ame-miR-34 Modulates the Larval Body Weight and Immune Response of Apis mellifera Workers to Ascosphara apis Invasion
Abstract
:1. Introduction
2. Results
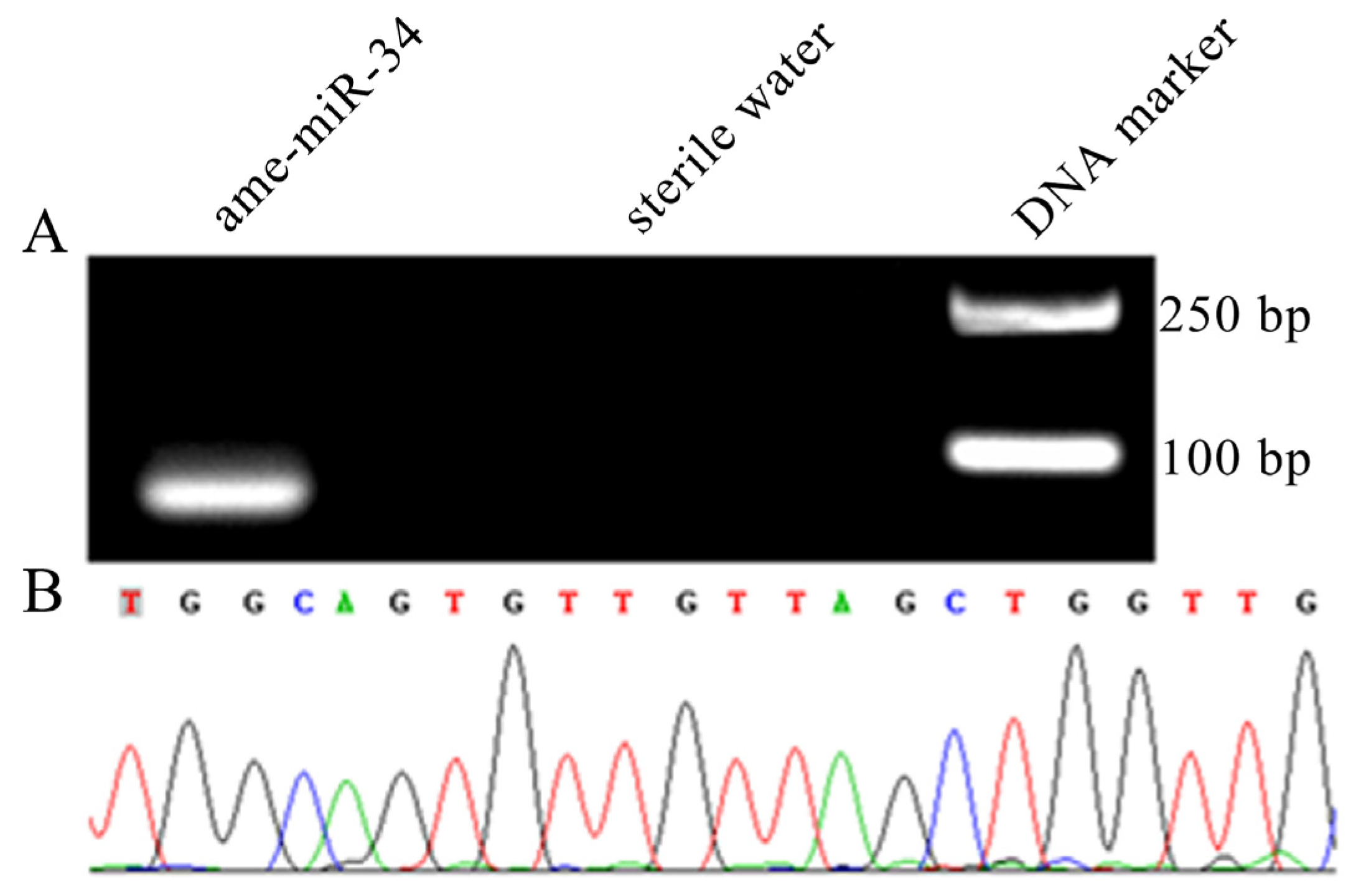
2.1. Molecular Verification of ame-miR-34 Expression and Sequence
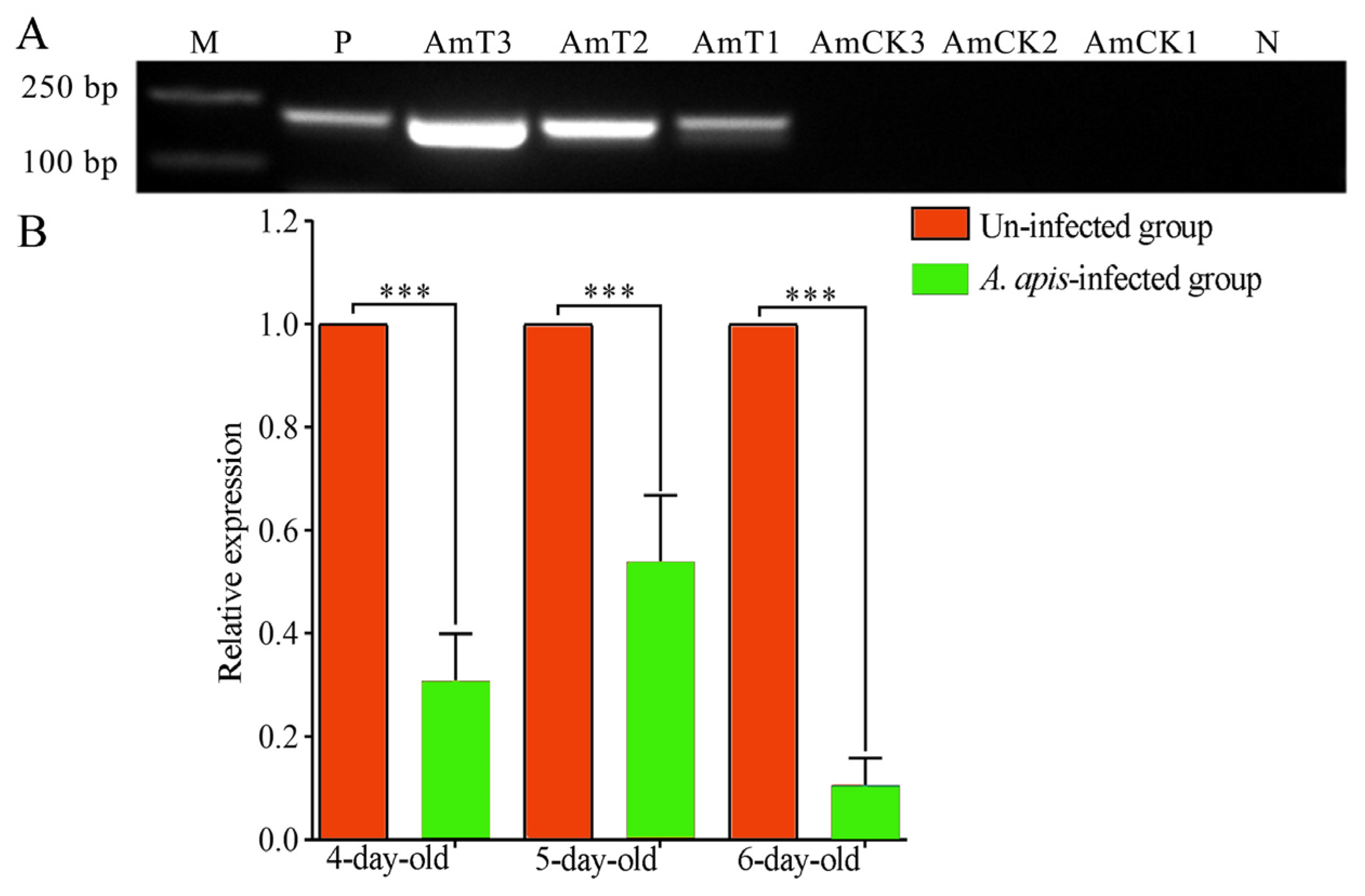
2.2. Expression Level of ame-miR-34 in the Larval Guts was Altered Due to A. apis Infection
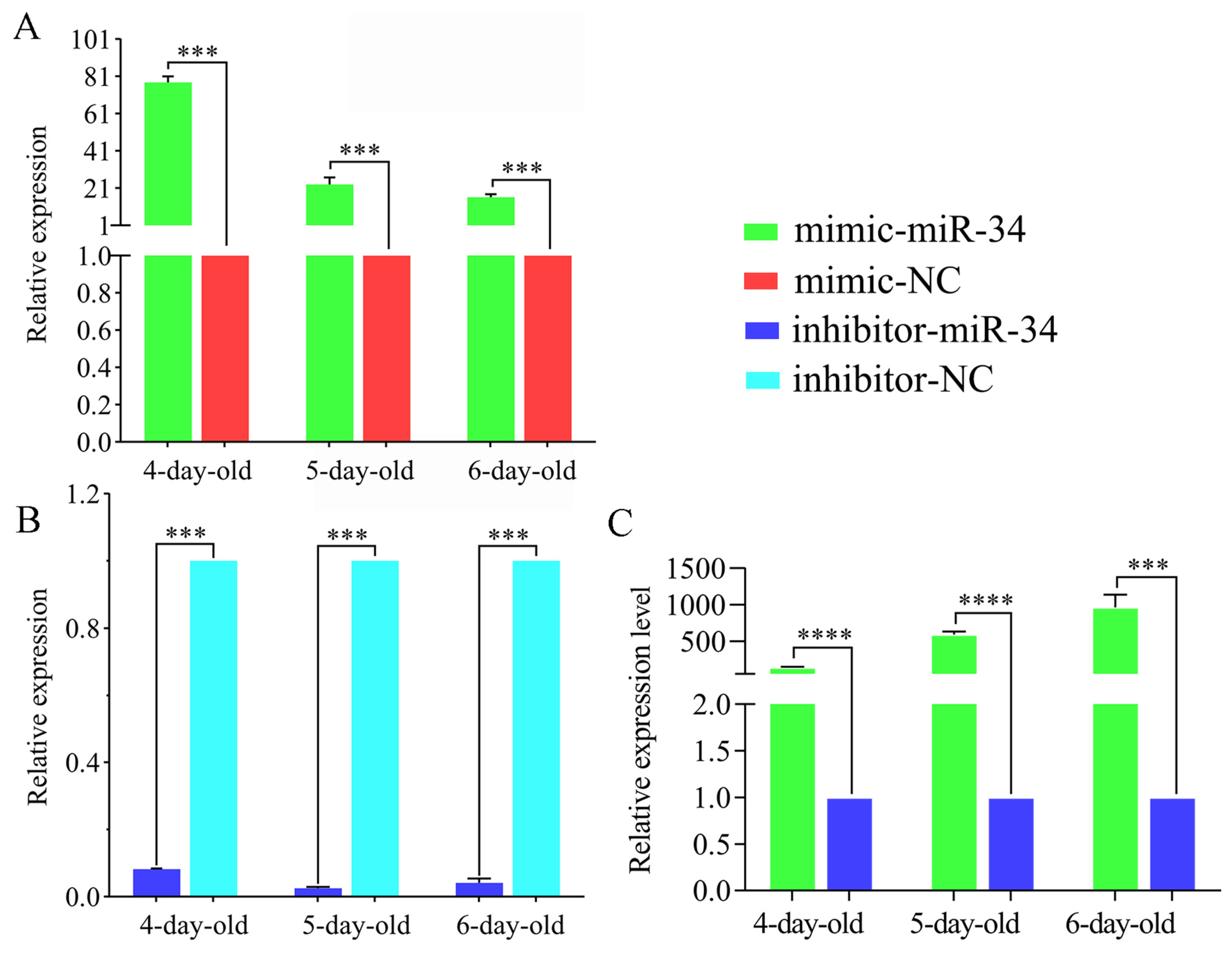
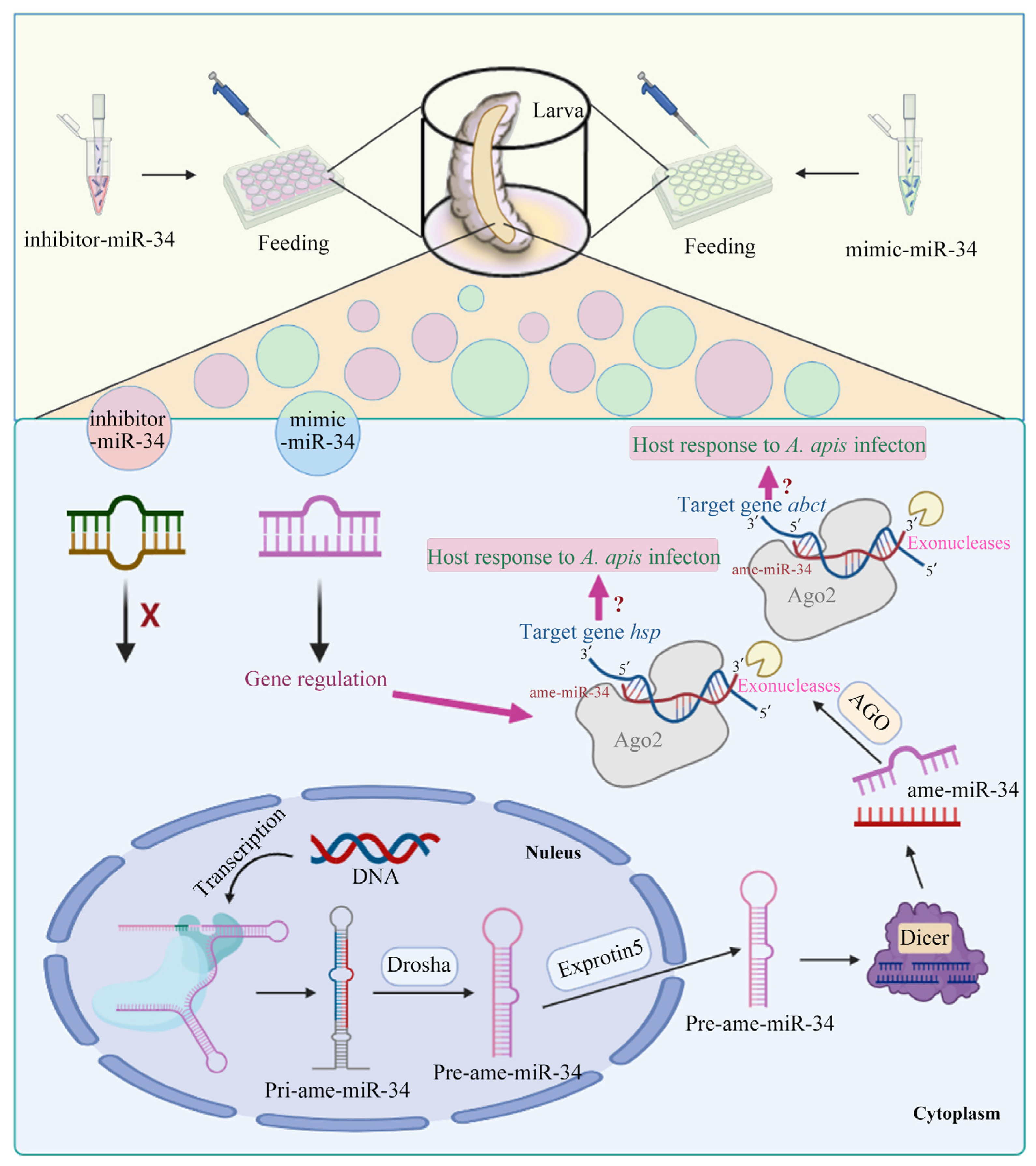
2.3. Overexpression and Knockdown of ame-miR-34 in Uninfected and A. apis-Infected Larval Guts
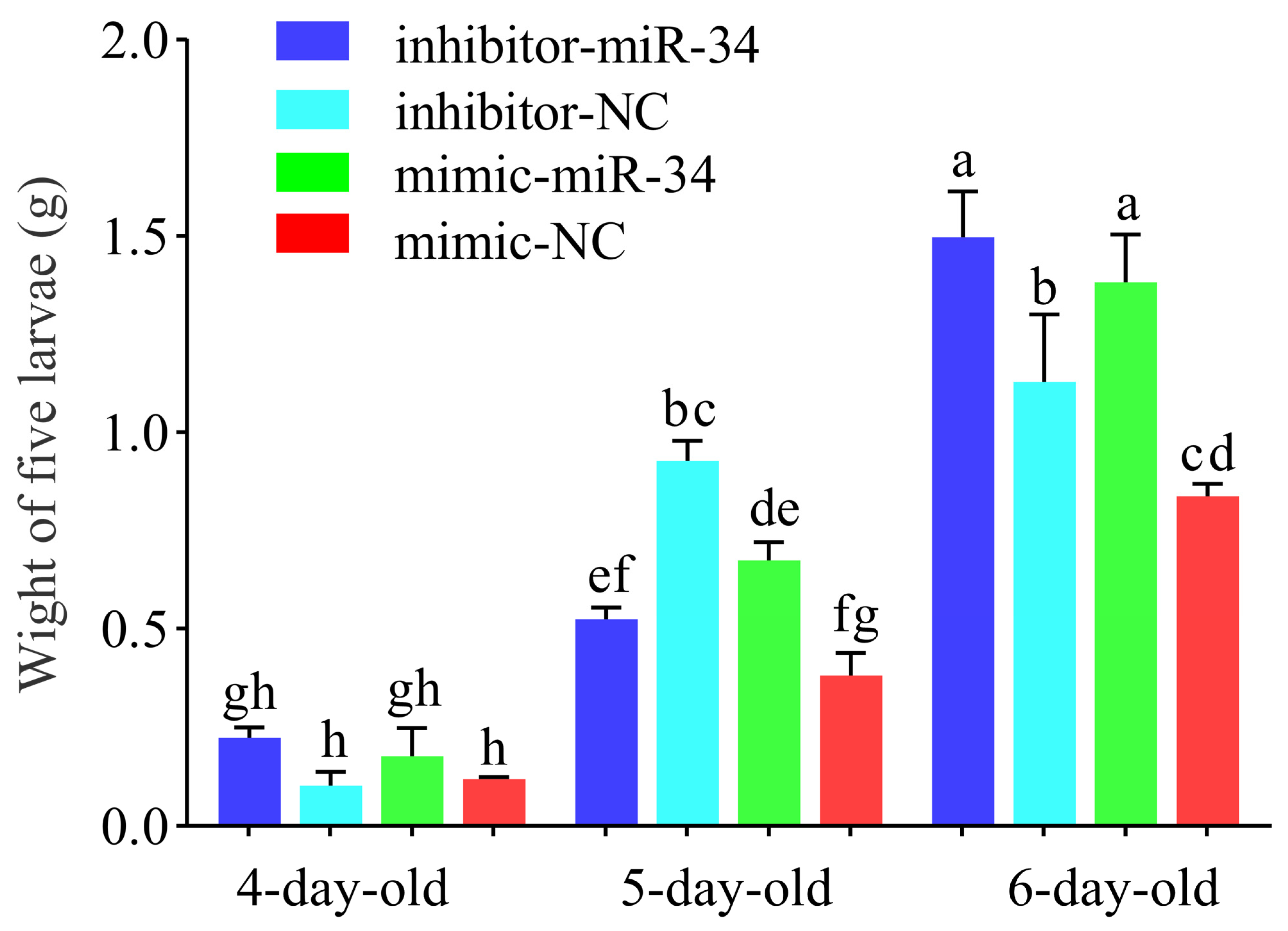
2.4. Effect of ame-miR-34 Overexpression and Knockdown on Body Weights of A. mellifera Larvae
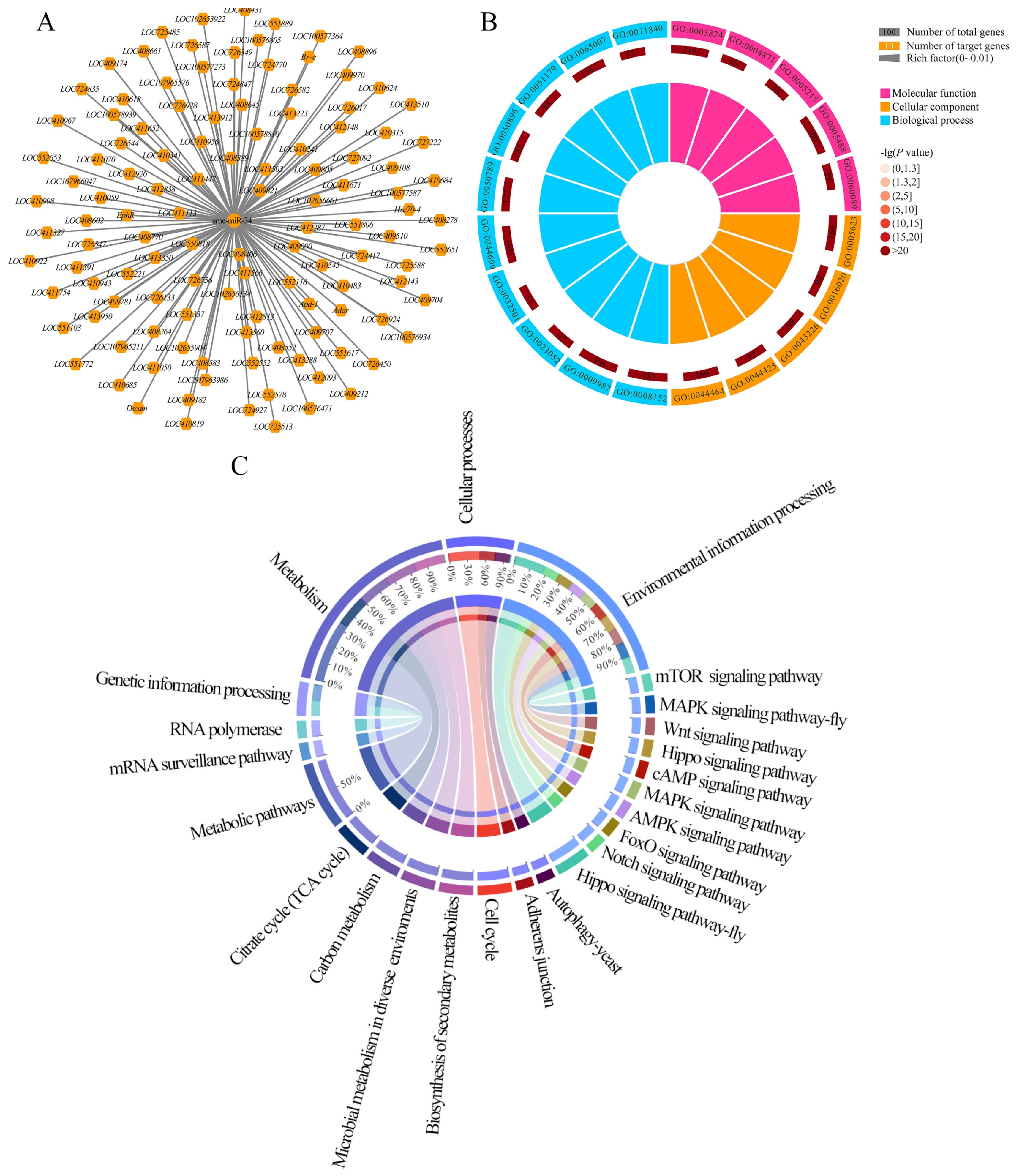
2.5. Analysis of ame-miR-34-Targeted Genes and the Corresponding Regulatory Network
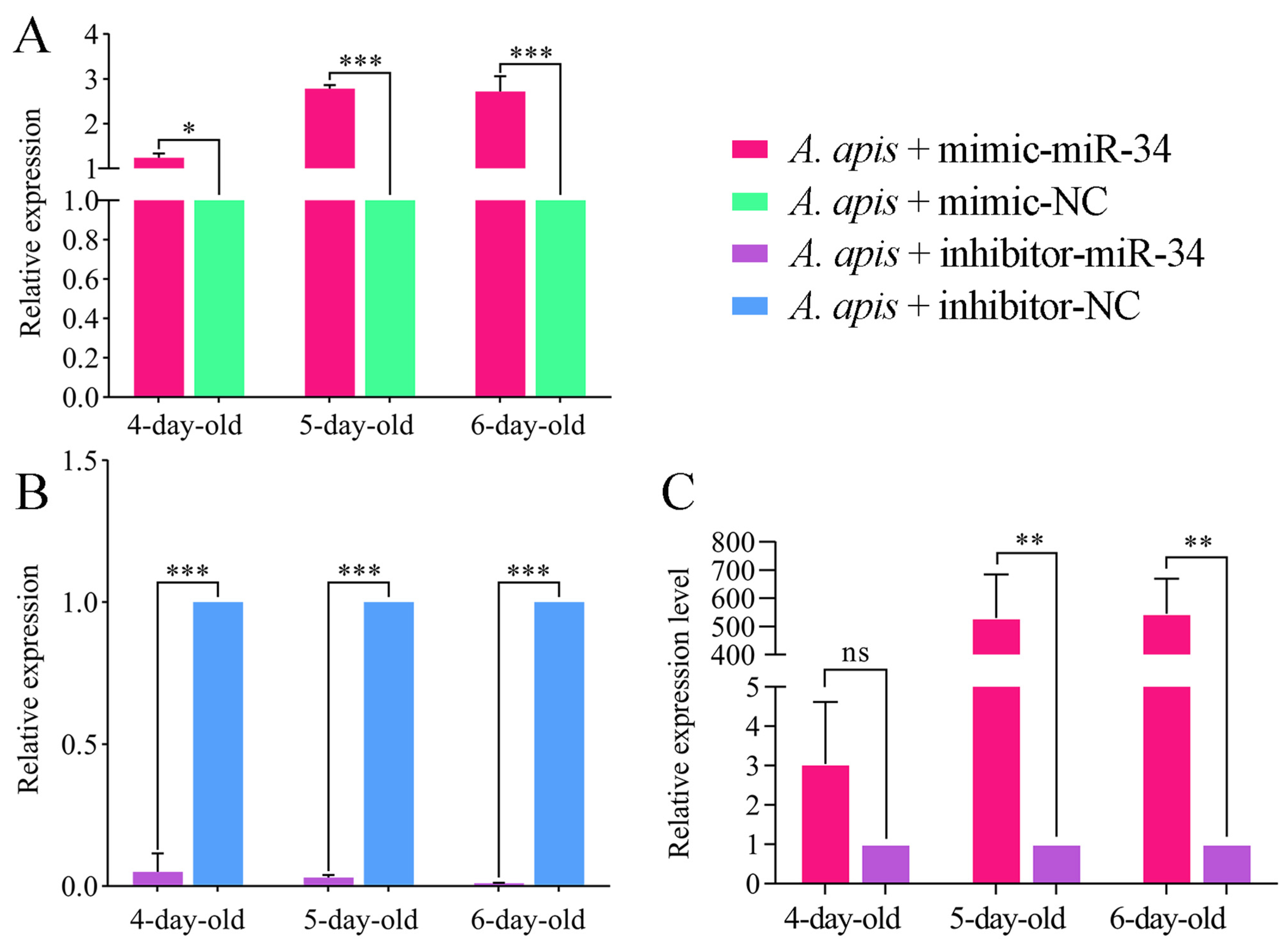
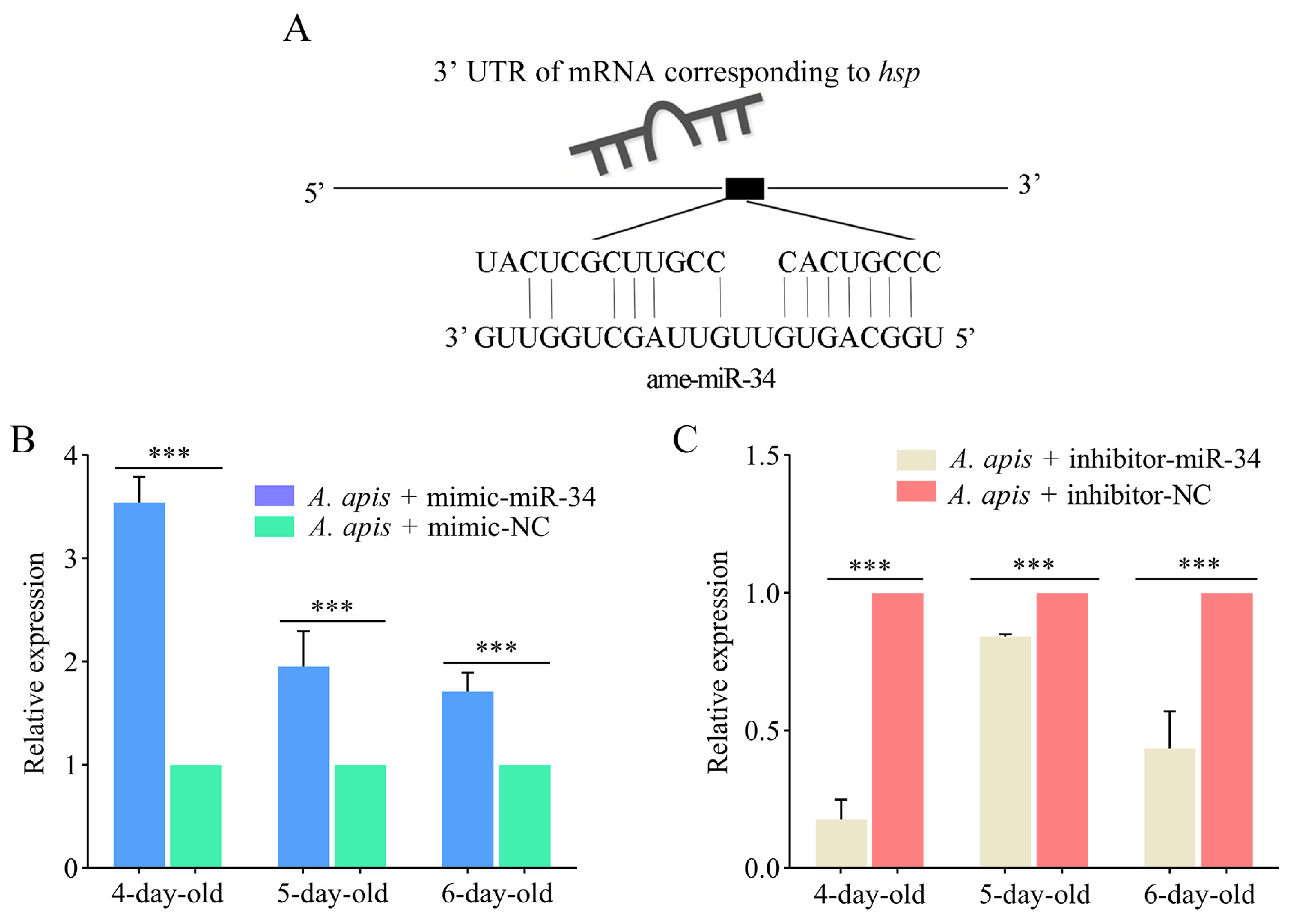
2.6. Effect of ame-miR-34 Overexpression and Knockdown on the Expression of hsp and abct in A. apis-Infected Larval Guts
3. Discussion
4. Materials and Methods
4.1. Honey Bee and Microsporidian Spore
4.2. Stem-Loop RT-PCR and Sanger Sequencing of ame-miR-34
4.3. Experimental Inoculation and Gut Sample Preparation
4.4. RT-qPCR Detection of ame-miR-34
4.5. Overexpression and Knockdown of ame-miR-34 in Normal Larval Guts
4.6. Measurement of Body Weight
4.7. RNA Isolation, cDNA Synthesis, and RT-qPCR Detection
4.8. Overexpression and Knockdown of ame-miR-34 in A. apis-Inoculated Larval Guts
4.9. Target Prediction and Analysis of ame-miR-34
4.10. RT-qPCR Determination of ame-miR-34-Targeted Genes
4.11. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morfin, N.; Anguiano-Baez, R.; Guzman-Novoa, E. Honey Bee (Apis mellifera) Immunity. Vet. Clin. North Am. Food Anim. Pract. 2021, 37, 521–533. [Google Scholar] [CrossRef]
