Clonal Spreading of ST42 Staphylococcus haemolyticus Strains Occurs Possibly Due to fusB and tetK Resistant Genes and Capsule-Related Genes
Abstract
:1. Introduction
2. Results
2.1. Comparative Analysis of Different Molecular Types of S. haemolyticus Genome Structures
2.2. Distribution of Antibiotic-Resistant Genotypes and Phenotypes among the Collected ST3 and ST42 Clinical Isolates
2.3. Distribution of Virulence Factor Genes between ST3 and ST42 S. haemolyticus
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Whole-Genome Sequencing and Annotation
4.3. Antimicrobial Susceptibilities Assay
4.4. Drug-Resistant Genotype and Virulence Factors Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, P.V.; Cruz, R.S.; Keim, L.S.; Paula, G.R.; Carvalho, B.T.; Coelho, L.R.; Carvalho, M.C.; Rosa, J.M.; Figueiredo, A.M.; Teixeira, L.A. The antimicrobial susceptibility, biofilm formation and geno-typic profiles of staphylococcus haemolyticus from bloodstream infections. Mem. Instig. Oswaldo Cruz 2013, 108, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Teeraputon, S.; Santanirand, P.; Wongchai, T.; Songjang, W.; Lapsomthob, N.; Jaikrasun, D.; Toonkaew, S.; Tophon, P. Prevalence of methicillin resistance and macrolide-lincosamide-streptogramin B resistance in Staphylococcus haemolyticus among clinical strains at a tertiary-care hospital in Thailand. New Microbes New Infect. 2017, 19, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Liu, T.P.; Huang, P.Y.; Lin, S.Y.; Lin, J.F.; Yeh, C.F.; Chang, S.C.; Wu, T.S.; Lu, J.J. Clinical features, outcomes, and molecular characteristics of an outbreak of staphylococcus haemo-lyticus infection, among a mass-burn casualty patient group, in a tertiary center in northern Taiwan. J. Microbiol. Immunol. Infect 2018, 51, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Liu, T.-P.; Chang, S.-C.; Lu, J.-J. Characterization of new Staphylococcus haemolyticus ST42 populations in northern Taiwan. Microb. Drug Resist. 2022, 28, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Fredheim, E.G.A.; Klingenberg, C.; Rohde, H.; Frankenberger, S.; Gaustad, P.; Flægstad, T.; Sollid, J.E. Biofilm formation by Staphylococcus haemolyticus. J. Clin. Microbiol. 2009, 47, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiology 2015, 161, 2061–2068. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with anti-microbial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-00017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-negative staphylococci pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef] [Green Version]
