Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting
Abstract
:1. Introduction
2. Iron-Series Electrocatalysts for Water Splitting
3. Strategies for Tuning the D-Band Center of Materials Based on Iron-Series Electrocatalysts
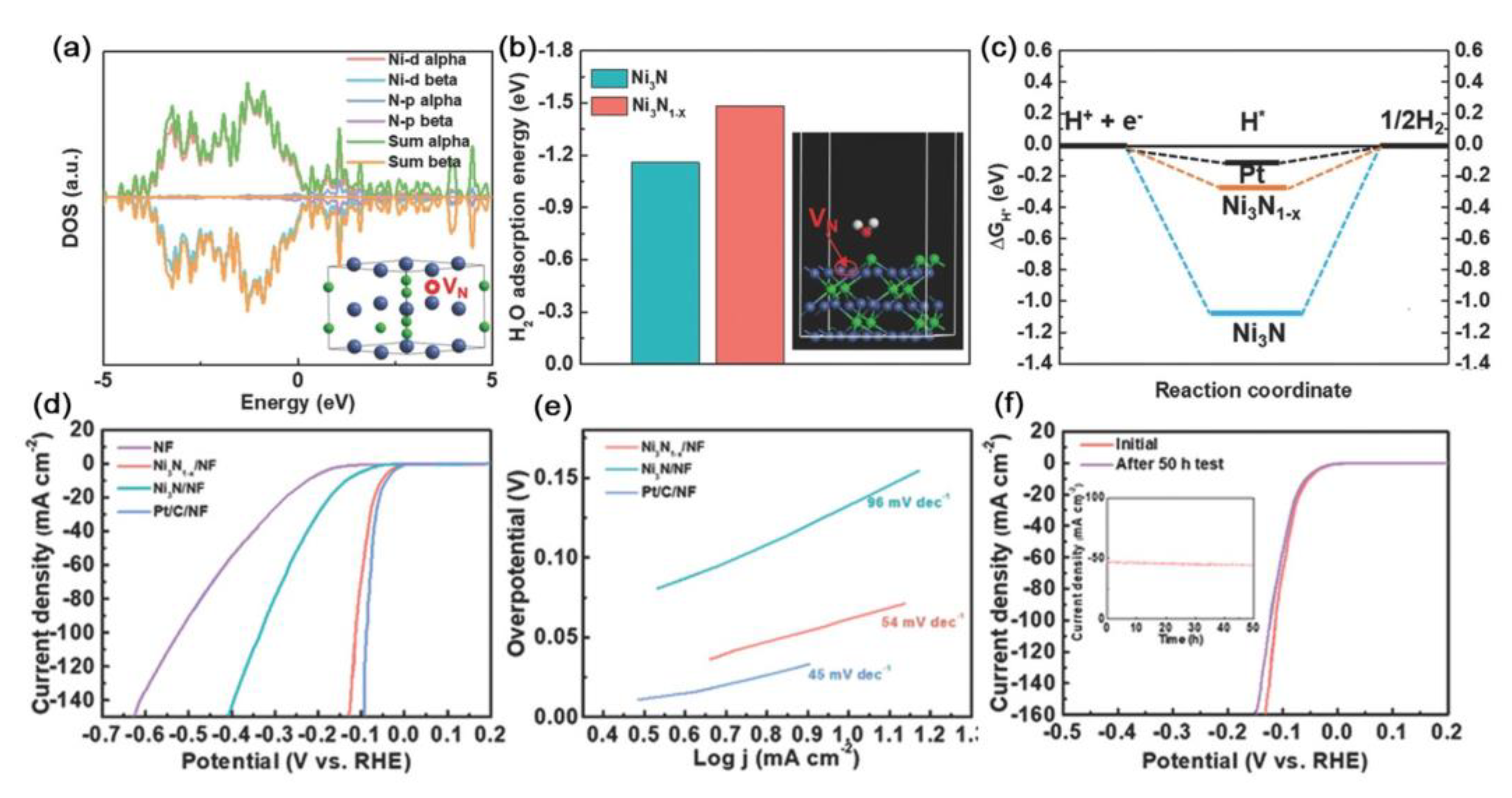
3.1. Introduction of Defects/Vacancies
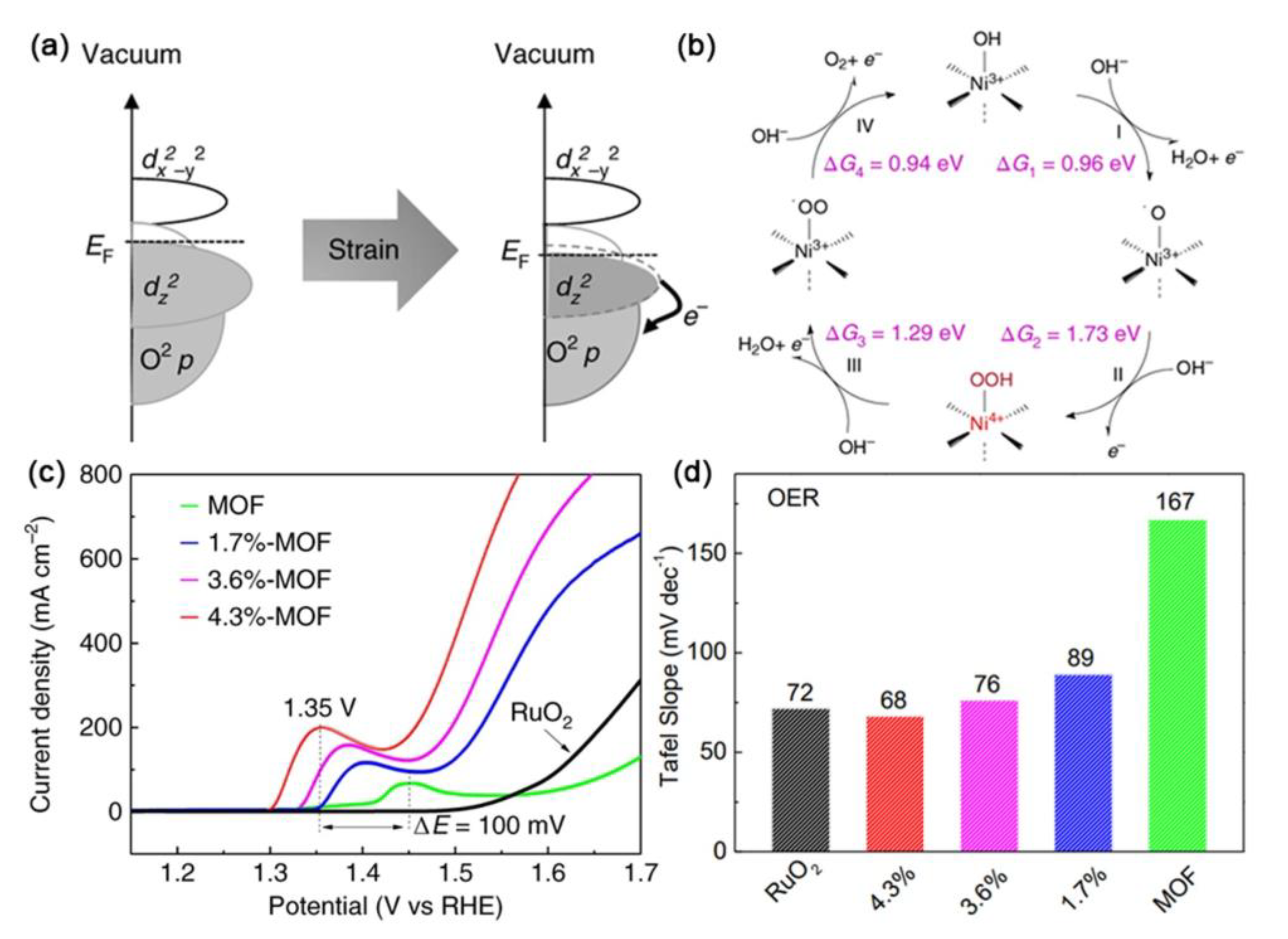
3.2. Strain Engineering
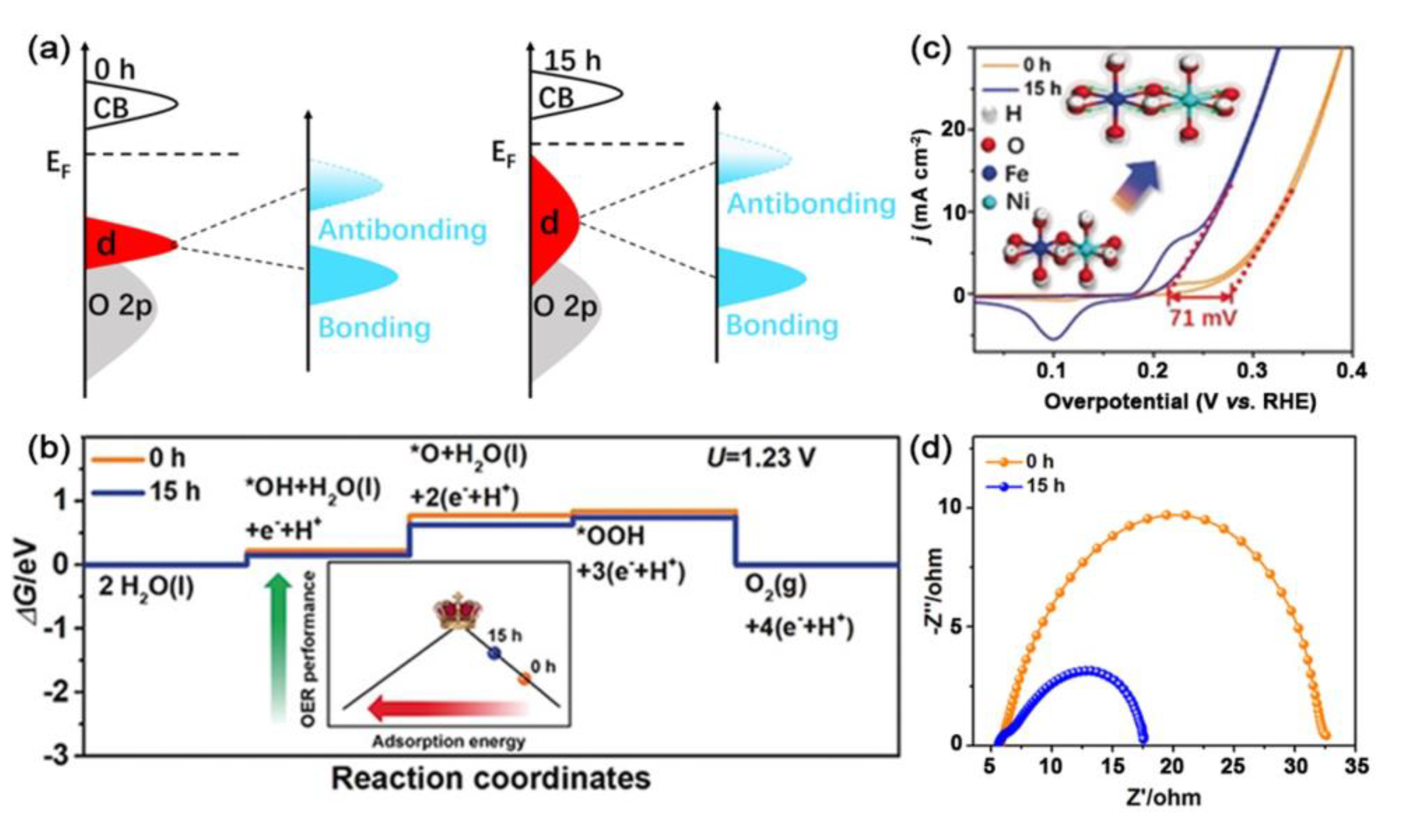
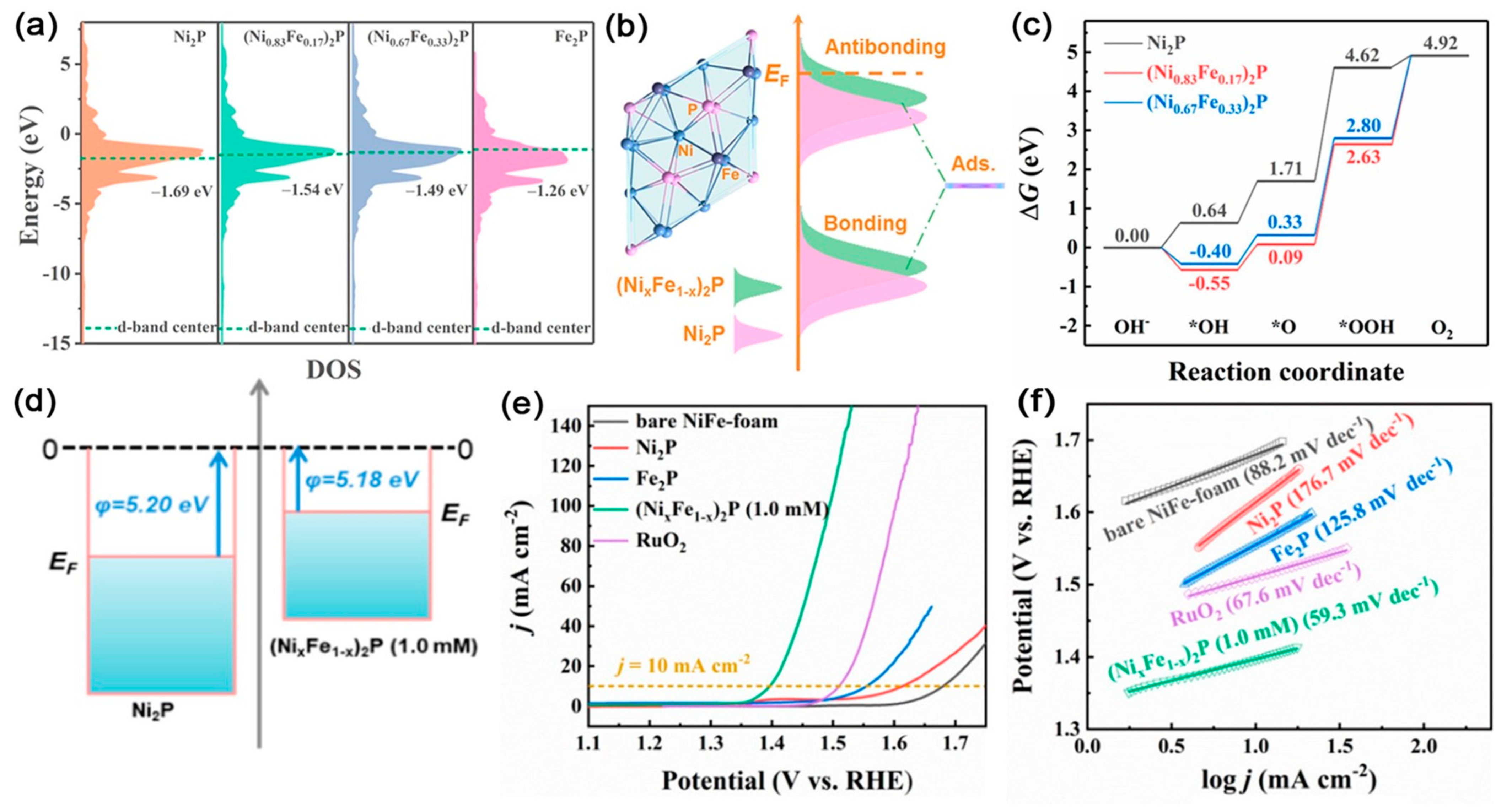
3.3. Element Doping and Element Substitution
3.4. Alloying
3.5. Composite of Two or More Iron Transition Series Metal-Based Compounds
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, B.; Chen, L.; Shi, J. Anion-Containing Noble-Metal-Free Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, M.; Chen, G.; Dong, R.; Feng, X. Two-Dimensional Conjugated Metal–Organic Frameworks for Electrocatalysis: Opportunities and Challenges. ACS Nano 2022, 16, 1759–1780. [Google Scholar] [CrossRef]
- Lim, K.; Handoko, A.; Nemani, S.; Wyatt, B.; Jiang, H.; Tang, J.; Anasori, B.; Seh, Z. Rational Design of Two-Dimensional Transition Metal Carbide/Nitride (MXene) Hybrids and Nanocomposites for Catalytic Energy Storage and Conversion. ACS Nano 2020, 14, 10834–10864. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent Development in Electrocatalysts for Hydrogen Production through Water Electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent Advances in Electrocatalysts for Neutral and Large-Current-Density Water Electrolysis. Nano Energy 2021, 80, 105545. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nano-Micro Lett. 2022, 14, 43. [Google Scholar] [CrossRef]
- Yang, H.; Driess, M.; Menezes, P. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Farràs, P.; Strasser, P.; Cowan, A. Water Electrolysis: Direct from the Sea or Not To Be? Joule 2021, 5, 1921–1923. [Google Scholar] [CrossRef]
- Lee, W.; Ko, Y.; Kim, J.; Choi, C.; Chae, K.; Kim, H.; Hwang, Y.; Min, B.; Strasser, P.; Oh, H. High Crystallinity Design of Ir-based Catalysts Drives Catalytic Reversibility for Water Electrolysis and Fuel Cells. Nat. Commun. 2021, 12, 4271. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Su, Z.; Chen, Y.; Srinivas, K.; Gao, J.; Zhang, W.; Wang, Z.; Lin, H. Electronic Modulation of Hierarchical Spongy Nanosheets toward Efficient and Stable Water Electrolysis. Small 2021, 17, 2006881. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, J.; Rajaei, H. Voltage Losses in Zero-Gap Alkaline Water Electrolysis. J. Power Sources 2021, 497, 229864. [Google Scholar] [CrossRef]
- Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D. Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction in Water Electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Oh, N.; Seo, J.; Lee, S.; Kim, H.; Kim, U.; Lee, J.; Han, Y.; Park, H. Highly Efficient and Robust Noble-Metal Free Bifunctional Water Electrolysis Catalyst Achieved Via Complementary Charge Transfer. Nat. Commun. 2021, 12, 4606. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in Noble Metal (Ru, Rh, and Ir) Doping for Boosting Water Splitting Electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, X.; Li, X.; Long, X.; Chang, C.; Yang, Z. Stable and High-Performance Ir Electrocatalyst with Boosted Utilization Efficiency in Acidic Overall Water Splitting. Chem. Eng. J. 2021, 424, 130337. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J. Regulating Electron Redistribution of Intermetallic Iridium Oxide by Incorporating Ru for Efficient Acidic Water Oxidation. Adv. Energy Mater. 2021, 11, 2102883. [Google Scholar] [CrossRef]