- Zeng, Z.J. Apiculture, 3rd ed.; China Agriculture Press: Beijing, China, 2017; pp. 10–11. (In Chinese) [Google Scholar]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honey bees. J. Invertebr. Pathol. 2010, 103, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Klinge, C.M. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol. Cell. Endocrinol. 2015, 418 Pt 3, 273–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Freter, C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Heikkinen, L.; Wang, C.L.; Yang, Y.; Sun, H.Y.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, A.; Petrek, M. Roles of macrophage polarization and macrophage-derived miRNAs in pulmonary fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Shi, T.F.; Zhu, Y.J.; Liu, P.; Ye, L.; Jiang, X.C.; Cao, H.Q.; Yu, L.S. Age and behavior-dependent differential miRNAs expression in the hypopharyngeal glands of Honeybees (Apis mellifera L.). Insects 2021, 12, 764. [Google Scholar] [CrossRef]
- Vieira, J.; Freitas, F.; Cristino, A.S.; Moda, L.; Martins, J.R.; Bitondi, M.; Simões, Z.; Barchuk, A.R. miRNA-34 and miRNA-210 target hexamerin genes enhancing their differential expression during early brain development of honeybee (Apis mellifera) castes. Insect Mol. Biol. 2021, 30, 594–604. [Google Scholar] [CrossRef]
- Chen, X.; Fu, J. The microRNA miR-14 regulates egg-laying by targeting EcR in Honeybees (Apis mellifera). Insects 2021, 12, 351. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Li, W.F.; Li, Z.G.; Pan, J.; Yan, L.M.; Zhang, S.W.; Huang, Z.Y.; Su, S.K. Differences in microRNAs and their expressions between foraging and dancing honey bees, Apis mellifera L. J. Insect Physiol. 2012, 58, 1438–1443. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Guidugli-Lazzarini, K.R.; de Freitas, N.; Message, D.; Bitondi, M.; Simões, Z.; Teixeira, É.W. Immunity and physiological changes in adult honey bees (Apis mellifera) infected with Nosema ceranae: The natural colony environment. J. Insect Physiol. 2021, 131, 104237. [Google Scholar] [CrossRef] [PubMed]
- Cristino, A.S.; Barchuk, A.R.; Freitas, F.C.; Narayanan, R.K.; Biergans, S.D.; Zhao, Z.; Simoes, Z.L.; Reinhard, J.; Claudianos, C. Neuroligin-associated microRNA-932 targets actin and regulates memory in the honeybee. Nat. Commun. 