Safety and Immunogenicity of a Chimeric Subunit Vaccine against Shiga Toxin-Producing Escherichia coli in Pregnant Cows
Abstract
:1. Introduction
2. Results
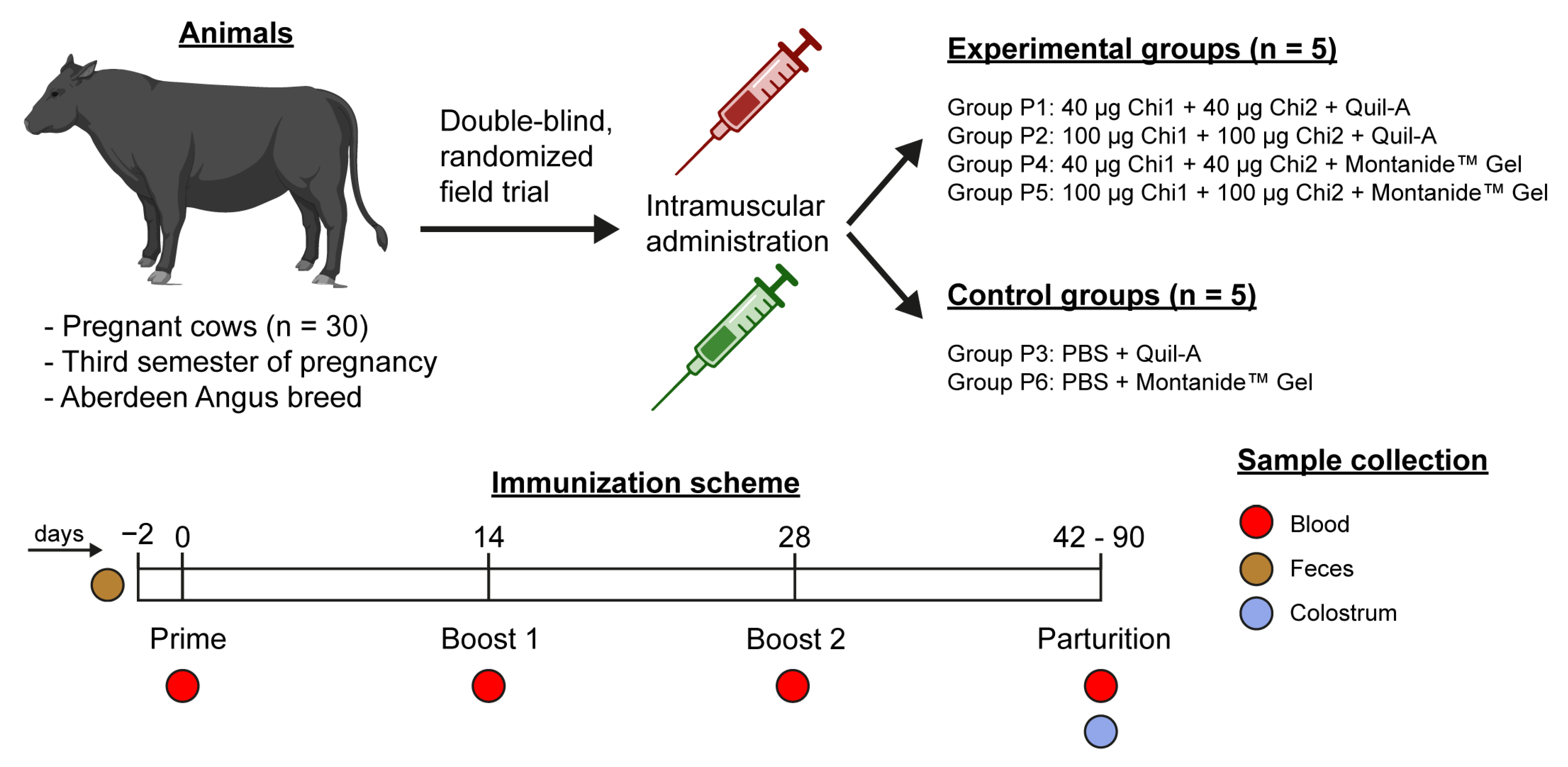
2.1. Experimental Design
2.2. Safety
2.3. Serum Antibody Response
2.4. Colostrum Antibody Response
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Production of Vaccine Formulations and Immunization Protocol
4.3. Sample Collection
4.4. Hematological and Blood Biochemical Parameters
4.5. Humoral Immune Responses
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Tesh, V.L. Roles of shiga toxins in immunopathology. Toxins 2019, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; Schijvens, A.M.; Rossen, J.W.A.; Kooistra-Smid, A.M.D.; Schreuder, M.F.; van de Kar, N.C.A.J. Unusual severe case of hemolytic uremic syndrome due to Shiga toxin 2d-producing E. coli O80:H2. Pediatr. Nephrol. 2017, 32, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Gaytán, M.O.; Martínez-Santos, V.I.; Soto, E.; González-Pedrajo, B. Type Three Secretion System in Attaching and Effacing Pathogens. Front. Cell Infect. Microbiol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Montero, D.A.; Velasco, J.; Del Canto, F.; Puente, J.L.; Padola, N.L.; Rasko, D.A.; Farfán, M.; Salazar, J.C.; Vidal, R. Locus of Adhesion and Autoaggregation (LAA), a pathogenicity island present in emerging Shiga Toxin-producing Escherichia coli strains. Sci. Rep. 2017, 7, 7011. [Google Scholar] [CrossRef]
- Michelacci, V.; Tozzoli, R.; Caprioli, A.; Martínez, R.; Scheutz, F.; Grande, L.; Sánchez, S.; Morabito, S. A new pathogenicity island carrying an allelic variant of the Subtilase cytotoxin is common among Shiga toxin producing Escherichia coli of human and ovine origin. Clin. Microbiol. Infect. 2013, 19, E149–E156. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Del Canto, F.; Velasco, J.; Colello, R.; Padola, N.L.; Salazar, J.C.; Martin, C.S.; Oñate, A.; Blanco, J.; Rasko, D.A.; et al. Cumulative acquisition of pathogenicity islands has shaped virulence potential and contributed to the emergence of LEE-negative Shiga toxin-producing Escherichia coli strains. Emerg. Microbes Infect. 2019, 8, 486–502. [Google Scholar] [CrossRef]
- Persad, A.K.; LeJeune, J.T. Animal Reservoirs of Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2, 1–14. [Google Scholar] [CrossRef]
- Etcheverría, A.I.; Padola, N.L. Shiga toxin-producing Escherichia coli: Factors involved in virulence and cattle colonization. Virulence 2013, 4, 366–372. [Google Scholar] [CrossRef]
- Jahan, N.A.; Lindsey, L.L.; Larsen, P.A. The Role of Peridomestic Rodents as Reservoirs for Zoonotic Foodborne Pathogens. Vector-Borne Zoonotic Dis. 2021, 21, 133–148. [Google Scholar] [CrossRef]