- Li, Q.; Huang, F.; Li, S.; Zhang, H.; Yu, X. Oxygen Vacancy Engineering Synergistic with Surface Hydrophilicity Modification of Hollow Ru Doped CoNi-LDH Nanotube Arrays for Boosting Hydrogen Evolution. Small 2022, 18, 2104323. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, J.; Li, X.; Yang, S.; Luo, W.; Zhang, Y.; Kim, S.; Kim, D.; Shinde, S.; Li, Y.; et al. In-Situ Reconstructed Ru Atom Array on α-MnO2 with Enhanced Performance for Acidic Water Oxidation. Nat. Catal. 2021, 4, 1012–1023. [Google Scholar] [CrossRef]
- Chen, P.; Hu, X. High-Efficiency Anion Exchange Membrane Water Electrolysis Employing Non-Noble Metal Catalysts. Adv. Energy Mater. 2020, 10, 2002285. [Google Scholar] [CrossRef]
- Khan, M.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef]
- Peng, S.; Gong, F.; Li, L.; Yu, D.; Ji, D.; Zhang, T.; Hu, Z.; Zhang, Z.; Chou, S.; Du, Y.; et al. Necklace-like Multishelled Hollow Spinel Oxides with Oxygen Vacancies for Efficient Water Electrolysis. J. Am. Chem. Soc. 2018, 140, 13644–13653. [Google Scholar] [CrossRef] [PubMed]
- Merrill, M.; Dougherty, R. Metal Oxide Catalysts for the Evolution of O2 from H2O. J. Phys. Chem. C 2008, 112, 3655–3666. [Google Scholar] [CrossRef]
- Koshikawa, H.; Murase, H.; Hayashi, T.; Nakajima, K.; Mashiko, H.; Shiraishi, S.; Tsuji, Y. Single Nanometer-Sized NiFe-Layered Double Hydroxides as Anode Catalyst in Anion Exchange Membrane Water Electrolysis Cell with Energy Conversion Efficiency of 74.7 % at 1.0 A cm−2. ACS Catal. 2020, 10, 1886–1893. [Google Scholar] [CrossRef]
- Chen, L.; Dong, X.; Wang, Y.; Xia, Y. Separating Hydrogen and Oxygen Evolution in Alkaline Water Electrolysis Using Nickel Hydroxide. Nat. Commun. 2016, 7, 11741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Ma, S.; Huang, W.; Boyjoo, Y.; Bai, S.; Liu, J. Ca2+-doped Ultrathin Cobalt Hydroxyl Oxides Derived from Coordination Polymers as Efficient Electrocatalysts for the Oxidation of Water. J. Mater. Chem. A 2019, 7, 19415–19422. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.; Xu, H.; Wang, S.; Niu, Z.; Jia, Q.; Yang, L.; Cao, R.; Zheng, L.; Cao, D. Dual Active Site Tandem Catalysis of Metal Hydroxyl Oxides and Single Atoms for Boosting Oxygen Evolution Reaction. Appl. Catal. B Environ. 2021, 297, 120451. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, R.; Cui, L.; Liu, D.; Hao, S.; Ma, Y.; Du, G.; Asiri, A.; Sun, X. High-Performance Electrolytic Oxygen Evolution in Neutral Media Catalyzed by a Cobalt Phosphate Nanoarray. Angew. Chem. Int. Ed. 2017, 56, 1064–1068. [Google Scholar] [CrossRef]
- Menezes, P.; Panda, C.; Walter, C.; Schwarze, M.; Driess, M. A Cobalt-Based Amorphous Bifunctional Electrocatalysts for Water-Splitting Evolved from a Single-Source Lazulite Cobalt Phosphate. Adv. Funct. Mater. 2019, 29, 1808632. [Google Scholar] [CrossRef]
- Giovanni, C.; Reyes, Á.; Coursier, A.; Nowak, S.; Grenèche, J.; Lecoq, H.; Mouton, L.; Rozière, J.; Jones, D.; Peron, J.; et al. Low-Cost Nanostructured Iron Sulfide Electrocatalysts for PEM Water Electrolysis. ACS Catal. 2016, 6, 2626–2631. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, 1806682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.; Yuan, Z.; Wu, M.; Zhou, H. Oxygen Defect-Stabilized Heterogeneous Single Atom Catalysts: Preparation, Properties and Catalytic Application. J. Mater. Chem. A 2021, 9, 3855–3879. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Wu, S.; Li, W.; Wu, B.; Liu, G.; Chen, G.; Xu, B.; Kang, B.; Li, Y.; et al. Engineering of the d-Band Center of Perovskite Cobaltite for Enhanced Electrocatalytic Oxygen Evolution. ChemSusChem 2020, 13, 2671–2676. [Google Scholar] [CrossRef]