2014, 5, 5529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, F.C.; Pires, C.V.; Claudianos, C.; Cristino, A.S.; Simões, Z.L. MicroRNA-34 directly targets pair-rule genes and cytoskeleton component in the honey bee. Sci. Rep. 2017, 7, 40884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michely, J.; Kraft, S.; Müller, U. miR-12 and miR-124 contribute to defined early phases of long-lasting and transient memory. Sci. Rep. 2017, 7, 7910. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Shi, T.F.; Yin, W.; Su, X.; Qi, L.; Huang, Z.Y.; Zhang, S.W.; Yu, L.S. The microRNA ame-miR-279a regulates sucrose responsiveness of forager honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2017, 90, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, K.M.; Coates, B.S.; Pittendrigh, B.R. Post-transcriptional modulation of cytochrome P450s, Cyp6g1 and Cyp6g2, by miR-310s cluster is associated with DDT-resistant Drosophila melanogaster strain 91-R. Sci. Rep. 2020, 10, 14394. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The role of microRNAs in organismal and skin aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef]
- Zhang, G.B.; Liu, Z.G.; Wang, J.; Fan, W. MiR-34 promotes apoptosis of lens epithelial cells in cataract rats via the TGF-β/Smads signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3485–3491. [Google Scholar]
- Ye, X.H.; Xu, L.; Li, X.; He, K.; Hua, H.X.; Cao, Z.H.; Xu, J.D.; Ye, W.Y.; Zhang, J.; Yuan, Z.T.; et al. miR-34 modulates wing polyphenism in planthopper. PLoS Genet. 2019, 15, e1008235. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.P.; Kurthkoti, K.; Chang, K.Y.; Li, J.L.; Ren, X.; Ni, J.Q.; Rana, T.M.; Zhou, R. miR-34 modulates innate immunity and ecdysone signaling in Drosophila. PLoS Pathog. 2016, 12, e1006034. [Google Scholar] [CrossRef]
- Lai, Y.W.; Chu, S.Y.; Wei, J.Y.; Cheng, C.Y.; Li, J.C.; Chen, P.L.; Chen, C.H.; Yu, H.H. Drosophila microRNA-34 impairs axon pruning of mushroom body γ neurons by downregulating the expression of ecdysone receptor. Sci. Rep. 2016, 6, 39141. [Google Scholar] [CrossRef]