- Blanco Crivelli, X.; Rumi, M.V.; Carfagnini, J.C.; Degregorio, O.; Bentancor, A.B. Synanthropic rodents as possible reservoirs of shigatoxigenic Escherichia coli strains. Front. Cell Infect. Microbiol. 2012, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Pruimboom-Brees, I.M.; Morgan, T.W.; Ackermann, M.R.; Nystrom, E.D.; Samuel, J.E.; Cornick, N.A.; Moon, H.W. Cattle lack vascular receptors for Escherichia coli 0157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 2000, 97, 10325–10329. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.A.; Smith, D.R. Attaching-effacing Escherichia coli Infections in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Dean-Nystrom, E.A.; Bosworth, B.T.; Moon, H.W.; O’Brien, A.D. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 1998, 66, 4560–4563. [Google Scholar] [CrossRef] [PubMed]
- Menge, C. The Role of Escherichia coli Shiga Toxins in STEC Colonization of Cattle. Toxins 2020, 12, 607. [Google Scholar] [CrossRef]
- Hoffman, M.A.; Menge, C.; Casey, T.A.; Laegreid, W.; Bosworth, B.T.; Dean-Nystrom, E.A. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 2006, 13, 1322–1327. [Google Scholar] [CrossRef]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; McKendrick, I.J.; Smith, D.G.E.; Gally, D.L. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef]
- Hamm, K.; Barth, S.A.; Stalb, S.; Geue, L.; Liebler-Tenorio, E.; Teifke, J.P.; Lange, E.; Tauscher, K.; Kotterba, G.; Bielaszewska, M.; et al. Experimental Infection of Calves with Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2016, 6, 32812. [Google Scholar] [CrossRef]
- Cornick, N.A.; Booher, S.L.; Moon, H.W. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 2002, 70, 2704–2707. [Google Scholar] [CrossRef]
- Dziva, F.; van Diemen, P.M.; Stevens, M.P.; Smith, A.J.; Wallis, T.S. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 2004, 150, 3631–3645. [Google Scholar] [CrossRef] [Green Version]
- Kudva, I.T.; Hovde, C.J.; John, M. Adherence of Non-O157 shiga toxin-producing Escherichia coli to bovine recto-anal junction squamous epithelial cells appears to be mediated by mechanisms distinct from those used by O157. Foodborne Pathog. Dis. 2013, 10, 375–381. [Google Scholar] [CrossRef]
- Barth, S.A.; Menge, C.; Eichhorn, I.; Semmler, T.; Wieler, L.H.; Pickard, D.; Belka, A.; Berens, C.; Geue, L. The accessory genome of Shiga toxin-producing Escherichia coli defines a persistent colonization type in cattle. Appl. Environ. Microbiol. 2016, 82, 5455–5464. [Google Scholar] [CrossRef]
- Matthews, L.; Reeve, R.; Gally, D.L.; Low, J.C.; Woolhouse, M.E.J.; McAteer, S.P.; Locking, M.E.; Chase-Topping, M.E.; Haydon, D.T.; Allison, L.J.; et al. Predicting the public health benefit of vaccinating cattle against Escherichia coli O157. Proc. Natl. Acad. Sci. USA 2013, 110, 16265–16270. [Google Scholar] [CrossRef]