- Gou, W.; Li, J.; Gao, W.; Xia, Z.; Zhang, S.; Ma, Y. Downshifted d-Band Center of Ru/MWCNTs by Turbostratic Carbon Nitride for Efficient and Robust Hydrogen Evolution in Alkali. ChemCatChem 2019, 11, 1970–1976. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Z.; Wang, X.; Gai, H.; Chen, Z.; Guo, T.; Hou, X.; Xu, L.; Hu, X.; Huang, M.; et al. Discovery of Quantitative Electronic Structure-OER Activity Relationship in Metal-Organic Framework Electrocatalysts Using an Integrated Theoretical-Experimental Approach. Adv. Funct. Mater. 2021, 31, 2102066. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, T.; Zhao, X.; Jiang, W.; Pan, H.; Gao, D.; Xu, C. Expediting in-Situ Electrochemical Activation of Two-Dimensional Metal–Organic Frameworks for Enhanced OER Intrinsic Activity by Iron Incorporation. ACS Catal. 2019, 9, 7356–7364. [Google Scholar] [CrossRef]
- Kwon, J.; Han, H.; Jo, S.; Choi, S.; Chung, K.; Ali, G.; Park, K.; Paik, U.; Song, T. Amorphous Nickel–Iron Borophosphate for a Robust and Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2021, 11, 2100624. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Chen, H.; Hu, Z.; Hung, S.; Ma, N.; Dai, J.; Lin, H.; Chen, C.; Zhou, W.; et al. An Amorphous Nickel–Iron-Based Electrocatalyst with Unusual Local Structures for Ultrafast Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1900883. [Google Scholar] [CrossRef]
- Chakraborty, B.; Beltrán-Suito, R.; Hausmann, J.; Garai, S.; Driess, M.; Menezes, P. Enabling Iron-Based Highly Effective Electrochemical Water-Splitting and Selective Oxygenation of Organic Substrates through In Situ Surface Modification of Intermetallic Iron Stannide Precatalyst. Adv. Energy Mater. 2020, 10, 2001377. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.; Sun, S.; Liu, Z.; Xi, S.; Xu, Z. Engineering High-Spin State Cobalt Cations in Spinel Zinc Cobalt Oxide for Spin Channel Propagation and Active Site Enhancement in Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 14536–14544. [Google Scholar] [CrossRef]
- Menezes, P.; Yao, S.; Suito, R.; Hausmann, J.; Menezes, P.; Driess, M. Facile Access to an Active γ-NiOOH Electrocatalyst for Durable Water Oxidation Derived From an Intermetallic Nickel Germanide Precursor. Angew. Chem. Int. Ed. 2021, 60, 4640–4647. [Google Scholar] [CrossRef]
- Xu, Z.; Moore, J. Rapid Construction of Large-size Phenylacetylene Dendrimers up to 12.5 Nanometers in Molecular Diameter. Angew. Chem. Int. Ed. 1993, 32, 1354–1357. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.; Yan, D.; Mao, J.; Liu, H.; Hu, W.; Du, X.; Ling, T.; Qiao, S. Engineering Oxygen Vacancy on NiO Nanorod Arrays for Alkaline Hydrogen Evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Nardi, K.; Yang, N.; Dickens, C.; Strickler, A.; Bent, S. Creating Highly Active Atomic Layer Deposited NiO Electrocatalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2015, 5, 1500412. [Google Scholar] [CrossRef]
- Kuai, C.; Zhang, Y.; Han, L.; Xin, H.; Sun, C.; Nordlund, D.; Qiao, S.; Du, X.; Lin, F. Creating Compressive Stress at the NiOOH/NiO Interface for Water Oxidation. J. Mater. Chem. A 2020, 8, 10747–10754. [Google Scholar] [CrossRef]
- Liang, J.; Tan, H.; Xiao, C.; Zhou, G.; Guo, S.; Ding, S. Hydroxyl-Riched Halloysite Clay Nanotubes Serving as Substrate of NiO Nanosheets for High-Performance Supercapacitor. J. Power Sources 2015, 285, 210–216. [Google Scholar] [CrossRef]
- Mota, M.; Bajdich, M.; Viswanathan, V.; Vojvodic, A.; Bell, A.; Nørskov, J. Importance of Correlation in Determining Electrocatalytic Oxygen Evolution Activity on Cobalt Oxides. J. Phys. Chem. C 2012, 116, 21077–21082. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.; Wang, H.; Jiao, Y.; Hu, Z.; Zheng, Y.; Zheng, L.; Mao, J.; Liu, H.; Du, X.; et al. Activating Cobalt(II) Oxide Nanorods for Efficient Electrocatalysis by Strain Engineering. Nat. Commun. 2017, 8, 1509. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Li, K.; Chen, M.; Wang, F. A Melamine Formaldehyderesin Route to In Situ Encapsulate Co2O3 into Carbon Black for Enhanced Oxygen Reduction in Alkaline Media. Int. J. Hydrogen Energy 2017, 42, 25960–25968. [Google Scholar] [CrossRef]