- Zhu, K.G.; Liu, M.H.; Fu, Z.; Zhou, Z.; Kong, Y.; Liang, H.W.; Lin, Z.G.; Luo, J.; Zheng, H.Q.; Wan, P.; et al. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genet. 2017, 13, e1006946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.W.; Wang, J.; Long, Q.; Xu, Y.J.; Feng, R.R.; Liu, J.M.; Zhao, H.D.; Zhu, L.R.; Hou, H.Q.; Chen, D.F.; et al. Impact of overexpression and knockdown of ame-miR-13b on the expression of genes in larval gut of Apis mellifera ligustica. Acta Entomol. Sin. 2022, 65, 460–468. (In Chinese) [Google Scholar]
- Zhang, K.Y.; Liu, J.M.; Zhang, W.D.; Hu, Y.; Kang, Y.X.; Wang, Z.X.; Wang, S.Y.; Qian, J.J.; Zhao, X.; Zhang, J.X.; et al. ame-miR-79 negatively regulates the expression of target genes CYP450 and FG in the larval guts of Apis mellifera ligustica workers. Acta Entomol. Sin. 2022, 65, 1256–1265. (In Chinese) [Google Scholar]
- Hu, Y.; Zhang, W.D.; Wang, S.Y.; Zhang, K.Y.; Ren, Z.M.; Ji, T.; Lin, Z.G.; Zhang, H.X.; Chen, D.F.; Guo, R. Regulation of the expression of target genes USP and P300 by ame-miR-bantam in the larval gut of Apis mellifera ligustica workers. Acta Entomol. Sin. 2022, 65, 1401–1410. (In Chinese) [Google Scholar]
- Zhang, Q.; Dou, W.; Taning, C.N.T.; Yu, S.S.; Yuan, G.R.; Shang, F.; Smagghe, G.; Wang, J.J. miR-309a is a regulator of ovarian development in the oriental fruit fly Bactrocera dorsalis. PLoS Genet. 2022, 18, e1010411. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, X.; Li, Y.; Zhang, J.; Fu, Y. miR-34-5p, encoded by Spodoptera frugiperda, participates in anti-baculovirus by regulating innate immunity in the insect host. Int. J. Biol. Macromol. 2022, 222, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, X.; Liu, X.; Michaud, J.P.; Wu, Y.; Zhang, H.; Li, Y.; Li, Z. Molecular characterization of insulin receptor (IR) in oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae), and elucidation of its regulatory roles in glucolipid homeostasis and metamorphosis through interaction with miR-982490. Insect Mol. Biol. 2022, 31, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Takamatsu, M.; Kobayashi, S.; Ogawa, M.; Shiwa, Y.; Watanabe, S.; Chibazakura, T.; Yoshikawa, H. Novel heat shock response mechanism mediated by the initiation nucleotide of transcription. J. Gen Appl. Microbiol. 2022, 68, 95–108. [Google Scholar] [CrossRef]