- Furi, L.; Haigh, R.; Al Jabri, Z.J.; Morrissey, I.; Ou, H.Y.; Leon-Sampedro, R.; Martinez, J.L.; Coque, T.M.; Oggioni, M.R. Dissemination of novel antimicrobial resistance mechanisms through the insertion sequence mediated spread of metabolic genes. Front. Microbiol. 2016, 7, 1008. [Google Scholar] [CrossRef] [Green Version]
- Maria, P.; Erik, H.; Claus, K.; Jorunn, P.C. Comparative genomic analysis of staphylococcus haemo-lyticus reveals key to hospital adaptation and pathogenicity. Front. Microbiol. 2019, 10, 2096. [Google Scholar] [CrossRef]
- Qin, M.; Chen, P.; Deng, B.; He, R.; Wu, Y.; Yang, Y.; Deng, W.; Ding, X.; Yang, F.; Xie, C.; et al. The emergence of a multidrug-resistant and pathogenic ST42 lineage of staphylococcus haemolyticus from a hospital in China. Microbiol. Spectr. 2022, 10, e0234221. [Google Scholar] [CrossRef] [PubMed]
- Nakakido, M.; Aikawa, C.; Nakagawa, I.; Tsumoto, K. The staphylococcal elastin-binding protein regulates zinc-dependent growth/biofilm formation. J. Biochem. 2014, 156, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Murray, S.; Pascoe, B.; Bray, J.; Meric, G.; Mageiros, L.; Wilkinson, T.S.; Jeeves, R.; Rohde, H.; Schwarz, S.; et al. Biofilm morphotypes and population structure among staphylococcus epidermidis from commensal and clinical samples. PLoS ONE 2016, 11, e0151240. [Google Scholar] [CrossRef]
- Shibuya, R.; Uehara, Y.; Baba, T.; Teruya, K.; Satou, K.; Hirano, T.; Kirikae, T.; Hiramatsu, K. Complete genome sequence of a methicillin-resistant staphylococcus lugdunensis strain and characteristics of its staphylococcal cassette chromosome mec. Sci. Rep. 2020, 10, 8682. [Google Scholar] [CrossRef]
- Jeon, J.; D’Souza, R.; Hong, S.K.; Lee, Y.; Yong, D.; Choi, J.; Lee, K.; Chong, Y. Complete genome sequence of the siphoviral bacteriophage ymc/09/04/r1988 mrsa bp: A lytic phage from a methicillin-resistant staphylococcus aureus isolate. FEMS Microbiol. Lett. 2014, 359, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-J.; Tsai, J.-C.; Hung, W.-C.; Tseng, S.-P.; Hsueh, P.-R.; Teng, L.-J. Identification of fusB-mediated fusidic acid resistance islands in staphylococcus epidermidis isolates. Antimicrob. Agents Chemother. 2011, 55, 5842–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, K.A.; Mulcahy, M.E.; Towell, A.M.; Geoghegan, J.A.; McLoughlin, R.M. Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target. PLOS Pathog. 2019, 15, e1007713. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.C.; de Lencastre, H. Multiplex pcr strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef] [Green Version]