- Cai, Z.; Bi, Y.; Hu, E.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X.; Kuang, Y.; Li, Y.; Yang, X.; et al. Single-Crystalline Ultrathin Co3O4 Nanosheets with Massive Vacancy Defects for Enhanced Electrocatalysis. Adv. Energy Mater. 2018, 8, 1701694. [Google Scholar] [CrossRef]
- Xu, Q.; Huo, W.; Li, S.; Fang, J.; Li, L.; Zhang, B.; Zhang, F.; Zhang, Y.; Li, S.-W. Crystal Phase Determined Fe Active Sites on Fe2O3 (γ- and α-Fe2O3) Yolk-Shell Microspheres and Their Phase Dependent Electrocatalytic Oxygen Evolution Reaction. Appl. Surf. Sci. 2020, 533, 147368. [Google Scholar] [CrossRef]
- Cai, M.; Pan, R.; Liu, W.; Luo, X.; Chen, C.; Zhang, H.; Zhong, M. Laser-Assisted Doping and Architecture Engineering of Fe3O4 Nanoparticles for Highly Enhanced Oxygen Evolution Reaction. ChemSusChem 2019, 12, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xia, Y.; Li, Q.; Dong, S.; Jiao, X.; Chen, D. Interface Engineering of Co(OH)2/Ag/FeP Hierarchical Superstructure as Efficient and Robust Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 7936–7945. [Google Scholar] [CrossRef]
- Sayeed, M.; Herd, T.; O’mullane, A. Direct Electrochemical Formation of Nanostructured Amorphous Co(OH)2 on Gold Electrodes with Enhanced Activity for the Oxygen Evolution Reaction. J. Mater. Chem. A 2016, 4, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Liu, D.; Chen, S.; Moses, O.; Chen, X.; Xu, W.; Wu, C.; Zheng, L.; Chu, S.; Jiang, H.; et al. Operando X-ray Spectroscopy Visualizing the Chameleon-like Structural Reconstruction on an Oxygen Evolution Electrocatalyst. Energy Environ. Sci. 2021, 14, 906–915. [Google Scholar] [CrossRef]
- Mavrič, A.; Fanetti, M.; Lin, Y.; Valant, M.; Cui, C. Spectroelectrochemical Tracking of Nickel Hydroxide Reveals Its Irreversible Redox States upon Operation at High Current Density. ACS Catal. 2020, 10, 9451–9457. [Google Scholar] [CrossRef]
- Duan, H. In-Situ Formation of Highly Active Electrocatalysts for Water Splitting. Ph.D. Thesis, Hong Kong University of Science and Technoloty, Hong Kong, China, 2017. [Google Scholar]
- Nguyen, T.; Lee, J.; Bae, J.; Lim, B. Binary FeCo Oxyhydroxide Nanosheets as Highly Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Chem. Eur. J. 2018, 24, 4724–4728. [Google Scholar] [CrossRef]
- Francàs, L.; Corby, S.; Selim, S.; Lee, D.; Mesa, C.; Godin, R.; Pastor, E.; Stephens, I.; Choi, K.; Durrant, J. Spectroelectrochemical Study of Water Oxidation on Nickel and Iron Oxyhydroxide Electrocatalysts. Nat. Commun. 2019, 10, 5208. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; You, S.; Li, X.; Tang, B.; Jiang, B.; Yu, Y.; Cai, Z.; Ren, N.; Zou, J. In Situ Crystallization of Active NiOOH/CoOOH Heterostructures with Hydroxide Ion Adsorption Sites on Velutipes-like CoSe/NiSe Nanorods as Catalysts for Oxygen Evolution and Cocatalysts for Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The Roles of Oxygen Vacancies in Electrocatalytic Oxygen Evolution Reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Xu, W.; Lyu, F.; Bai, Y.; Gao, A.; Feng, J.; Cai, Z.; Yin, Y. Porous Cobalt Oxide Nanoplates Enriched with Oxygen Vacancies for Oxygen Evolution Reaction. Nano Energy 2018, 43, 110–116. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Huang, Y.; Wei, Z.; Dong, C.; Ma, J.; Shen, S.; Li, Y.; Wang, S. Filling the Oxygen Vacancies in Co3O4 with Phosphorus: An Ultra-Efficient Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, C.; Hua, J.; Hong, X.; Sun, C.; Li, H.; Xu, X.; Mai, L. Engineering Oxygen Vacancies in a Polysulfide-Blocking Layer with Enhanced Catalytic Ability. Adv. Mater. 