- Musa, M.; Dionisio, P.A.; Casqueiro, R.; Milosevic, I.; Raimundo, N.; Krisko, A. Lack of peroxisomal catalase affects heat shock response in Caenorhabditis elegans. Life Sci. Alliance 2022, 6, e202201737. [Google Scholar] [CrossRef]
- Al-Ghzawi, A.A.A.; Al-Zghoul, M.B.; Zaitoun, S.; Al-Omary, I.M.; Alahmad, N.A. Dynamics of heat shock proteins and heat shock factor expression during heat stress in daughter workers in pre-heat-treated (rapid heat hardening) Apis mellifera mother queens. J. Therm. Biol. 2022, 104, 103194. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Miao, J.; He, J.; Chen, Q.; Qian, J.; Li, H.; Xu, Y.; Ma, D.; Zhao, Y.; Tian, X.; et al. Characterization of the wheat heat shock factor TaHsfA2e-5D conferring heat and drought tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2784. [Google Scholar] [CrossRef] [PubMed]
- Dores-Silva, P.R.; Cauvi, D.M.; Coto, A.; Silva, N.; Borges, J.C.; De Maio, A. Human heat shock cognate protein (HSC70/HSPA8) interacts with negatively charged phospholipids by a different mechanism than other HSP70s and brings HSP90 into membranes. Cell Stress Chaperones. 2021, 26, 671–684. [Google Scholar] [CrossRef]
- Tian, F.; Hu, X.L.; Yao, T.; Yang, X.; Chen, J.G.; Lu, M.Z.; Zhang, J. Recent advances in the roles of HSFs and HSPs in heat stress response in woody plants. Front. Plant Sci. 2021, 12, 704905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.F.; Cai, J.Z.; Wu, J.F.; Tang, Y.T.; Zhao, K.; Liang, F.; Li, F.L.; Yang, X.Y.; He, Z.H.; Billiar, T.R.; et al. Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science 2022, 376, 609–615. [Google Scholar] [CrossRef]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy--cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cao, H.; Kimari, M.; Maronitis, G.; Williams, M.J.; Schiöth, H.B. Multidrug resistance like protein 1 activity in malpighian tubules regulates lipid homeostasis in Drosophila. Membranes 2021, 11, 432. [Google Scholar] [CrossRef]
