C.; Winkvist, A.; Augustin, H. Changes in Cortical Volumetric Bone Mineral Density and Thickness, and Trabecular Thickness in Lactating Women Postpartum. J. Clin. Endocrinol. Metab. 2015, 100, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørnerem, A.; Ghasem-Zadeh, A.; Wang, X.; Bui, M.; Walker, S.P.; Zebaze, R.; Seeman, E. Irreversible Deterioration of Cortical and Trabecular Microstructure Associated with Breastfeeding. J. Bone Miner. Res. 2016, 32, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Yazici, S.; Korkmaz, U.; Erkan, M.; Korkmaz, N.; Erdem Baki, A.; Alçelik, A.; Önder, E.; Ataoglu, S. The effect of breast-feeding duration on bone mineral density in postmenopausal Turkish women: A population-based study. Arch. Med. Sci. 2011, 73, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.M.; Magaziner, J.; Sherwin, R.; Scott, J.C.; Plato, C.C.; Nevitt, M.; Cummings, S. Reproductive correlates of bone mass in elderly women. Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1993, 8, 901–908. [Google Scholar] [CrossRef]
- Cooke-Hubley, S.; Gao, Z.; Mugford, G.; Kaiser, S.M.; Goltzman, D.; Leslie, W.D.; Davison, K.S.; Brown, J.P.; Probyn, L.; Lentle, B.; et al. Parity and lactation are not associated with incident fragility fractures or radiographic vertebral fractures over 16 years of follow-up: Canadian Multicentre Osteoporosis Study (CaMos). Arch. Osteoporos. 2019, 14, 49. [Google Scholar] [CrossRef]
- Song, S.Y.; Kim, Y.; Park, H.; Kim, Y.J.; Kang, W.K.; Kim, E.Y. Effect of parity on bone mineral density: A systematic review and meta-analysis. Bone 2017, 101, 70–76. [Google Scholar] [CrossRef]
- Alderman, B.W.; Weiss, N.S.; Daling, J.R.; Ure, C.; Ballard, J.H. Reproductive history and postmenopausal risk of hip and forearm fracture. Am. J. Epidemiol. 1986, 124, 262–267. [Google Scholar] [CrossRef]
- Kreiger, N.; Kelsey, J.L.; Holford, T.R.; O’Connor, T. An epidemiologic study of hip fracture in postmenopausal women. Am. J. Epidemiol. 1982, 116, 141–148. [Google Scholar] [CrossRef]
- Hanzen, C. Endocrine regulation of postpartum ovarian activity in cattle: A review. Reprod. Nutr. Dev. 1986, 26, 1219–1239. [Google Scholar] [CrossRef] [Green Version]
- Koskinen, E.; Huhtinen, M.; Katila, T. Serum Progesterone Levels in Mares in Winter and during Transitional Periods. Acta Vet. Scand. 1996, 37, 409–414. [Google Scholar] [CrossRef]
- Rhodes, F.M.; McDougall, S.; Burke, C.R.; Verkerk, G.A.; Macmillan, K. Invited Review: Treatment of Cows with an Extended Postpartum Anestrous Interval. J. Dairy Sci. 2003, 86, 1876–1894. [Google Scholar] [CrossRef]
- Quesnel, H.; Prunier, A. Endocrine bases of lactational anoestrus in the sow. Reprod. Nutr. Dev. 1995, 35, 395–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzies, B.R.; Hildebrandt, T.B.; Renfree, M.B. Unique reproductive strategy in the swamp wallaby. Proc. Natl. Acad. Sci. USA 2020, 117, 5938–5942. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country of the Observational Study | Duration of Amenorrhea with LAM | Women Amenorrheic after Six Months | Main Results of the Study |
|---|---|---|---|---|
| [89] | Australia | Median: >8.5 months | - | Breastfeeding is an effective contraception method during the first six months after the delivery. |
| [90] | Uganda | - | 62.7% | It is possible to use LAM as a contraception method for most women. More support from health workers is needed. |
| [85] | Multicenter study (Egypt, Mexico, Nigeria: Jos, Nigeria: Sagamu, Philippines, Germany/Italy, Sweden, United Kingdom, United States) | - | 65.2% | LAM method use is highly satisfactory and effective without extensive supervision. |
| [74] | Mexico | Mean: 5.5 months (mean duration of LAM use was 4.3 + 0.2 months) | - | In developing countries, LAM use might be improved by regular supervision. |
| [91] | Turkey | - | 56.2% (women who had 6-month-old infants) | Prenatal and postnatal counseling is needed for effective LAM use because of low LAM criteria fulfillment. |
| [92] | Nigeria | - | - | There is a need for correct information about natural family planning methods. |
| [93] | Niger | - | - | The improvement of women’s education about LAM criteria and better access to health services is needed. |
| [94] | Ethiopia | - | - | The low level of knowledge about LAM might be improved with home-to-home counseling. |
| [95] | Tanzania | - | - | Women do not know about LAM. Future counseling should address their misconceptions, concerns, and knowledge gaps. |
| Study by | Time of Observation | Lumbar Spine | Femoral Neck | Radius |
|---|---|---|---|---|
| Laskey [108] | 3 ms | 3.96% | 2.39% | n/d |
| Atkinson [113] | 100 days | n/d | 2.2% | n/d |
| Sowers [114] | 6 ms | 5.1% | 4.8% | n/d |
| Hayslip [110] | 6 ms | 6.5% | n/d | NS |
| Affinito [109] | 3 ms | 6% | n/d | 2% |
| 6 ms | 7.5% | n/d | 5% | |
| Cross [101] | 3 ms | 4.3% | n/d | +5.7% |
| Kolthof [107] | 3 ms | 5.2% | n/d | n/d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calik-Ksepka, A.; Stradczuk, M.; Czarnecka, K.; Grymowicz, M.; Smolarczyk, R. Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1633. https://doi.org/10.3390/ijms23031633
Calik-Ksepka A, Stradczuk M, Czarnecka K, Grymowicz M, Smolarczyk R. Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover. International Journal of Molecular Sciences. 2022; 23(3):1633. https://doi.org/10.3390/ijms23031633
Chicago/Turabian StyleCalik-Ksepka, Anna, Monika Stradczuk, Karolina Czarnecka, Monika Grymowicz, and Roman Smolarczyk. 2022. "Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover" International Journal of Molecular Sciences 23, no. 3: 1633. https://doi.org/10.3390/ijms23031633
APA StyleCalik-Ksepka, A., Stradczuk, M., Czarnecka, K., Grymowicz, M., & Smolarczyk, R. (2022). Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover. International Journal of Molecular Sciences, 23(3), 1633. https://doi.org/10.3390/ijms23031633