2020, 32, 1907444. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zheng, Y.; Guo, Z.; Zhu, Y.; Chen, H.; Li, F.; Liu, P.; Yu, B.; Wang, X.; et al. Uncovering the Effect of Lattice Strain and Oxygen Deficiency on Electrocatalytic Activity of Perovskite Cobaltite Thin Films. Adv. Sci. 2019, 6, 1801898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Du, W.; Zhou, W.; Wang, C.; Lu, S.; Lu, S.; Zhang, B. Unveiling the Promotion of Surface-Adsorbed Chalcogenate on the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 22470–22474. [Google Scholar] [CrossRef]
- Hu, J.; Salihy, A.; Wang, J.; Li, X.; Fu, Y.; Li, Z.; Han, X.; Song, B.; Xu, P. Improved Interface Charge Transfer and Redistribution in CuO-CoOOH p-n Heterojunction Nanoarray Electrocatalyst for Enhanced Oxygen Evolution Reaction. Adv. Sci. 2021, 8, 2103314. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Q.; Song, F.; Yu, L.; Mechler, A.; Schlögl, R.; Heumann, S. Oxygen Evolution Reaction at Carbon Edge Sites: Investigation of Activity Evolution and Structure–Function Relationships with Polycyclic Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2019, 58, 8917–8921. [Google Scholar] [CrossRef] [Green Version]
- Somorjai, G.; Blakely, D. Mechanism of Catalysis of Hydrocarbon Reactions by Platinum Surfaces. Nature 1975, 258, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Chen, A.; Huang, T.; Li, J.; Tian, Z. Electrocatalytic Properties of Pt(111), Pt(332), Pt(331) and Pt(110) Single Crystal Electrodes towards Ethylene Glycol Oxidation in Sulphuric Acid Solutions. J. Electroanal. Chem. 1992, 340, 213–226. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.; Sun, S.; Ding, Y.; Wang, Z. Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-Atom Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, B.; Peng, Q.; Zhou, J.; Sun, Z. Mo2B2 MBene-Supported Single-Atom Catalysts as Bifunctional HER/OER and OER/ORR Electrocatalysts. J. Mater. Chem. A 2021, 9, 433–441. [Google Scholar] [CrossRef]
- Li, F.; Han, G.; Bu, Y.; Chen, S.; Ahmad, I.; Jeong, H.; Fu, Z.; Lu, Y.; Baek, J. Unveiling the Critical Role of Active Site Interaction in Single Atom Catalyst Towards Hydrogen Evolution Catalysis. Nano Energy 2022, 93, 106819. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Hu, J.; Zhang, Y.; Du, Y.; Han, X.; Liu, X.; Xu, P. Hollow FeCo-FeCoP@C Nanocubes Embedded in Nitrogen-Doped Carbon Nanocages for Efficient Overall Water Splitting. J. Energy Chem. 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Hu, J.; Li, S.; Du, Y.; Han, X.; Xu, P. Metal–Organic Frameworks Derived Interconnected Bimetallic Metaphosphate Nanoarrays for Efficient Electrocatalytic Oxygen Evolution. Adv. Funct. Mater. 2020, 30, 1910498. [Google Scholar] [CrossRef]
- Jiang, K.; Luo, M.; Peng, M.; Yu, Y.; Lu, Y.; Chan, T.; Liu, P.; De Groot, F.; Tan, Y. Dynamic Active-Site Generation of Atomic Iridium Stabilized on Nanoporous Metal Phosphides for Water Oxidation. Nat. Commun. 2020, 11, 2701. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kim, D.; Zhang, Z.; Lee, M.; Yong, K. MOF-Derived NiCoZnP Nanoclusters Anchored on Hierarchical N-doped Carbon Nanosheets Array as Bifunctional Electrocatalysts for Overall Water Splitting. Chem. Eng. J. 2021, 422, 130533. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wang, Y.; Xu, C.; Liu, S.; Chen, C.; Peng, Q.; Zhuang, Z.; Xiao, H.; Pan, Y.; Lu, S.; et al. Three-Dimensional Open Nano-Netcage Electrocatalysts for Efficient pH-Universal Overall Water Splitting. Nat. Commun. 2019, 10, 4875. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.; Song, D.; Oh, S.; Chang, K.; Cho, E. Promotion of Electrochemical Oxygen Evolution Reaction by Chemical Coupling of Cobalt to Molybdenum Carbide. Appl. Catal. B Environ. 2018, 227, 340–348. [Google Scholar] [CrossRef]