- Wang, L.X.; Tao, S.; Zhang, Y.C.; Pei, X.G.; Gao, Y.; Song, X.Y.; Yu, Z.T.; Gao, C.F. Overexpression of ATP-binding cassette transporter Mdr49-like confers resistance to imidacloprid in the field populations of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2022, 78, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Sinakevitch, I.; Lei, H.; Smith, B.H. Comparison of RNAi knockdown effect of tyramine receptor 1 induced by dsRNA and siRNA in brains of the honey bee, Apis mellifera. J. Insect Physiol. 2018, 111, 47–52. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Heerman, M.C.; Cook, S.C.; Evans, J.D.; DeGrandi-Hoffman, G.; Banmeke, O.; Zhang, Y.; Huang, S.; Hamilton, M.; Chen, Y.P. Transferrin-mediated iron sequestration suggests a novel therapeutic strategy for controlling Nosema disease in the honey bee, Apis mellifera. PLoS Pathog. 2021, 17, e1009270. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Yu, K.J.; Zhao, X.; Qian, J.J.; Zhao, H.D.; Zhang, J.; Zhang, Y.; Zhao, H.X.; Xu, X.J.; Luo, Q.; et al. Bioinformatic analysis and functional study of nkd gene in larvae of Apis mellifera ligustica workers. Acta Microbiol. Sin. 2022, 62, 5005–5017. (In Chinese) [Google Scholar]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, D.F.; Chen, H.Z.; Fu, Z.M.; Xiong, C.L.; Hou, C.S.; Zheng, Y.Z.; Guo, Y.L.; Wang, H.P.; Du, Y.; et al. Systematic investigation of circular RNAs in Ascosphaera apis, a fungal pathogen of honeybee larvae. Gene 2018, 678, 17–22. [Google Scholar] [CrossRef]
- Guo, R.; Chen, D.F.; Xiong, C.L.; Hou, C.S.; Zheng, Y.Z.; Fu, Z.M.; Diao, Q.Y.; Zhang, L.; Wang, H.Q.; Hou, Z.X.; et al. Identification of long non-coding RNAs in the chalkbrood disease pathogen Ascospheara apis. J. Invertebr. Pathol. 2018, 156, 1–5. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Fu, Z.M.; Long, Q.; Du, Y.; Zhang, W.D.; Hu, Y.; Zhao, X.; Shi, X.Y.; Xu, X.J.; Chen, D.F.; et al. Expression profiles and potential function of three miRNAs during the pupal development process of Apis mellifera ligustica worker. Acta Entomol. Sin. 2018, 65, 53–62. (In Chinese) [Google Scholar]