- McManus, B.A.; Coleman, D.C.; Deasy, E.C.; Brennan, G.I.; O’Connell, B.; Monecke, S.; Ehricht, R.; Leggett, B.; Leonard, N.; Shore, A.C. Comparative genotypes, staphylococcal cassette chromosome mec (sccmec) genes and antimicrobial resistance amongst staphylococcus epidermidis and staphylococcus haemolyticus isolates from infections in humans and companion animals. PLoS ONE 2015, 10, e0138079. [Google Scholar] [CrossRef] [Green Version]
- Hosseinkhani, F.; Tammes Buirs, M.; Jabalameli, F.; Emaneini, M.; van Leeuwen, W.B. High diversity in sccmec elements among multidrug-resistant staphylococcus haemolyticus strains originating from paediatric patients; characterization of a new composite island. J. Med. Microbiol. 2018, 67, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Lin, L.-C.; Lu, J.-J. Comparative genomic analyses reveal potential factors responsible for the ST6 oxacillin-resistant Staphylococcus lugdunensis endemic in a hospital. Front. Microbiol. 2021, 12, 765437. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.; Peters, B.M.; Li, L.; Li, B.; Xu, Z.; Shirliff, M.E. Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2016, 101, 56–67. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. An analysis of the is6/is26 family of insertion sequences: Is it a single family? Microb. Genom. 2019, 5, e000291. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Fusidic acid: A bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, M.; Watters, A.A.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Occurrence and molecular characterization of fusidic acid resistance mechanisms among Staphylococcus spp. from European countries (2008). J. Antimicrob. Chemother. 2010, 65, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.-C.; Chen, H.-J.; Lin, Y.-T.; Tsai, J.-C.; Chen, C.-W.; Lu, H.-H.; Tseng, S.-P.; Jheng, Y.-Y.; Leong, K.H.; Teng, L.-J. Skin commensal staphylococci may act as reservoir for fusidic acid resistance genes. PLoS ONE 2015, 10, e0143106. [Google Scholar] [CrossRef]
- Yazdankhah, S.P.; Åsli, A.W.; Sørum, H.; Oppegaard, H.; Sunde, M. Fusidic acid resistance, mediated by fusB, in bovine coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 58, 1254–1256. [Google Scholar] [CrossRef]
- Chen, H.J.; Hung, W.C.; Tseng, S.P.; Tsai, J.C.; Hsueh, P.R.; Teng, L.J. Fusidic acid resistance determinants in staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2010, 54, 4985–4991. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, M.; Watters, A.A.; Bell, J.M.; Turnidge, J.D.; Jones, R.N. Fusidic acid resistance rates and prevalence of resistance mechanisms among staphylococcus spp. Isolated in north America and Australia, 2007–2008. Antimicrob. Agents Chemother. 