- Jie, Y.; Jin, J.; Zhang, H.; Lu, M.; Peng, Y.; Huang, B.; Xi, P.; Yan, C. Atomic Arrangement in Metal-Doped NiS2 Boosts the Hydrogen Evolution Reaction in Alkaline Media. Angew. Chem. Int. Ed. 2019, 131, 18849–18855. [Google Scholar]
- Hammer, B.; Norskov, J. Why Gold is the Noblest of All the Metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the Design of Defect Electrocatalysts: From Electronic Structure to Adsorption Energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, W.; Song, E.; Gu, F.; Zheng, Z.; Zhao, X.; Zhao, Y.; Liu, J.; Zhang, W. Adsorption-Energy-Based Activity Descriptors for Electrocatalysts in Energy Storage Applications. Nat. Sci. Rev. 2018, 5, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Arandiyan, H.; Mofarah, S.; Wang, Y.; Cazorla, C.; Jampaiah, D.; Garbrecht, M.; Wilson, K.; Lee, A.; Zhao, C.; Maschmeyer, T. Impact of Surface Defects on LaNiO3 Perovskite Electrocatalysts for the Oxygen Evolution Reaction. Chem. Eur. J. 2021, 27, 14418–14426. [Google Scholar] [CrossRef]
- Jin, X.; Lee, T.; Tamakloe, W.; Patil, S.; Soon, A.; Kang, Y.; Hwang, S. In Situ Defect Engineering Route to Optimize the Cationic Redox Activity of Layered Double Hydroxide Nanosheet via Strong Electronic Coupling with Holey Substrate. Adv. Sci. 2021, 9, 2103368. [Google Scholar] [CrossRef]
- Arandiyan, H.; Mofarah, S.; Sorrell, C.; Doustkhah, E.; Sajjadi, B.; Hao, D.; Wang, Y.; Sun, H.; Ni, B.; Rezaei, M.; et al. Defect Engineering of Oxide Perovskites for Catalysis and Energy Storage: Synthesis of Chemistry and Materials Science. Chem. Soc. Rev. 2021, 50, 10116–10211. [Google Scholar] [CrossRef]
- Gao, N.; Liang, X.; Zhao, J.; Chen, Y. First-Principles Study of the Atomic Structures and Catalytic Properties of Monolayer TaS2 with Intrinsic Defects. J. Phys. Chem. C 2021, 125, 10362–10369. [Google Scholar] [CrossRef]
- Geng, S.; Tian, F.; Li, M.; Guo, X.; Yu, Y.; Yang, W.; Hou, Y. Hole-Rich CoP Nanosheets with an Optimized d-band Center for Enhancing pH-universal Hydrogen Evolution Electrocatalysis. J. Mater. Chem. A 2021, 9, 8561–8567. [Google Scholar] [CrossRef]
- Liu, B.; He, B.; Peng, H.; Zhao, Y.; Cheng, J.; Xia, J.; Shen, J.; Ng, T.; Meng, X.; Lee, C.; et al. Unconventional Nickel Nitride Enriched with Nitrogen Vacancies as a High-Efficiency Electrocatalyst for Hydrogen Evolution. Adv. Sci. 2018, 5, 1800406. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.; Zhang, M.; Zhou, Y.; Rao, D.; Zhong, C.; Zhang, J.; Han, X.; Hu, W.; Zhang, Y.; et al. Lattice-Strain Engineering of Homogeneous NiS0.5Se0.5 Core–Shell Nanostructure as a Highly Efficient and Robust Electrocatalyst for Overall Water Splitting. Adv. Mater. 2020, 32, 2000231. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Maiti, K.; Curnan, M.; Kim, K.; Noh, K.; Han, J. Engineering Electrocatalyst Nanosurfaces to Enrich the Activity by Inducing Lattice Strain. Energy Environ. Sci. 2021, 14, 3717–3756. [Google Scholar] [CrossRef]
- Jiao, S.; Fu, X.; Huang, H. Descriptors for the Evaluation of Electrocatalytic Reactions: D-Band Theory and Beyond. Adv. Funct. Mater. 2021, 32, 2107651. [Google Scholar] [CrossRef]
- Wu, T.; Sun, M.; Huang, B. Probing the Irregular Lattice Strain-Induced Electronic Structure Variations on Late Transition Metals for Boosting the Electrocatalyst Activity. Small 2020, 16, 2002434. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Tang, J.; Zhang, J.; Liu, D.; Zheng, L.; et al. NiFe Hydroxide Lattice Tensile Strain: Enhancement of Adsorption of Oxygenated Intermediates for Efficient Water Oxidation Catalysis. Angew. Chem. Int. Ed. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Kelsey, S.; Choi, W.; Jeen, H.; Lee, H.; Shao, Y. Role of Strain and Conductivity in Oxygen Electrocatalysis on LaCoO3 Thin Films. J. Phys. Chem. Lett. 2015, 6, 487–492. [Google Scholar]