- Walle, K.V.; Vanrompay, D.; Cox, E. Bovine innate and adaptive immune responses against Escherichia coli O157: H7 and vaccination strategies to reduce faecal shedding in ruminants. Vet. Immunol. Immunopathol. 2013, 152, 109–120. [Google Scholar] [CrossRef]
- O’Ryan, M.; Vidal, R.; del Canto, F.; Carlos Salazar, J.; Montero, D. Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part II: Vaccines for Shigella, Salmonella, enterotoxigenic E. coli (ETEC) enterohemorragic E. coli (EHEC) and Campylobacter jejuni. Hum. Vaccin. Immunother. 2015, 11, 601–619. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; Renter, D.G.; Cull, C.A.; Paddock, Z.D.; Shi, X.; Nagaraja, T.G. Fecal shedding of non-O157 serogroups of shiga toxin-producing Escherichia coli in feedlot cattle vaccinated with an Escherichia coli O157:H7 SRP vaccine or fed a lactobacillus-based direct-fed microbial. J. Food Prot. 2014, 77, 732–737. [Google Scholar] [CrossRef]
- Montero, D.; Orellana, P.; Gutiérrez, D.; Araya, D.; Salazar, J.C.; Prado, V.; Oñate, A.; Del Canto, F.; Vidal, R. Immunoproteomic analysis to identify Shiga toxin-producing Escherichia coli outer membrane proteins expressed during human infection. Infect. Immun. 2014, 82, 4767–4777. [Google Scholar] [CrossRef]
- Colello, R.; Vélez, M.V.; González, J.; Montero, D.A.; Bustamante, A.V.; Del Canto, F.; Etcheverría, A.I.; Vidal, R.; Padola, N.L. First report of the distribution of Locus of Adhesion and Autoaggregation (LAA) pathogenicity island in LEE-negative Shiga toxin-producing Escherichia coli isolates from Argentina. Microb. Pathog. 2018, 123, 259–263. [Google Scholar] [CrossRef]
- Montero, D.A.; Del Canto, F.; Salazar, J.C.; Céspedes, S.; Cádiz, L.; Arenas-Salinas, M.; Reyes, J.; Oñate, Á.; Vidal, R.M. Immunization of mice with chimeric antigens displaying selected epitopes confers protection against intestinal colonization and renal damage caused by Shiga toxin-producing Escherichia coli. NPJ Vaccines 2020, 5, 20. [Google Scholar] [CrossRef]
- Vilte, D.A.; Larzábal, M.; Cataldi, Á.A.; Mercado, E.C. Bovine colostrum contains immunoglobulin G antibodies against intimin, EspA, and EspB and inhibits hemolytic activity mediated by the type three secretion system of attaching and effacing Escherichia coli. Clin. Vaccine Immunol. 2008, 15, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Widiasih, D.A.; Matsuda, I.; Omoe, K.; Hu, D.L.; Sugii, S.; Shinagawa, K. Passive transfer of antibodies to shiga toxin-producing Escherichia coli O26, O111 and O157 antigens in neonatal calves by feeding colostrum. J. Vet. Med. Sci. 2004, 66, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, B.C.; Gerhardt, E.; Tironi Farinati, C.; Abdala, A.; Galarza, R.; Vilte, D.A.; Ibarra, C.; Cataldi, A.; Mercado, E.C. Vaccination of pregnant cows with EspA, EspB, γ-intimin, and Shiga toxin 2 proteins from Escherichia coli O157: H7 induces high levels of specific colostral antibodies that are transferred to newborn calves. J. Dairy Sci. 2012, 95, 3318–3326. [Google Scholar] [CrossRef] [PubMed]