2010, 54, 3614–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lina, G.; Quaglia, A.; Reverdy, M.-E.; Leclercq, R.; Vandenesch, F.; Etienne, J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999, 43, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatermann, S.G.; Koschinski, T.; Friedrich, S. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin. Microbiol. Infect. 2007, 13, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Moxon, E.R.; Kroll, J.S. The role of bacterial polysaccharide capsules as virulence factors. Curr. Top Microbiol. Immunol. 1990, 150, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Roberts, I.S. Capsular polysaccharides and their role in virulence. Contrib. Microbiol. 2005, 12, 55–66. [Google Scholar] [CrossRef]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [Green Version]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Paharik, A.E.; Horswill, A.R. The staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef] [Green Version]
- Entenza, J.M.; Foster, T.J.; Ni Eidhin, D.; Vaudaux, P.; Francioli, P.; Moreillon, P. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 2000, 68, 5443–5446. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Jena, S.; Sharma, S.; Dhawan, B.; Nath, G.; Singh, D.V. Identification of novel sequence types among staphylococcus haemolyticus isolated from variety of infections in India. PLoS ONE 2016, 11, e0166193. [Google Scholar] [CrossRef] [Green Version]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Boetzer, M.; Pirovano, W. Sspace-longread: Scaffolding bacterial draft genomes using long read sequence information. BMC Bioinform. 2014, 15, 211. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. Quast: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayne, P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; Volume M100Ed32. [Google Scholar]
- Warsa, U.C.; Nonoyama, M.; Ida, T.; Okamoto, R.; Okubo, T.; Shimauchi, C.; Kuga, A.; Inoue, M. Detection of tet(K) and tet(M) in staphylococcus aureus of Asian countries by the polymerase chain reaction. J. Antibiot. 1996, 49, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, M.; Sistla, S.; Ray, P. Prevalence and molecular determinants of antimicrobial resistance in clinical isolates of Staphylococcus haemolyticus from India. Microb. Drug Resist. 2021, 27, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.C.; Hidrosollo, J.H.; Lin, L.C.; Ou, Y.H.; Kao, C.Y.; Lu, J.J. Characterization of oxacillin-resistant staphylococcus lugdunensis isolated from sterile body fluids in a medical center in Taiwan: A 12-year longitudinal epidemiological study. J. Microbiol. Immunol. Infect. 2022, 56, 292–298. [Google Scholar] [CrossRef]
