Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage
Abstract
:1. Introduction
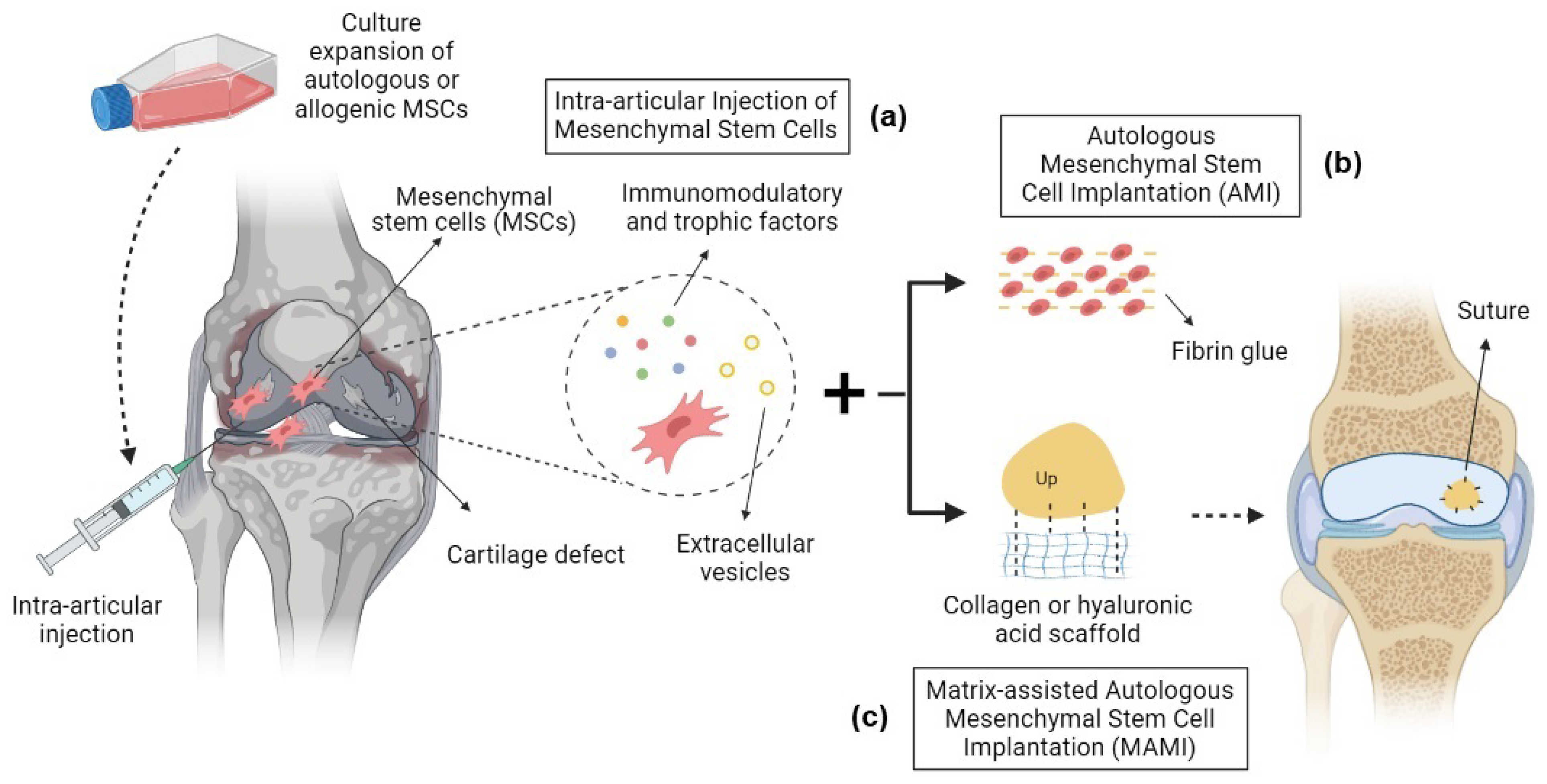
2. Mesenchymal Stem Cell Therapies
2.1. Bone-Marrow-Derived Mesenchymal Stem Cells
2.2. Adipose-Derived Mesenchymal Stem Cells
2.3. Synovium- and Peripheral-Blood-Derived Mesenchymal Stem Cells
2.4. Umbilical-Cord- and Placenta-Derived Mesenchymal Stem Cells
3. Adverse Events of MSC Therapies in OA
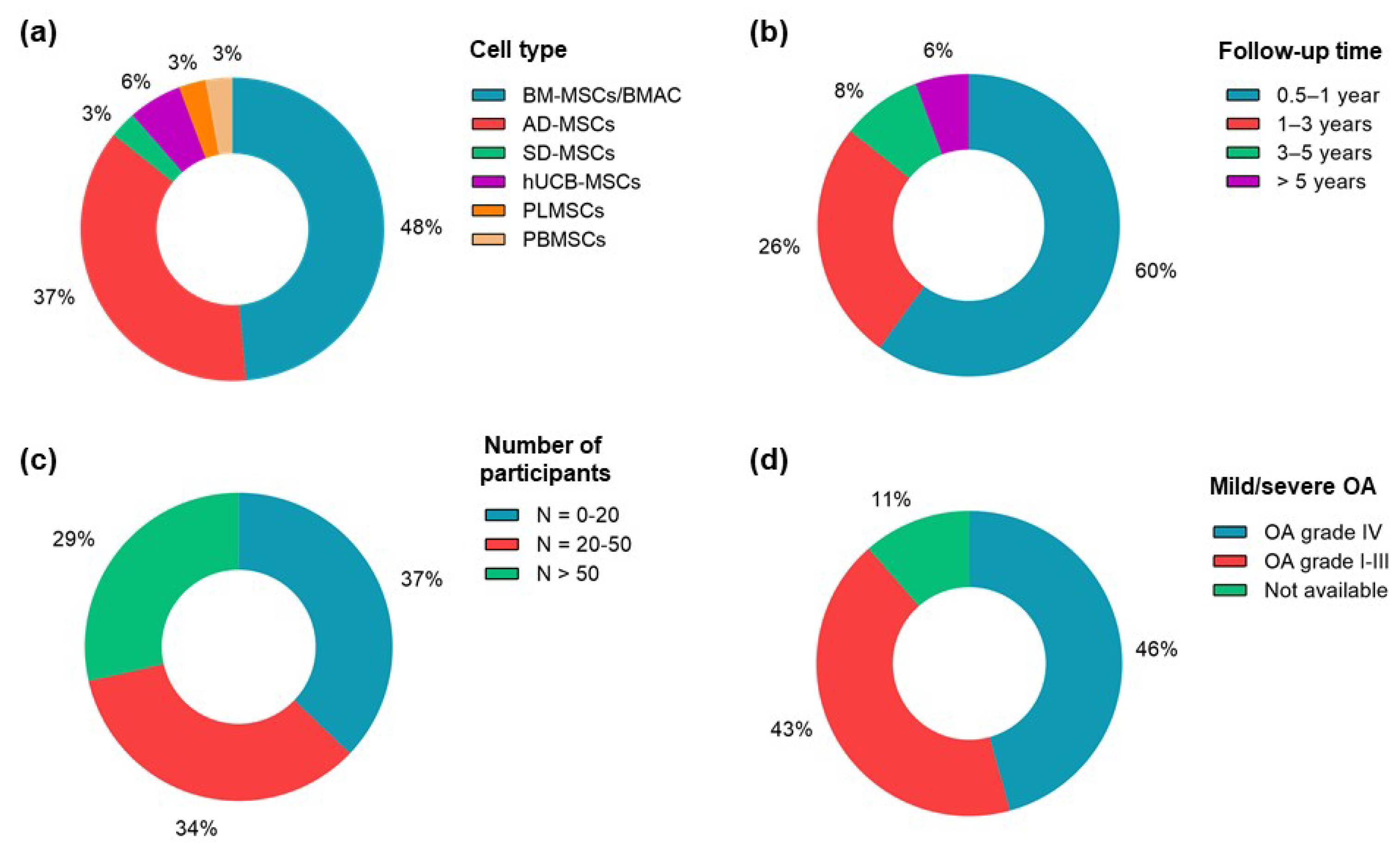
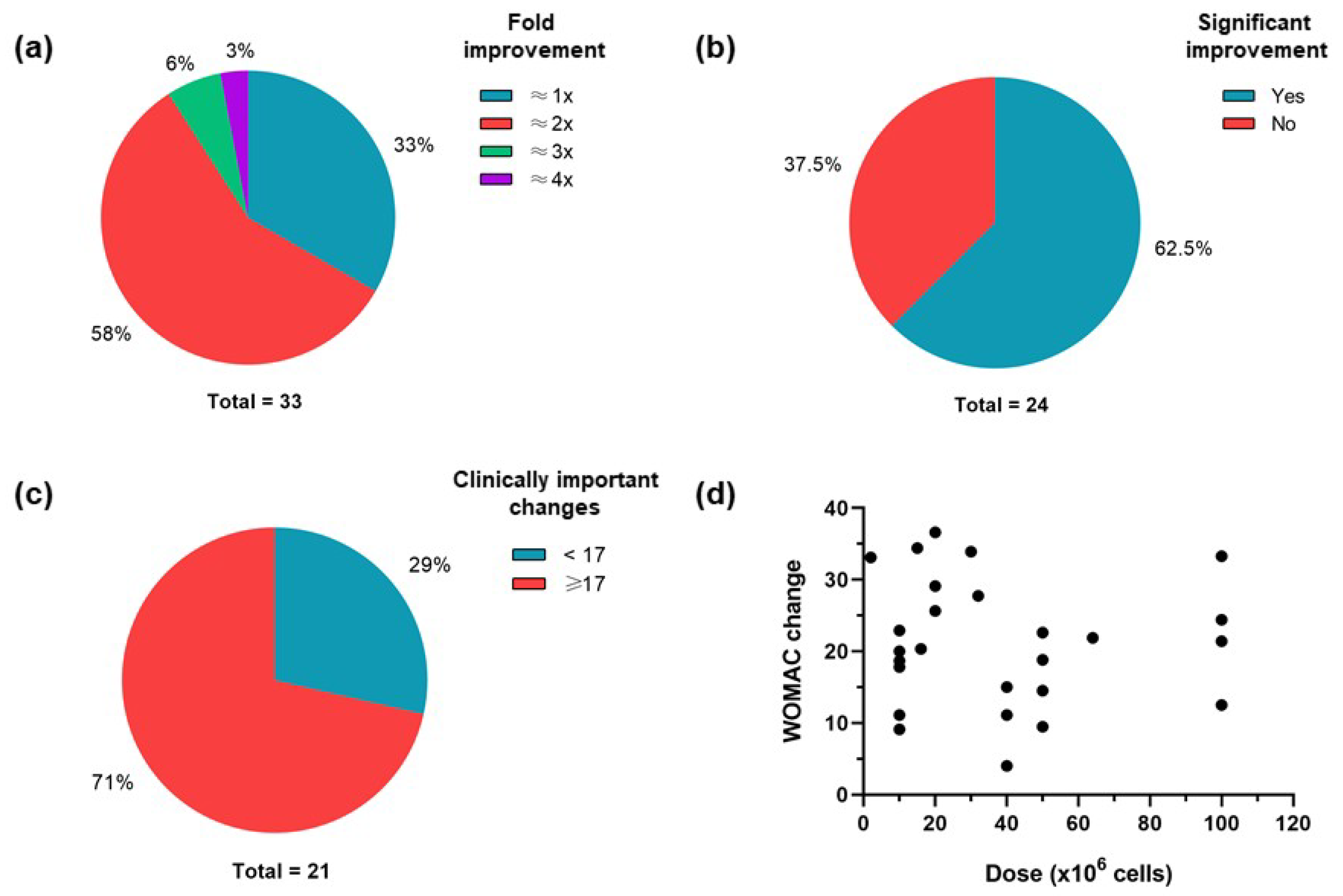
4. Clinical Variables and Outcomes
5. Quality Assurance of Clinical Trials and MSC Manufacturing
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arden, N.; Cooper, C. Osteoarthritis Handbook; Arden, N., Cooper, C., Eds.; Taylor and Francis: Abingdon, UK, 2006. [Google Scholar]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An Update on the Pathophysiology of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Erratum: Pluripotency of Mesenchymal Stem Cells Derived from Adult Marrow. Nature 2002, 418, 41–49, Erratum in Nature 2007, 447, 879–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagata, K.; Nakayamada, S.; Tanaka, Y. Use of Mesenchymal Stem Cells Seeded on the Scaffold in Articular Cartilage Repair. Inflamm. Regen. 2018, 38, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.; Mitchell, K.; Soans, J.; Kim, L.; Zaidi, R. The Use of Mesenchymal Stem Cells for Cartilage Repair and Regeneration: A Systematic Review. J. Orthop. Surg. Res. 2017, 12, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, L.A. Osteoarthritis Year in Review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Gold, G.E.; Cicuttini, F.; Crema, M.D.; Eckstein, F.; Guermazi, A.; Kijowski, R.; Link, T.M.; Maheu, E.; Martel-Pelletier, J.; Miller, C.G.; et al. OARSI Clinical Trials Recommendations: Hip Imaging in Clinical Trials in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 716–731. [Google Scholar] [CrossRef] [Green Version]