- Chen, D.F.; Guo, R.; Xu, X.J.; Xiong, C.L.; Liang, Q.; Zheng, Y.Z.; Luo, Q.; Zhang, Z.N.; Huang, Z.J.; Kumar, D.; et al. Uncovering the immune responses of Apis mellifera ligustica larval gut to Ascosphaera apis infection utilizing transcriptome sequencing. Gene 2017, 621, 40–50. [Google Scholar] [CrossRef]
- Jensen, A.B.; Aronstein, K.; Flores, J.M.; Vojvodic, S.; Palacio, M.A.; Spivak, M. Standard methods for fungal brood disease research. J. Apic. Res. 2013, 52. [Google Scholar] [CrossRef] [Green Version]
- Brødsgaard, C.F.; Ritter, W.; Hansen, H. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae spores. Apidologie 1998, 29, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Chen, D.; Diao, Q.; Xiong, C.; Zheng, Y.; Hou, C. Transcriptomic investigation of immune responses of the Apis cerana cerana larval gut infected by Ascosphaera apis. J. Invertebr. Pathol. 2019, 166, 107210. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.R.; Li, X.M.; Pu, Q.; Luo, C.Y.; Xu, L.L.; Peng, X.Y.; Liu, S.P. Genetic manipulation of micrornas in the silk gland of Silkworm, Bombyx Mori. Biol. Proced. Online 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Mussen, E.; Fong, A.; Montague, M.A.; Tyler, T. Effect of chlortetracycline of honeybee worker larvae reared in vitro. J. Invertebr. Pathol. 1992, 60, 127–133. [Google Scholar] [CrossRef]
- Borsuk, G.; Ptaszyńska, A.A.; Olszewski, K.; Domaciuk, M.; Krutmuang, P.; Paleolog, J. A new method for quick and easy hemolymph collection from apidae adults. PLoS ONE 2017, 12, e0170487. [Google Scholar] [CrossRef]
- Xiong, C.L.; Du, Y.; Feng, R.R.; Jiang, H.B.; Shi, X.Y.; Wang, H.P.; Fan, X.X.; Wang, J.; Zhu, Z.W.; Fan, Y.C.; et al. Differential expression pattern and regulation network of microRNAs in Ascosphaera apis invading Apis cerana cerana 6-day-old larvae. Acta Microbiol. Sin. 2020, 60, 992–1009. (In Chinese) [Google Scholar]
- Jagla, T.; Dubińska-Magiera, M.; Poovathumkadavil, P.; Daczewska, M.; Jagla, K. Developmental expression and functions of the small heat shock proteins in Drosophila. Int. J. Mol. Sci. 2018, 19, 3441. [Google Scholar] [CrossRef] [PubMed]

| Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| ame-miR-34-F | TGGCAGTGTTGTTA | Reverse transcription of ame-miR-34 |
| ame-miR-34-Loop | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCAACCAGC | |
| ame-miR-34-R | CTCAACTGGTGTCGTGGA | |
| AmU6-F | GTTAGGCTTTGACGATTTCG | Internal reference for qPCR of ame-miR-34 |
| AmU6-R | GGCATTTCTCCACCAGGTA | |
| actin-F | ATGCCAACACTGTCCTTTCTGG | Internal reference for qPCR of target genes |
| actin-R | GACCCACCAATCCATACGGA | |
| hsp-F | TCCTGTGTTGGTGTATTCCAGCATG | qPCR detection |
| hsp-R | GCAACTTGGTTCTTGGCAGCATC | |
| abct-F | ACGACGACTATACCTGGCAGTGG | |
| abct-R | CAGTTGAGACGAGACAGCATCCG |
| Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| mimic-miR-34-sense | UGGCAGUGUUGUUAGCUGGUUG | ame-miR-34 overexpression |
| mimic-miR-34-antisense | ACCAGCUAACAACACUGCCAUU | |
| inhibitor-miR-34 | CAACCAGCUAACAACACUGCCA | ame-miR-34 knockdown |
| mimic-NC-sense | UUCUCCGAACGUGUCACGUTT | Negative control for ame-miR-34 overexpression |
| mimic-NC-antisense | ACGUGACACGUUCGGAGAATT | |
| inhibitor-NC | CAGUACUUUUGUGUAGUACAA | Negative control for ame-miR-34 knockdown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Guo, Y.; Fan, X.; Zhao, H.; Zhang, Y.; Guo, S.; Jing, X.; Liu, Z.; Feng, P.; Liu, X.; et al. ame-miR-34 Modulates the Larval Body Weight and Immune Response of Apis mellifera Workers to Ascosphara apis Invasion. Int. J. Mol. Sci. 2023, 24, 1214. https://doi.org/10.3390/ijms24021214
Wu Y, Guo Y, Fan X, Zhao H, Zhang Y, Guo S, Jing X, Liu Z, Feng P, Liu X, et al. ame-miR-34 Modulates the Larval Body Weight and Immune Response of Apis mellifera Workers to Ascosphara apis Invasion. International Journal of Molecular Sciences. 2023; 24(2):1214. https://doi.org/10.3390/ijms24021214
Chicago/Turabian StyleWu, Ying, Yilong Guo, Xiaoxue Fan, Haodong Zhao, Yiqiong Zhang, Sijia Guo, Xin Jing, Zhitan Liu, Peilin Feng, Xiaoyu Liu, and et al. 2023. "ame-miR-34 Modulates the Larval Body Weight and Immune Response of Apis mellifera Workers to Ascosphara apis Invasion" International Journal of Molecular Sciences 24, no. 2: 1214. https://doi.org/10.3390/ijms24021214