- Chen, H.J.; Chang, Y.C.; Tsai, J.C.; Hung, W.C.; Lin, Y.T.; You, S.J.; Tseng, S.P.; Teng, L.J. New structure of phage-related islands carrying fusb and a virulence gene in fusidic acid-resistant staphy-lococcus epidermidis. Antimicrob. Agents Chemother. 2013, 57, 5737–5739. [Google Scholar] [CrossRef] [Green Version]

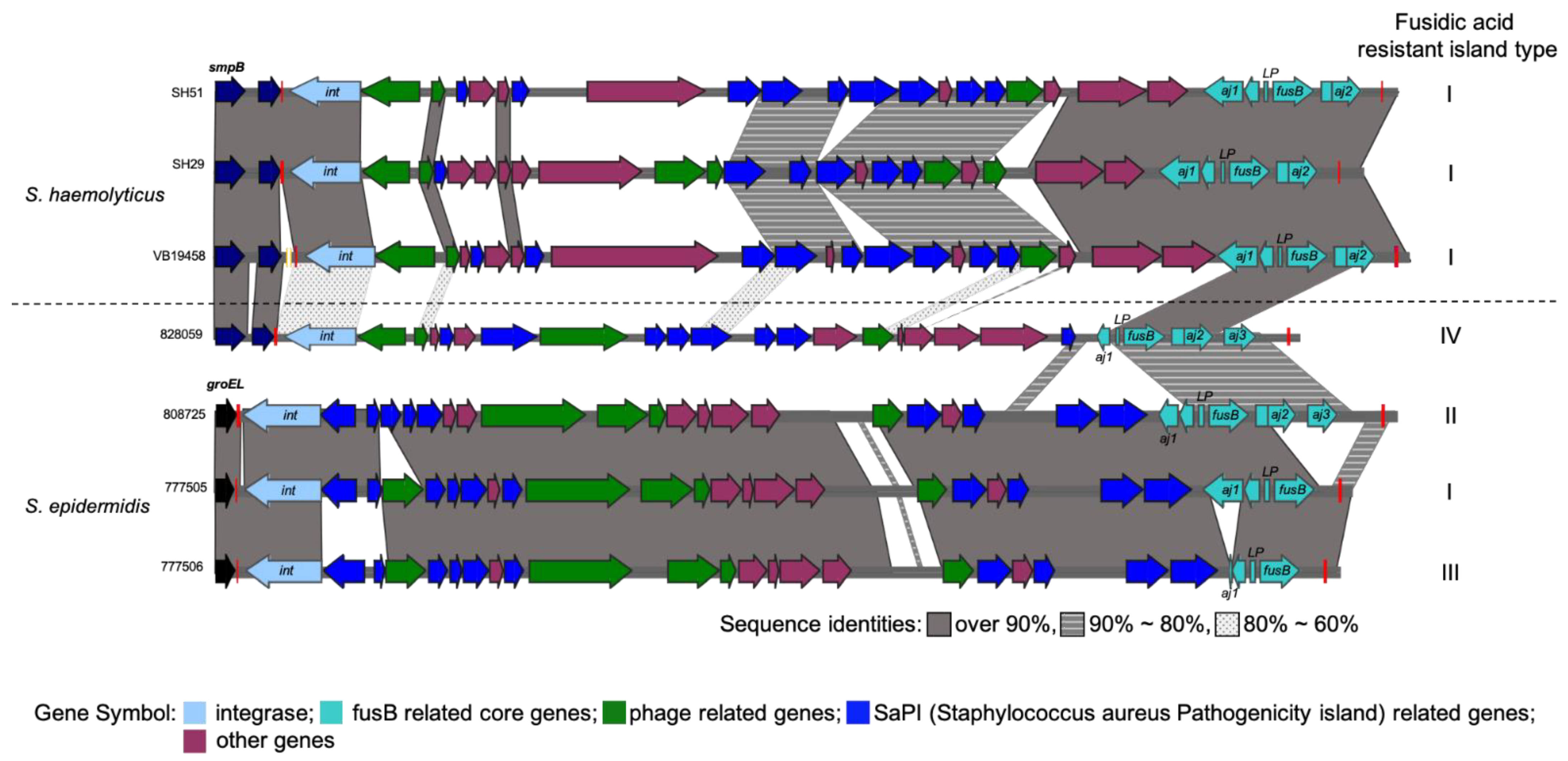
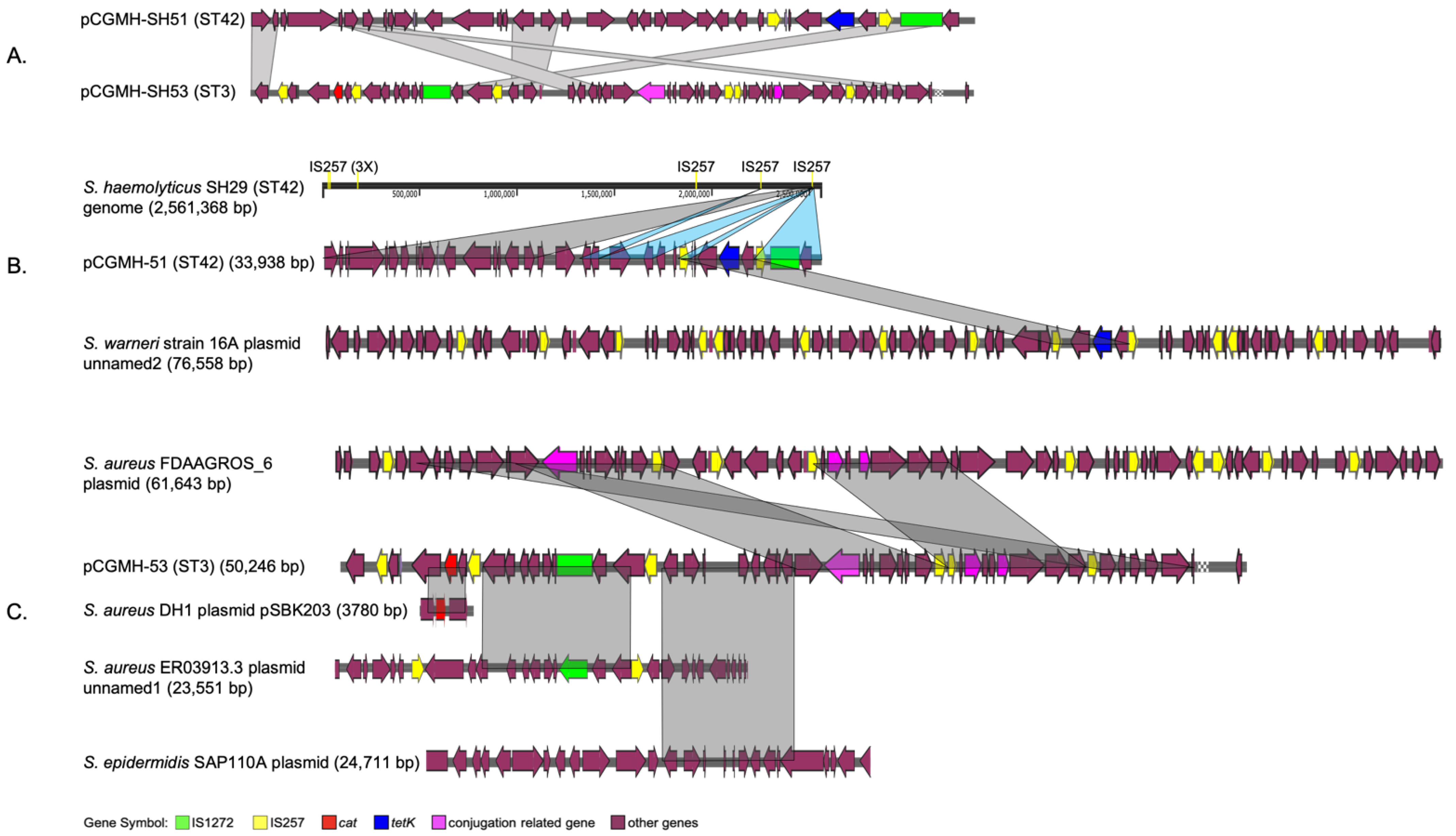
| Strain | CGMH_SH51 | SH 29 | CGMH_SH53 | VB19458 | JCSC1435 |
|---|---|---|---|---|---|
| MLST | ST42 | ST42 | ST3 | ST3 | ST2 |
| Size (bp) | 2,563,044 | 2,561368 | 2,586,626 | 2,699,210 | 2,685,015 |
| Clinical origin | blood | blood | blood | blood | unknown |
| Number of CDS | 2618 | 2456 | 2593 | 2568 | 2678 |
| tmRNA | 1 | 1 | 1 | 1 | 1 |
| tRNA | 61 | 60 | 63 | 60 | 59 |
| rRNA | 19 | 16 | 16 | 19 | 16 |
| G+C content | 33% | 33% | 33% | 33% | 33% |
| SCCmec | + | + | + | + | + |
| Plasmid | 1 | 0 | 1 | 0 | 3 |
| Prophage | PHAGE_Staphy_YMC/09/04/R1988_NC_022758 (1559323–1600746 bp) | PHAGE_Staphy_YMC/09/04/R1988_NC_022758 (1,552,815–1,609,358 bp) | PHAGE_Staphy_YMC/09/04/R1988_NC_022758 (1,584,557–1,625,922 bp) | PHAGE_Staphy_YMC/09/04/R1988_NC_022758 (1,652,339–1,698,371 bp) | PHAGE_Staphy_CNPx_NC_031241 (2,346,384–2,410,301 bp) |
| PHAGE_Staphy_SPbeta_like_NC_029119 (2,502,492–2,527,281 bp) | PHAGE_Staphy_SPbeta_like_NC_029119 (2,464,491–2,514,683 bp) | Staphy_IME_SA4_NC_029025 (2,125,445–2,197,547 bp) | |||
| ARGs (Antimicrobial Resistant Genes) | |||||
| mphC | + | + | - | - | + |
| msr(A) | + | + | - | - | + |
| blaZ | + (prophage) | + (prophage) | + (prophage) | + (prophage) | + |
| mecA | + (SCCmec) | + (SCCmec) | + (SCCmec) | + (SCCmec) | + (SCCmec) |
| aac(6′)-aph(2″) | + | + | + | + | + |
| aph(3′)-III | + (prophage) | + (prophage) | + (prophage) | + (prophage) | - |
| ant(6)-Ia | + (prophage) | + (prophage) | + (prophage) | + (prophage) | - |
| dfrG | + | + | + | + | - |
| fusB | + | + | - | + | - |
| tetK | plasmid | - | - | - | - |
| cat | - | - | plasmid | - | - |
| ermC | - | - | - | - | plasmid |
| Virulence Factors | |||||
| Cap8E | + | + | - | - | + |
| Cap8G | + | + | - | - | + |
| Cap8M | + | + | - | - | - |
| ClfB | + | + | - | - | - |
| ST42 [n, (%)] | ST3 [n, (%)] | ||||
|---|---|---|---|---|---|
| Antibiotics | Resistant Gene | Phenotypic Distribution | Genotypic Distribution | Phenotypic Distribution | Genotypic Distribution |
| Fusidic acid | fusB | 43 (46.7) | 42 (45.6) | 11 (22.9) | 11 (22.9) |
| Tetracycline | Tet(A) | 65 (70.1) | 65 (70.1) | 2 (4.2) | 2 (4.2) |
| Chloramphenicol | cat | 4 (4.3) | 4 (4.3) | 7 (14.6) | 7 (14.6) |