- Orth, P.; Madry, H. Complex and Elementary Histological Scoring Systems for Articular Cartilage Repair. Histol. Histopathol. 2015, 30, 911–919. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Van Damme, B.; Nesic, D.; Knutsen, G.; Kandel, R.; Roberts, S. A New Histology Scoring System for the Assessment of the Quality of Human Cartilage Repair: ICRS II. Am. J. Sport. Med. 2010, 38, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and Tissue Engineering Techniques for Articular Cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.W.; Locker, P.; Friel, N.A.C.B. Articular Cartilage Injury and Adult Osteochondritis Dissecans: Treatment Options and Decision Making. In Insall & Scott Surgery of the Knee; Elsevier: New York, NY, USA, 2017; pp. 401–411. [Google Scholar]
- Chilelli, B.; Farr, J.; Gomoll, A. Articular Cartilage Repair With Bioscaffolds. In Insall & Scott Surgery of the Knee; Elsevier: New York, NY, USA, 2017; pp. 454–461. [Google Scholar]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse Events, Side Effects and Complications in Mesenchymal Stromal Cell-Based Therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal Stem Cell Related Therapies for Cartilage Lesions and Osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Song, Y.; Zhang, J.; Xu, H.; Lin, Z.; Chang, H.; Liu, W.; Kong, L. Mesenchymal Stem Cells in Knee Osteoarthritis Treatment: A Systematic Review and Meta-Analysis. J. Orthop. Transl. 2020, 24, 121–130. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells: A Pilot Study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; López, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final Results of a Phase I–II Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H.P. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-Up. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Al Abdullah, A.; et al. Intra-Articular Injection of Expanded Autologous Bone Marrow Mesenchymal Cells in Moderate and Severe Knee Osteoarthritis Is Safe: A Phase I/II Study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef] [Green Version]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal Stem Cell Therapy for Knee Osteoarthritis: 5 Years Follow-up of Three Patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-Articular Injection of Two Different Doses of Autologous Bone Marrow Mesenchymal Stem Cells versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: Long-Term Follow up of a Multicenter Randomized Controlled Clinical Trial (Phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.J.F.C.; Schott, V.; Balduino, A.; Bastos, R.; Miguel Oliveira, J.; Reis, R.L.; Rodeo, S.; et al. Intra-Articular Injection of Culture-Expanded Mesenchymal Stem Cells with or without Addition of Platelet-Rich Plasma Is Effective in Decreasing Pain and Symptoms in Knee Osteoarthritis: A Controlled, Double-Blind Clinical Trial. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-Articular Injections of Expanded Mesenchymal Stem Cells with and without Addition of Platelet-Rich Plasma Are Safe and Effective for Knee Osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis with Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and Safety of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells (Stempeucel®): Preclinical and Clinical Trial in Osteoarthritis of the Knee Joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The Effect of Intra-Articular Injection of Autologous Bone Marrow Stem Cells on Pain and Knee Function in Patients with Osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sport. Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Arthurs, J.R.; Heckman, M.G.; Bestic, J.M.; Kazmerchak, S.E.; Diehl, N.N.; Zubair, A.C.; O’Connor, M.I. Quantitative T2 MRI Mapping and 12-Month Follow-up in a Randomized, Blinded, Placebo Controlled Trial of Bone Marrow Aspiration and Concentration for Osteoarthritis of the Knees. Cartilage 2019, 10, 432–443. [Google Scholar] [CrossRef]
- Hernigou, P.; Bouthors, C.; Bastard, C.; Flouzat Lachaniette, C.H.; Rouard, H.; Dubory, A. Subchondral Bone or Intra-Articular Injection of Bone Marrow Concentrate Mesenchymal Stem Cells in Bilateral Knee Osteoarthritis: What Better Postpone Knee Arthroplasty at Fifteen Years? A Randomized Study. Int. Orthop. 2021, 45, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Delambre, J.; Quiennec, S.; Poignard, A. Human Bone Marrow Mesenchymal Stem Cell Injection in Subchondral Lesions of Knee Osteoarthritis: A Prospective Randomized Study versus Contralateral Arthroplasty at a Mean Fifteen Year Follow-Up. Int. Orthop. 2021, 45, 365–373. [Google Scholar] [CrossRef] [PubMed]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; van den Broek, M.P.H.; Nizak, R.; van Rijen, M.H.P.; de Weger, R.A.; Dhert, W.J.A.; Saris, D.B.F. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef] [PubMed]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Nizak, R.; van Rijen, M.H.P.; Saris, D.B.F. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Saris, T.F.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.H.; Saris, D.B.F. Five-Year Outcome of 1-Stage Cell-Based Cartilage Repair Using Recycled Autologous Chondrons and Allogenic Mesenchymal Stromal Cells: A First-in-Human Clinical Trial. Am. J. Sport. Med. 2021, 49, 941–947. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [Green Version]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-Articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sport. Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human Adipose-Derived Mesenchymal Stem Cells for Osteoarthritis: A Pilot Study with Long-Term Follow-up and Repeated Injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of Knee Osteoarthritis with Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Progenitor Cells: A Prospective, Randomized, Double-Blind, Active-Controlled, Phase IIb Clinical Trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Z.; Tang, J.; Yue, B.; Wang, J.; Zhang, J.; Xuan, L.; Dai, C.; Li, S.; Li, M.; Xu, C.; et al. Human Adipose-Derived Mesenchymal Progenitor Cells plus Microfracture and Hyaluronic Acid for Cartilage Repair: A Phase IIa Trial. Regen. Med. 2020, 15, 1193–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Dai, C.; Du, H.; Li, S.; Ye, P.; Zhang, L.; Wang, X.; Song, Y.; Togashi, R.; Vangsness, C.T.; et al. Intra-Articular Injections of Allogeneic Human Adipose-Derived Mesenchymal Progenitor Cells in Patients with Symptomatic Bilateral Knee Osteoarthritis: A Phase I Pilot Study. Regen. Med. 2020, 15, 1625–1636. [Google Scholar] [CrossRef]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-Derived Mesenchymal Stem Cells with Microfracture versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chung, P.K.; Suh, D.S.; Heo, D.B.; Tak, D.H.; Koh, Y.G. Implantation of Mesenchymal Stem Cells in Combination with Allogenic Cartilage Improves Cartilage Regeneration and Clinical Outcomes in Patients with Concomitant High Tibial Osteotomy. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Zhao, X.; Ruan, J.; Tang, H.; Li, J.; Shi, Y.; Li, M.; Li, S.; Xu, C.; Lu, Q.; Dai, C. Multi-Compositional MRI Evaluation of Repair Cartilage in Knee Osteoarthritis with Treatment of Allogeneic Human Adipose-Derived Mesenchymal Progenitor Cells. Stem Cell Res. Ther. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.F.; Hu, C.C.; Te Wu, C.; Wu, H.T.H.; Chang, C.S.; Hung, Y.P.; Tsai, C.C.; Chang, Y. Treatment of Knee Osteoarthritis with Intra-Articular Injection of Allogeneic Adipose-Derived Stem Cells (ADSCs) ELIXCYTE®: A Phase I/II, Randomized, Active-Control, Single-Blind, Multiple-Center Clinical Trial. Stem Cell Res. Ther. 2021, 12, 562. [Google Scholar] [CrossRef]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-Induced Autologous Mesenchymal Stem Cell Implantation versus Matrix-Induced Autologous Chondrocyte Implantation in the Treatment of Chondral Defects of the Knee: A 2-Year Randomized Study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef]
- Saw, K.Y.; Anz, A.W.; Ng, R.C.S.; Jee, C.S.Y.; Low, S.F.; Dorvault, C.; Johnson, K.B. Arthroscopic Subchondral Drilling Followed by Injection of Peripheral Blood Stem Cells and Hyaluronic Acid Showed Improved Outcome Compared to Hyaluronic Acid and Physiotherapy for Massive Knee Chondral Defects: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2502–2517. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Canintika, A.F.; Hanitya, A.L.; Pawitan, J.A.; Liem, I.K.; Pandelaki, J. Umbilical Cord-Derived Mesenchymal Stem Cells for Treating Osteoarthritis of the Knee: A Single-Arm, Open-Label Study. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 799–807. [Google Scholar] [CrossRef]
- Khalifeh Soltani, S.; Forogh, B.; Ahmadbeigi, N.; Hadizadeh Kharazi, H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and Efficacy of Allogenic Placental Mesenchymal Stem Cells for Treating Knee Osteoarthritis: A Pilot Study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. What Is the Minimum Clinically Important Difference for the Womac Index after TKA? Clin. Orthop. Relat. Res. 2018, 476, 2005–2014. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lopa, S.; Colombini, A.; Moretti, M.; de Girolamo, L. Injective Mesenchymal Stem Cell-Based Treatments for Knee Osteoarthritis: From Mechanisms of Action to Current Clinical Evidences. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- de Windt, T.S.; Saris, D.B.F.; Slaper-Cortenbach, I.C.M.; van Rijen, M.H.P.; Gawlitta, D.; Creemers, L.B.; de Weger, R.A.; Dhert, W.J.A.; Vonk, L.A. Direct Cell-Cell Contact with Chondrocytes Is a Key Mechanism in Multipotent Mesenchymal Stromal Cell-Mediated Chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef]