- Cheng, W.; Zhao, X.; Su, H.; Tang, F.; Che, W.; Zhang, H.; Liu, Q. Lattice-Strained Metal–Organic-Framework Arrays for Bifunctional Oxygen Electrocatalysis. Nat. Energy 2019, 4, 115–122. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhou, L.; Duan, W.; Song, Y.; Zhang, F.; Zhen, Y.; Zhang, J.; Bao, W.; Lu, Y.; et al. Refining d-band Center in Ni0.85Se by Mo Doping: A Strategy for Boosting Hydrogen Generation via Coupling Electrocatalytic Oxidation 5-Hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Zhao, J.; Dong, B.; Wang, F.; Wu, Z.; Wang, L.; Chai, Y.; Liu, C. Tailoring the D-band Centers of FeP Nanobelt Arrays by Fluorine Doping for Enhanced Hydrogen Evolution at High Current Density. Fuel 2022, 316, 123206. [Google Scholar] [CrossRef]
- Cao, B.; Hu, M.; Cheng, Y.; Jing, P.; Liu, B.; Zhou, B.; Wang, X.; Gao, R.; Sun, X.; Du, Y.; et al. Tailoring the D-band Center of N-doped Carbon Nanotube Arrays with Co4N Nanoparticles and Single-atom Co for a Superior Hydrogen Evolution Reaction. NPG Asia Mater. 2021, 13, 1. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, B.; Cai, H.; Hao, H.; Li, J.; Yang, T.; Yang, S. Understanding the Doping Effect on Hydrogen Evolution Activity of Transition-Metal Phosphides: Modeled with Ni2P. Appl. Catal. B Environ. 2021, 295, 120283. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the d-Band Centers Endows (NixFe1–x)2P Nanosheets with Efficient Oxygen Evolution Catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Deng, J.; Shen, S.; Meng, F.; Zhang, J.; Zhang, Q.; Zhong, W.; Gu, L. Elevating the d-Band Center of Six-Coordinated Octahedrons in Co9S8 through Fe-Incorporated Topochemical Deintercalation. Adv. Energy Mater. 2021, 11, 2003023. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-Band Centers Enables Co4N Nanosheets To Be Highly Active for Hydrogen Evolution Catalysis. Angew. Chem. Int. Ed. 2018, 130, 5170–5174. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Lin, Y.; Cao, X.; Cheng, Y.; Liu, S.; Zeng, L.; Cheong, W.; Zhao, D.; Wu, K.; et al. Electronic Structure and D-band Center Control Engineering over M-doped CoP (M = Ni, Mn, Fe) Hollow Polyhedron Frames for Boosting Hydrogen Production. Nano Energy 2019, 56, 411–419. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, T.; Ye, S.; Zheng, L.; Liao, P.; Xiong, W.; Hu, J.; Wang, Y.; Wang, J.; Ren, X.; et al. Engineering Defect-rich Fe-doped NiO Coupled Ni Cluster Nanotube Arrays with Excellent Oxygen Evolution Activity. Appl. Catal. B Environ. 2021, 285, 119809. [Google Scholar] [CrossRef]
- Shervedani, R.; Torabi, M.; Foroushani, M. Mixture Design of NiCoMo Ternary Alloy Nanoparticles Assembled on Graphene Nanosheets and Decorated with Ru Nanoparticles: A Pt/C-like Activity for Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 17621–17631. [Google Scholar] [CrossRef]
- Lupi, C.; Era, A.; Pasquali, M. Nickel–Cobalt Electrodeposited Alloys for Hydrogen Evolution in Alkaline Media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.; Bonde, J.; Chorken, D.; Nørskov, J. Computational High-throughput Screening of Electrocatalytic Materials for Hydrogen Evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Raj, I.; Vasu, K. Transition Metal-based Cathodes for Hydrogen Evolution in Alkaline Solution: Electrocatalysis on Nickel-based Ternary Electrolytic Codeposits. J. Appl. Electrochem. 1992, 22, 471–477. [Google Scholar] [CrossRef]