- Martorelli, L.; Garimano, N.; Fiorentino, G.A.; Vilte, D.A.; Garbaccio, S.G.; Barth, S.A.; Menge, C.; Ibarra, C.; Palermo, M.S.; Cataldi, A. Efficacy of a recombinant Intimin, EspB and Shiga toxin 2B vaccine in calves experimentally challenged with Escherichia coli O157:H7. Vaccine 2018, 36, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, B.C.; Vilte, D.A.; Larzábal, M.; Abdala, A.; Galarza, R.; Zotta, E.; Ibarra, C.; Mercado, E.C.; Cataldi, A. Physiopathological effects of Escherichia coli O157: H7 inoculation in weaned calves fed with colostrum containing antibodies to EspB and Intimin. Vaccine 2014, 32, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.N.; Chamorro-Veloso, N.; Costa, P.; Cádiz, L.; Del Canto, F.; Venegas, S.A.; López Nitsche, M.; Coloma-Rivero, R.F.; Montero, D.A.; Vidal, R.M. Deciphering Additional Roles for the EF-Tu, l-Asparaginase II and OmpT Proteins of Shiga Toxin-Producing Escherichia coli. Microorganisms 2020, 8, 1184. [Google Scholar] [CrossRef]
- Torres, A.G.; Perna, N.T.; Burland, V.; Ruknudin, A.; Blattner, F.R.; Kaper, J.B. Characterization of Cah, a calcium-binding and heat-extractable autotransporter protein of enterohaemorrhagic Escherichia coli. Mol. Microbiol. 2002, 45, 951–966. [Google Scholar] [CrossRef]
- Dalsgaard, K. Saponin adjuvants. Arch. Fuer Die Gesamte Virusforsch. 1974, 44, 243–254. [Google Scholar] [CrossRef]
- Parker, R.; Deville, S.; Dupuis, L.; Bertrand, F.; Aucouturier, J. Adjuvant formulation for veterinary vaccines: MontanideTM Gel safety profile. Procedia Vaccinol. 2009, 1, 140–147. [Google Scholar] [CrossRef]
- Mellado, M.; López, R.; de Santiago, Á.; Veliz, F.G.; Macías-Cruz, U.; Avendaño-Reyes, L.; García, J.E. Climatic conditions, twining and frequency of milking as factors affecting the risk of fetal losses in high-yielding Holstein cows in a hot environment. Trop. Anim. Health Prod. 2016, 48, 1247–1252. [Google Scholar] [CrossRef]
- Daniel Givens, M.; Marley, M.S.D. Infectious causes of embryonic and fetal mortality. Theriogenology 2008, 70, 270–285. [Google Scholar] [CrossRef]
- Albuja, C.; Ortiz, O.; López, C.; Hernández-Cerón, J. Economic impact of pregnancy loss in an intensive dairy farming system. Vet. Mex. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Diskin, M.G.; Waters, S.M.; Parr, M.H.; Kenny, D.A. Pregnancy losses in cattle: Potential for improvement. Reprod. Fertil. Dev. 2016, 28, 83–93. [Google Scholar] [CrossRef]
- Padola, N.L.; Sanz, M.E.; Blanco, J.E.; Blanco, M.; Blanco, J.; Etcheverria, A.I.; Arroyo, G.H.; Usera, M.A.; Parma, A.E. Serotypes and virulence genes of bovine Shigatoxigenic Escherichia coli (STEC) isolated from a feedlot in Argentina. Vet. Microbiol. 2004, 100, 3–9. [Google Scholar] [CrossRef]