| Clindamycin Erythromycin | mph(C) msrA | 56 (60.8) 92 (100) | 91 (99) | 32 (66.7) 48 (100) | 36 (75) |
| ermC | 80 (87) | 42 (87.5) | |||
| Virulence Factors | Prevalence of Virulence Factors | Prevalence of Virulence Factors | |||
| Cap8E | 87 (94.6) | 25 (59.5) | |||
| Cap8G | 87 (94.6) | 25 (59.5) | |||
| Cap8M | 86 (93.5) | 15 (35.7) | |||
| ClfB | 64 (69.6) | 2 (4.8) | |||
| MSLT | MIC (μg/mL) | No (%) |
|---|---|---|
| 3 | 4 | 3 (27.3%) |
| 8 | 8 (72.7%) | |
| 42 | 4 | 1 (2.3%) |
| 8 | 36 (83.7%) | |
| 16 | 5 (14%) |
| PCR Targets | Primer Name | Oligo Sequence (5′-3′) | Reference |
|---|---|---|---|
| fusB | FusB-F | TCATATAGATGACGATATTG | [31] |
| FusB-R | ACAATGAATGCTATCTCGAC | ||
| aj1-LP-fusB | aj1 606-577R | AGTAAAGAATAAGTTTTTAATCGTTAATGC | [17] |
| fusB 389-361R | TTCCGATTTGATGCAAGTTCATTCCATCC | ||
| mphC | mphC-F | GAGACTACCAAGAAGACCTGACG | this study |
| mphC-R | CATACGCCGATTCTCCTGAT | ||
| msrA | msrA-F | CCTATGCATACAACCGACAG | |
| msrA-R | CTACACCATTTGCACCTACG | ||
| ermA | ermA-F | GTTCAAGAACAATCAATACAGAG | [46] |
| ermA-R | GGATCAGGAAAAGGACATTTTAC | ||
| ermC | ermC-F | GGTGTAATTTCGTAACTGCC | [47] |
| ermC-R | TAATGCCAATGAGCGTTTTG | ||
| tetK | tet-F | TCGATAGGAACAGCAGTA | [45] |
| tet-R | CAGCAGATCCTACTCCTT | ||
| cat | cat-F | TGGTAACCATCACATACCGCA | this study |
| cat-R | GTGAGGGAAATTTGGGTTATTG | ||
| cap8E | cap8E-F | CTTTAACGGTGACAGATCCA | this study |
| cap8E-R | CACACTGTGCATACTCTTCT | ||
| cap8G | cap8G-F | TACTTAGAAGCAGTTGGCAG | this study |
| cap8G-R | TTCTTCGGGTACATTTTGGT | ||
| cap8M | cap8M-F | ACTCATAGTAGCTGGACCTT | this study |
| cap8M-R | CCCATAACTTGAGCTAGTCC | ||
| clfB | clfB-F | TTTTGAGGGTTGGATAACTGA | this study |
| clfB-R | TCTGCAGAACCATTACCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-C.; Chang, S.-C.; Ou, Y.-H.; Liu, T.-P.; Lu, J.-J. Clonal Spreading of ST42 Staphylococcus haemolyticus Strains Occurs Possibly Due to fusB and tetK Resistant Genes and Capsule-Related Genes. Int. J. Mol. Sci. 2023, 24, 6198. https://doi.org/10.3390/ijms24076198
Lin L-C, Chang S-C, Ou Y-H, Liu T-P, Lu J-J. Clonal Spreading of ST42 Staphylococcus haemolyticus Strains Occurs Possibly Due to fusB and tetK Resistant Genes and Capsule-Related Genes. International Journal of Molecular Sciences. 2023; 24(7):6198. https://doi.org/10.3390/ijms24076198
Chicago/Turabian StyleLin, Lee-Chung, Shih-Cheng Chang, Yu-Hsiang Ou, Tsui-Ping Liu, and Jang-Jih Lu. 2023. "Clonal Spreading of ST42 Staphylococcus haemolyticus Strains Occurs Possibly Due to fusB and tetK Resistant Genes and Capsule-Related Genes" International Journal of Molecular Sciences 24, no. 7: 6198. https://doi.org/10.3390/ijms24076198