- Ci, S.; Mao, S.; Hou, Y.; Cui, S.; Kim, H.; Ren, R.; Wen, Z.; Chen, J. Rational Design of Mesoporous NiFe-Alloy-Based Hybrids for Oxygen Conversion Electrocatalysis. J. Mater. Chem. A 2015, 3, 7986–7993. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, H.; Zhang, Y.; Wang, Q. NiFe Alloy Nanoparticles with hcp Crystal Structure Stimulate Superior Oxygen Evolution Reaction Electrocatalytic Activity. Angew. Chem. Int. Ed. 2019, 58, 6099–6103. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, X.; Paik, U. N-doped Graphene Layers Encapsulated NiFe Alloy Nanoparticles Derived from MOFs with Superior Electrochemical Performance for Oxygen Evolution Reaction. Sci. Rep. 2016, 6, 34004. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Zheng, F.; Zhang, C.; Du, C.; Fang, Z.; Zhang, Z.; Chen, W. High-Efficiency Bifunctional Electrocatalyst Based on 3D Freestanding Cu Foam in Situ Armored CoNi Alloy Nanosheet Arrays for Overall Water Splitting. J. Power Sources 2019, 427, 184–193. [Google Scholar] [CrossRef]
- Yu, X.; Hu, C.; Ji, P.; Ren, Y.; Zhao, H.; Liu, G.; Xu, R.; Zhu, X.; Li, Z.; Ma, Y.; et al. Optically Transparent Ultrathin NiCo Alloy Oxide Film: Precise Oxygen Vacancy Modulation and Control for enhanced Electrocatalysis of Water Oxidation. Appl. Catal. B Environ. 2022, 310, 121301. [Google Scholar] [CrossRef]
- Song, Q.; Li, J.; Wang, S.; Liu, J.; Liu, X.; Pang, L.; Li, H.; Liu, H. Enhanced Electrocatalytic Performance through Body Enrichment of Co-Based Bimetallic Nanoparticles In Situ Embedded Porous N-Doped Carbon Spheres. Small 2019, 15, 1903395. [Google Scholar] [CrossRef]
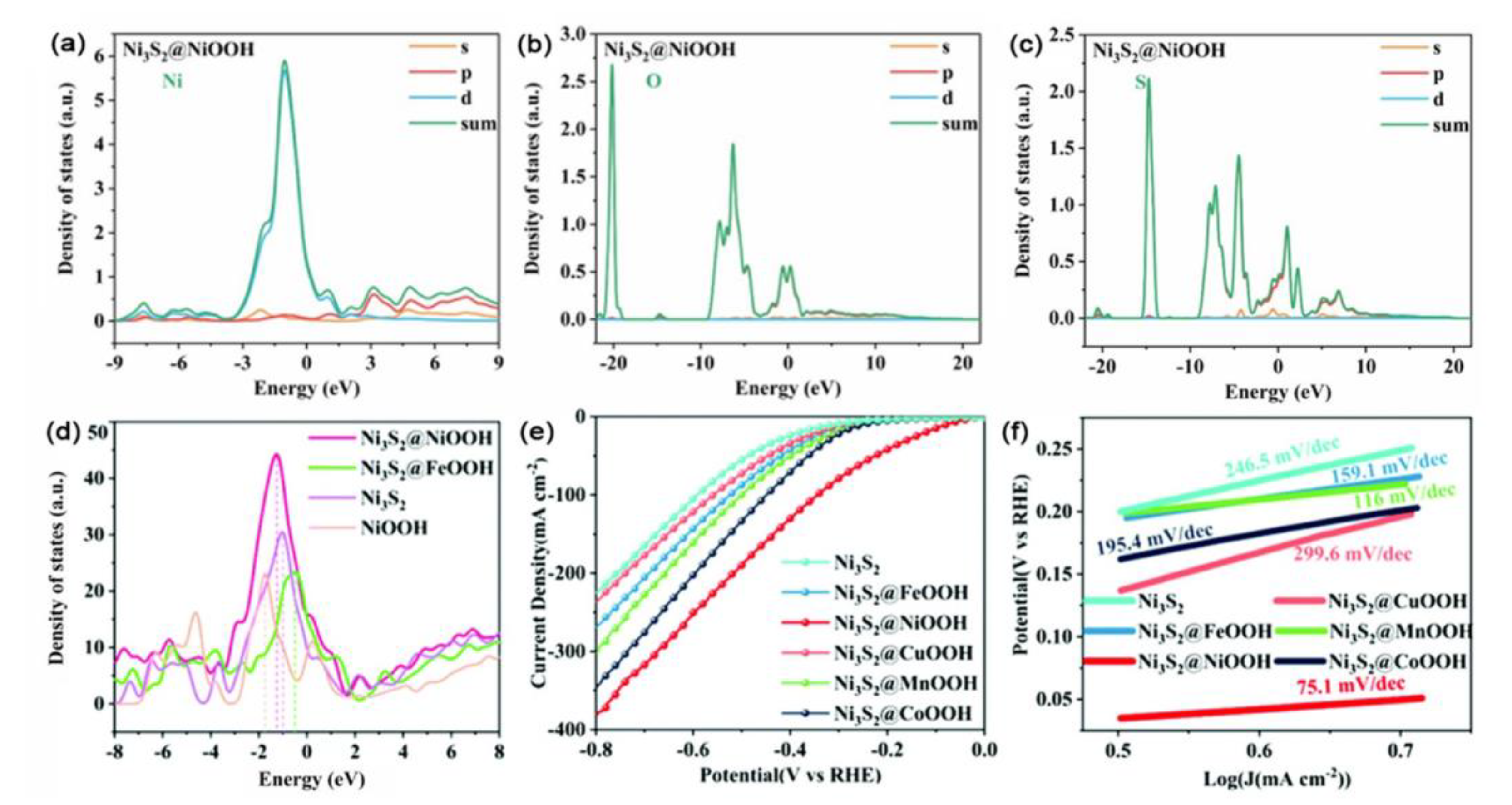
- Du, X.; Ma, G.; Wang, Y.; Han, X.; Zhang, X. Controllable Synthesis of Ni3S2@MOOH/NF (M = Fe, Ni, Cu, Mn and Co) Hybrid Structure for the Efficient Hydrogen Evolution Reaction. Dalton Trans. 2021, 50, 14001–14008. [Google Scholar] [CrossRef]
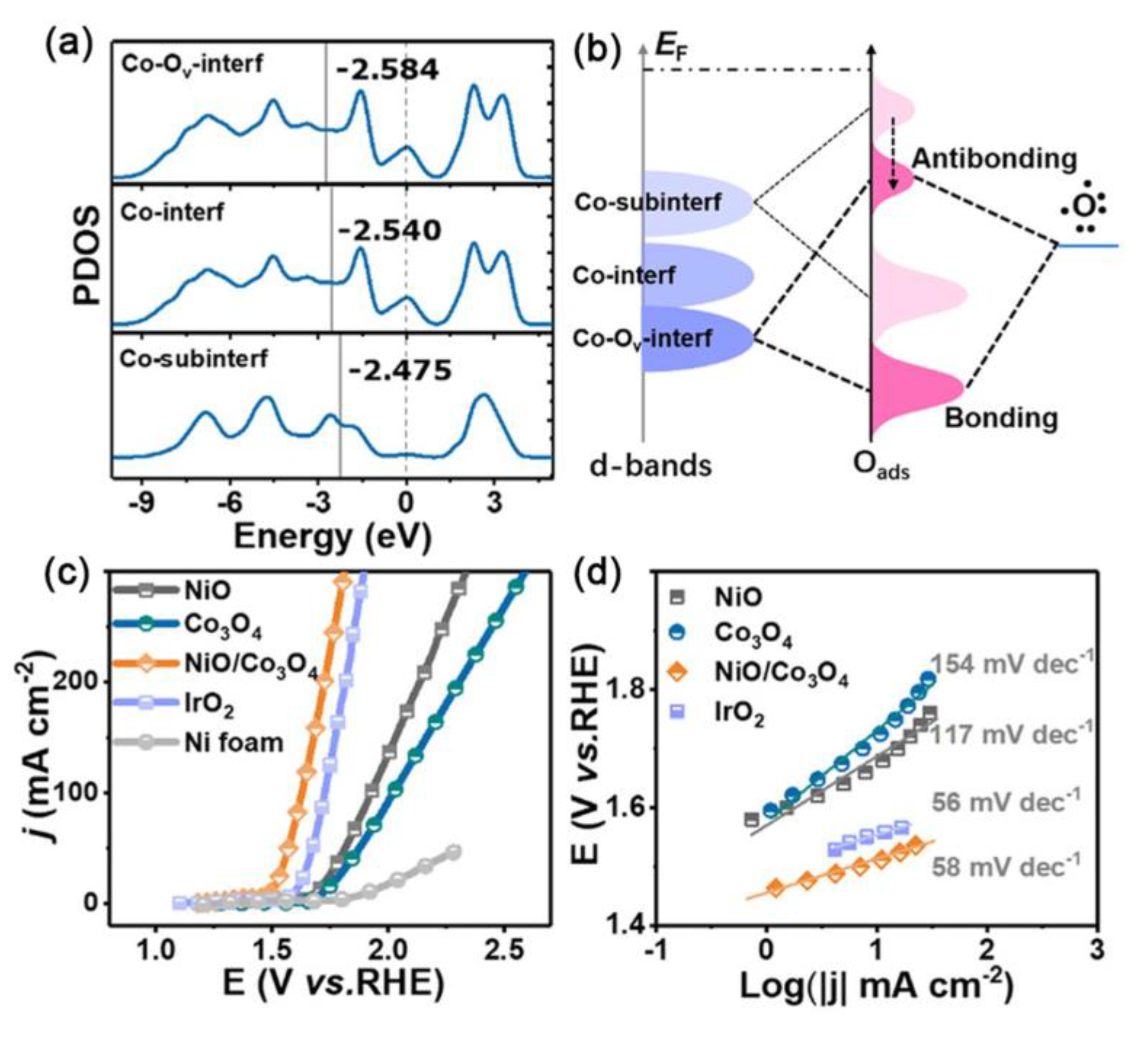
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]


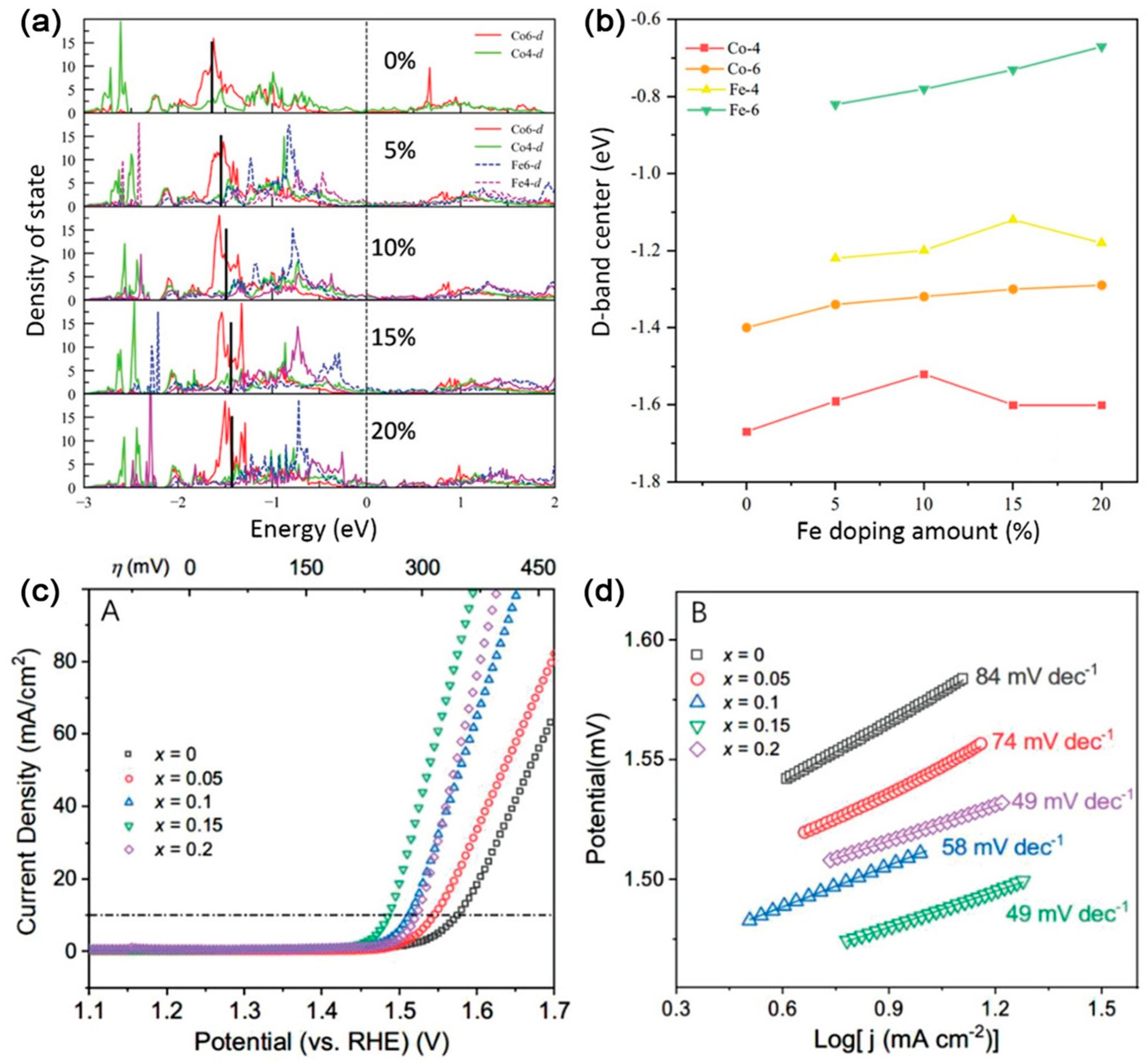
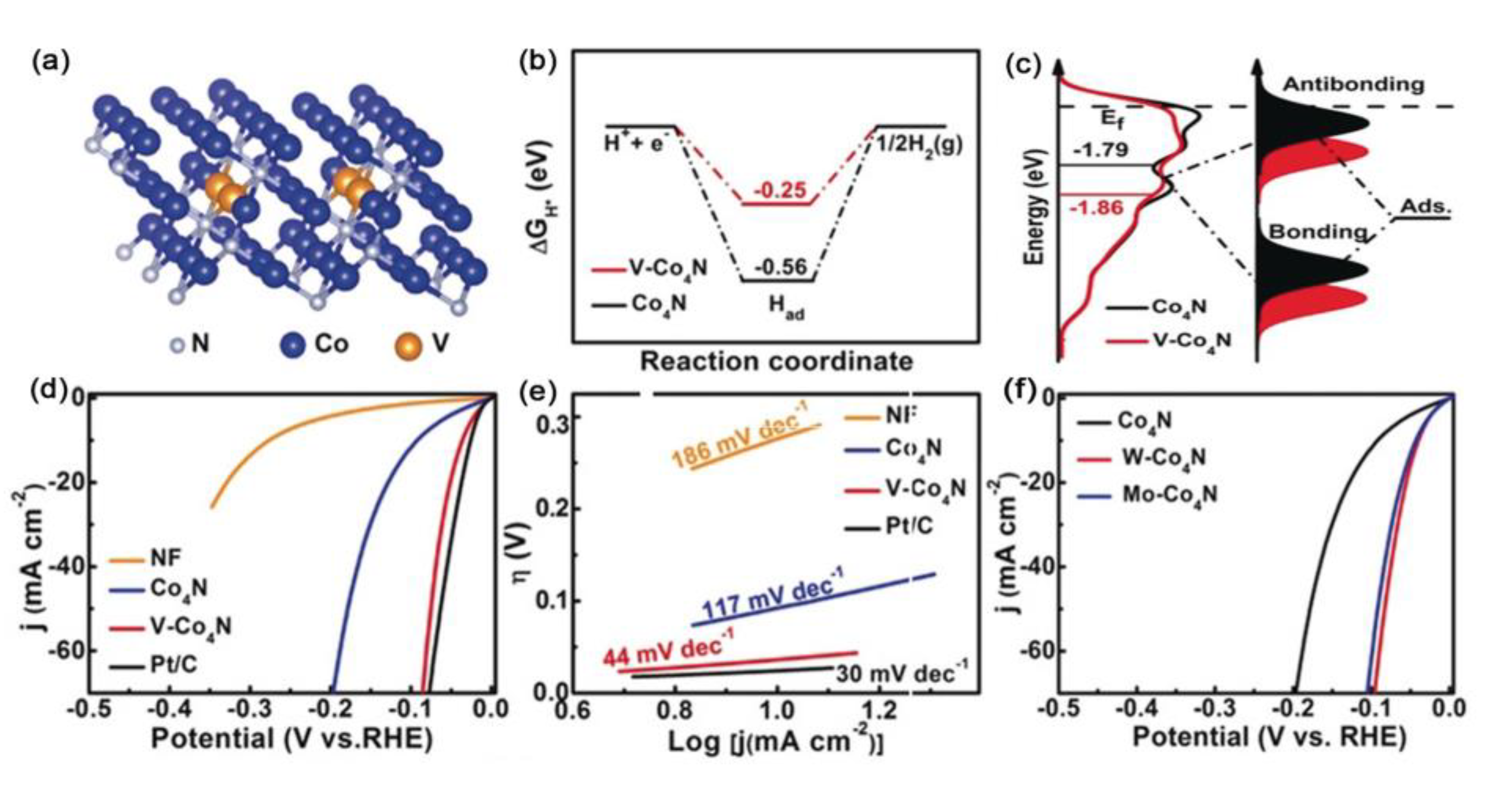

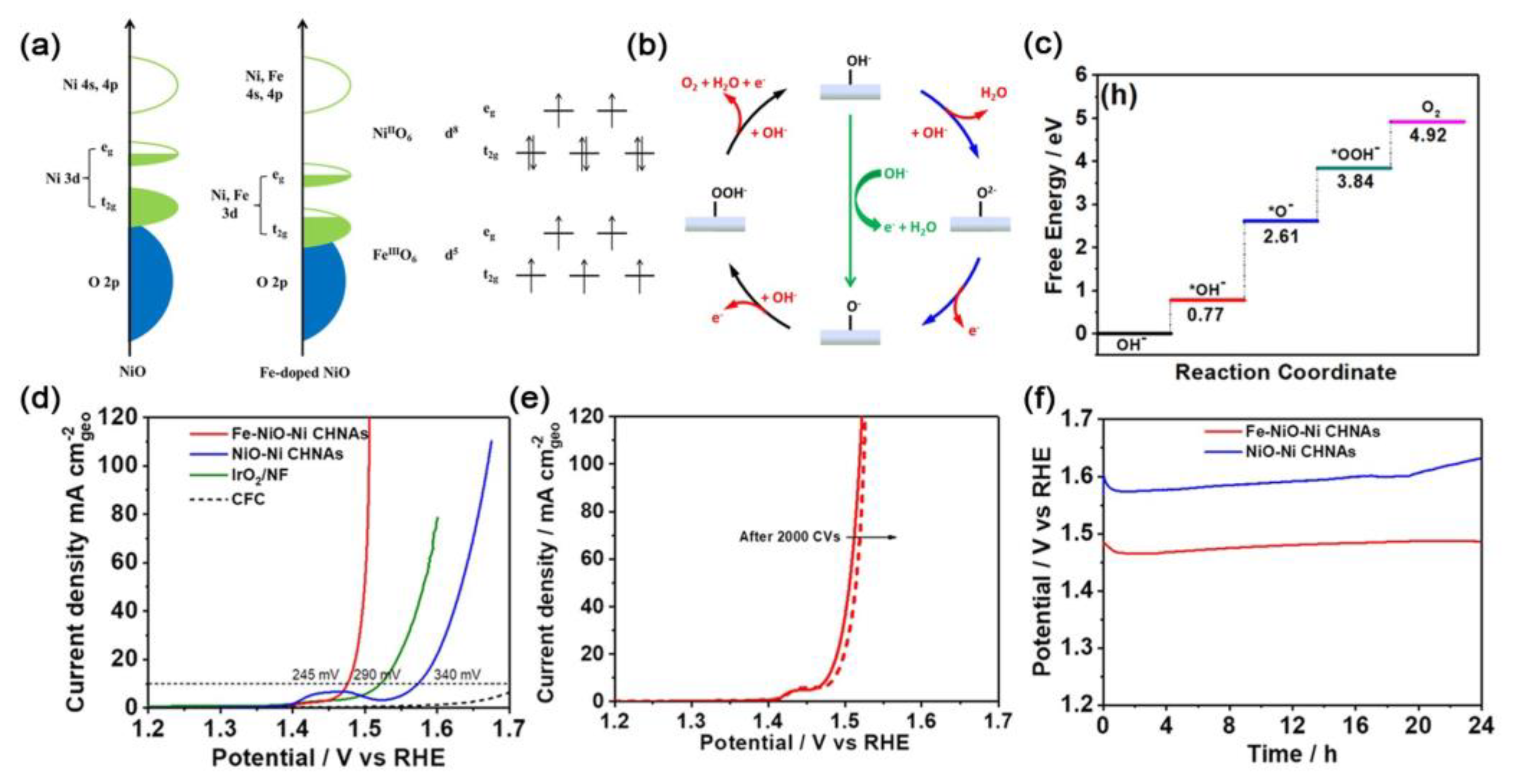
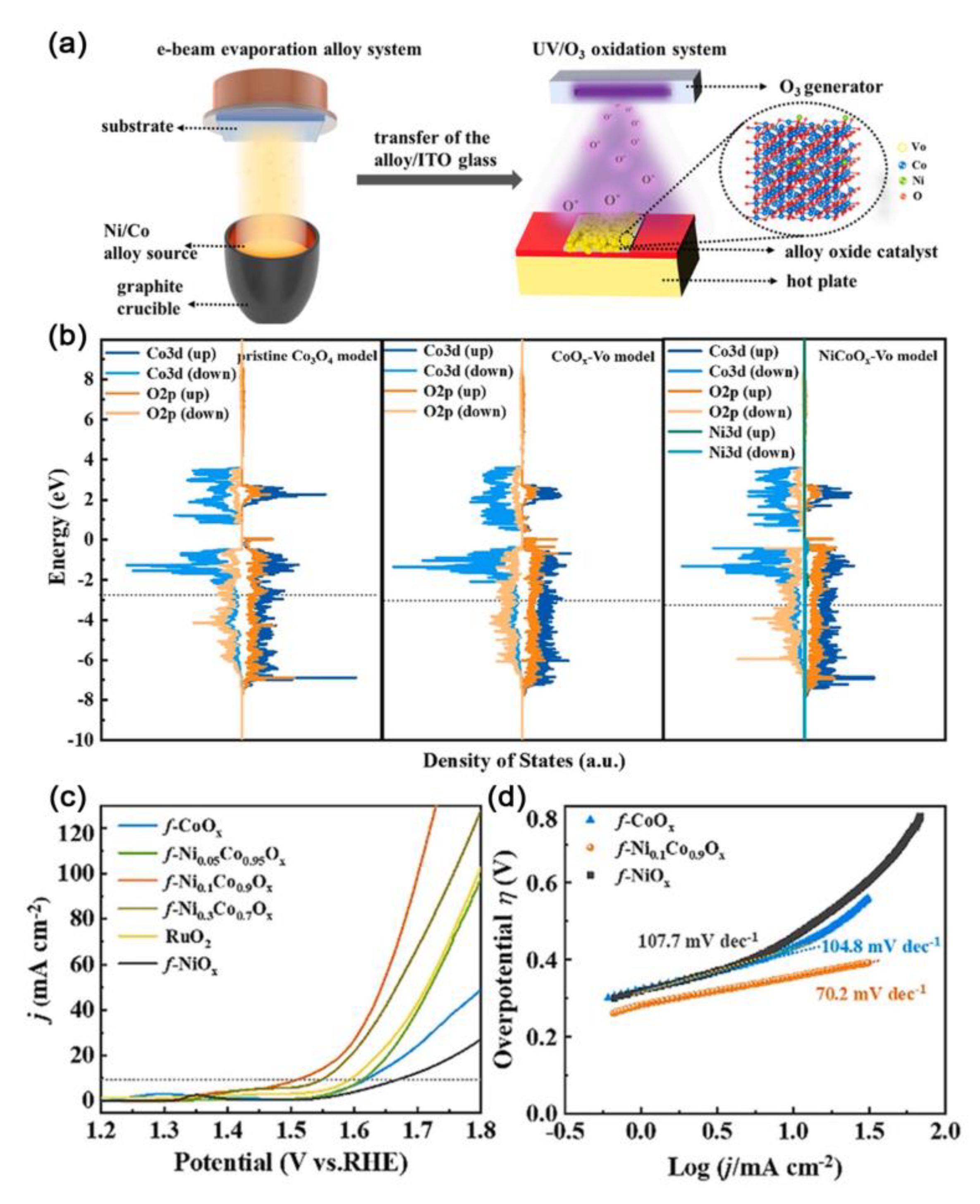

| Catalysts | D-Band Center | HER (at 10 mA cm−2) | OER (at 10 mA cm−2) | Ref. |
|---|---|---|---|---|
| NiCo2O4 | downward shift | 135 mV in 1 M KOH | 240 mV in 1 M KOH | [22] |
| CoP | upward shift | 84 and 94 mV in acidic and alkaline media, respectively | / | [93] |
| Ni3N1−x | downward shift | 55 mV in 1 M KOH | / | [94] |
| NiFe–LDH | upward shift | / | 270 mV in 1 M KOH | [99] |
| NiFe MOFs | negatively shifted | / | 210 mV at 200 mA cm−2 in 0.1 M KOH | [101] |
| Fe-substituted Ni2P | upward shift | / | 166 mV in 1 M KOH | [106] |
| Co9S8 | upward shift | / | 255 mV in 1 M KOH | [107] |
| V–Co4N | downward shift | 37 mV in 1 M KOH | / | [108] |
| M-doped CoP | downward shift | 144 mV in 0.5 M H2SO4 | 92 mV in 1 M KOH | [109] |
| Fe-doped NiO | downward shift | / | 245 mV in 1 M KOH | [110] |
| NiCo bimetallic alloy oxide | downward shift | / | 268 mV in 1 M KOH | [119] |
| Co-based bimetallic nanoparticles | tailored | / | 240 mV in 1 M KOH | [120] |
| Ni3S2@MOOH/NF | downward shift | 79 mV in 1 M KOH | / | [121] |
| NiO/Co3O4 | downward shift | / | 262 mV in 1 M KOH | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Al-Salihy, A.; Zhang, B.; Li, S.; Xu, P. Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting. Int. J. Mol. Sci. 2022, 23, 15405. https://doi.org/10.3390/ijms232315405
Hu J, Al-Salihy A, Zhang B, Li S, Xu P. Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting. International Journal of Molecular Sciences. 2022; 23(23):15405. https://doi.org/10.3390/ijms232315405
Chicago/Turabian StyleHu, Jing, Adel Al-Salihy, Bin Zhang, Siwei Li, and Ping Xu. 2022. "Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting" International Journal of Molecular Sciences 23, no. 23: 15405. https://doi.org/10.3390/ijms232315405