- Asper, D.J.; Karmali, M.A.; Townsend, H.; Rogan, D.; Potter, A.A. Serological response of shiga toxin-producing Escherichia coli type III secreted proteins in sera from vaccinated rabbits, naturally infected cattle, and humans. Clin. Vaccine Immunol. 2011, 18, 1052–1057. [Google Scholar] [CrossRef]
- Baintner, K. Transmission of antibodies from mother to young: Evolutionary strategies in a proteolytic environment. Vet. Immunol. Immunopathol. 2007, 117, 153–161. [Google Scholar] [CrossRef]
- Chucri, T.M.; Monteiro, J.M.; Lima, A.R.; Salvadori, M.L.B.; Junior, J.R.K.; Miglino, M.A. A review of immune transfer by the placenta. J. Reprod. Immunol. 2010, 87, 14–20. [Google Scholar] [CrossRef]
- Albanese, A.; Sacerdoti, F.; Seyahian, E.A.; Amaral, M.M.; Fiorentino, G.; Fernandez Brando, R.; Vilte, D.A.; Mercado, E.C.; Palermo, M.S.; Cataldi, A.; et al. Immunization of pregnant cows with Shiga toxin-2 induces high levels of specific colostral antibodies and lactoferrin able to neutralize E. coli O157:H7 pathogenicity. Vaccine 2018, 36, 1728–1735. [Google Scholar] [CrossRef]
- Engelen, F.; Thiry, D.; Devleesschauwer, B.; Mainil, J.; De Zutter, L.; Cox, E. Occurrence of ‘gang of five’ Shiga toxin-producing Escherichia coli serogroups on Belgian dairy cattle farms by overshoe sampling. Lett. Appl. Microbiol. 2021, 72, 415–419. [Google Scholar] [CrossRef]
- Fernández, D.; Sanz, M.E.; Parma, A.E.; Padola, N.L. Short communication: Characterization of Shiga toxin-producing Escherichia coli isolated from newborn, milk-fed, and growing calves in Argentina. J. Dairy Sci. 2012, 95, 5340–5343. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, R.M.; Montero, D.A.; Del Canto, F.; Salazar, J.C.; Arellano, C.; Alvarez, A.; Padola, N.L.; Moscuzza, H.; Etcheverría, A.; Fernández, D.; et al. Safety and Immunogenicity of a Chimeric Subunit Vaccine against Shiga Toxin-Producing Escherichia coli in Pregnant Cows. Int. J. Mol. Sci. 2023, 24, 2771. https://doi.org/10.3390/ijms24032771
Vidal RM, Montero DA, Del Canto F, Salazar JC, Arellano C, Alvarez A, Padola NL, Moscuzza H, Etcheverría A, Fernández D, et al. Safety and Immunogenicity of a Chimeric Subunit Vaccine against Shiga Toxin-Producing Escherichia coli in Pregnant Cows. International Journal of Molecular Sciences. 2023; 24(3):2771. https://doi.org/10.3390/ijms24032771
Chicago/Turabian StyleVidal, Roberto M., David A. Montero, Felipe Del Canto, Juan C. Salazar, Carolina Arellano, Alhejandra Alvarez, Nora L. Padola, Hernán Moscuzza, Analía Etcheverría, Daniel Fernández, and et al. 2023. "Safety and Immunogenicity of a Chimeric Subunit Vaccine against Shiga Toxin-Producing Escherichia coli in Pregnant Cows" International Journal of Molecular Sciences 24, no. 3: 2771. https://doi.org/10.3390/ijms24032771