- Kostanjsek, N. Use of the International Classification of Functioning, Disability and Health (ICF) as a Conceptual Framework and Common Language for Disability Statistics and Health Information Systems. BMC Public Health 2011, 11 (Suppl. 4), S3. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, C.; Alt, C.; Pearce, D.A.; Furia, J.P.; Maffulli, N.; Alt, E.U. Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review. Cells 2022, 11, 965. [Google Scholar] [CrossRef]
- Wiggers, T.G.H.; Winters, M.; Van Den Boom, N.A.C.; Haisma, H.J.; Moen, M.H. Autologous Stem Cell Therapy in Knee Osteoarthritis: A Systematic Review of Randomised Controlled Trials. Br. J. Sport. Med. 2021, 55, 1161–1169. [Google Scholar] [CrossRef]
- Toh, W.S.; Brittberg, M.; Farr, J.; Foldager, C.B.; Gomoll, A.H.; Hui, J.H.P.; Richardson, J.B.; Roberts, S.; Spector, M. Cellular Senescence in Aging and Osteoarthritis: Implications for Cartilage Repair. Acta Orthop. 2016, 87, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederichs, S.; Klampfleuthner, F.A.M.; Moradi, B.; Richter, W. Chondral Differentiation of Induced Pluripotent Stem Cells Without Progression Into the Endochondral Pathway. Front. Cell Dev. Biol. 2019, 7, 270. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- De Luna, A.; Otahal, A.; Nehrer, S. Mesenchymal Stromal Cell-Derived Extracellular Vesicles—Silver Linings for Cartilage Regeneration? Front. Cell Dev. Biol. 2020, 8, 593386. [Google Scholar] [CrossRef] [PubMed]
- Pitta, M.; Esposito, C.I.; Li, Z.; Lee, Y.; Wright, T.M.; Padgett, D.E. Failure After Modern Total Knee Arthroplasty: A Prospective Study of 18,065 Knees. J. Arthroplast. 2018, 33, 407–414. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Vassallo, V.; Tsianaka, A.; Alessio, N.; Grübel, J.; Cammarota, M.; Tovar, G.E.M.; Southan, A.; Schiraldi, C. Evaluation of Novel Biomaterials for Cartilage Regeneration Based on Gelatin Methacryloyl Interpenetrated with Extractive Chondroitin Sulfate or Unsulfated Biotechnological Chondroitin. J. Biomed. Mater. Res. Part A 2022, 110, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Otsuka, T.; Bhattacharjee, M.; Laurencin, C.T. Minimally Invasive Cellular Therapies for Osteoarthritis Treatment. Regen. Eng. Transl. Med. 2021, 7, 76–90. [Google Scholar] [CrossRef]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef]
| Reference | Follow-Up (Years) | AE | Number of Patients | Percentage of N | Duration of AE | Intensity of AE |
|---|---|---|---|---|---|---|
| [19] | 1 | Joint pain | 6 | 50% | 1–6 days | Mild |
| Joint inflammation | 3 | 25% | NA | Mild | ||
| Back pain | 3 | 25% | NA | Mild | ||
| Tendonitis | 1 | 8% | NA | Mild | ||
| [20] | 1 | Joint swelling | 2 | 13% | NA | Mild |
| Joint lock | 1 | 6% | NA | Mild | ||
| Back pain | 3 | 20% | 2–3 days | Mild | ||
| Arthralgia | 8 | 53% | 2–3 days | Mild | ||
| [22] | 1–2 | Joint pain | 2 | 15% | 1 day | Mild |
| Joint swelling | 1 | 8% | 2 days | Mild | ||
| [25] | 1 | Joint pain | 4 | 33% | 2–4 weeks | Mild |
| Joint swelling | 2 | 17% | 4 weeks | Mild | ||
| [30] | 0.5 | Joint pain and swelling | 1 | 2% | 2 days | Mild |
| [32] | 0.5 | Joint swelling | 3 | 12% | 6 months | Moderate |
| [33] | 1 | Joint swelling | 2 | 8% | 12 months | Moderate |
| [40] | 0.5 | Joint swelling | 5 | 28% | NA | Mild |
| [41] | 0.5 | Arthralgia | 6 | 50% | 6 months | Moderate |
| Joint swelling | 2 | 17% | 6 months | Moderate | ||
| [42] | 1 | Joint pain and swelling | 2 | 7% | 4 weeks | Mild |
| Discomfort and bruising | 18 | 60% | NA | Mild | ||
| [43] | 0.5–1 | Joint swelling | 1 | 3% | NA | Mild |
| [44] | 2 | Joint pain | 4 | 11% | NA | Mild |
| Joint swelling | 15 | 44% | NA | Mild | ||
| Joint edema and cramps | 1 | 3% | NA | Mild | ||
| [45] | 1 | Joint pain and swelling | 19 | 36% | 7 days | Mild |
| [46] | 2 | Joint pain and swelling | 5 | 17% | NA | Mild |
| Skin erythema | 2 | 7% | NA | Mild | ||
| [28] | 1 | Joint pain and swelling | 8 | 53% | 7 days | Mild |
| [29] | 1 | Joint pain and swelling | 4 | 7% | NA | Mild |
| Arthralgia | 2 | 3% | NA | Mild | ||
| [47] | 1 | Joint pain | 18 | 82% | 3 weeks | Moderate |
| Joint swelling | 3 | 14% | 3 weeks | Mild | ||
| Joint edema | 10 | 45% | 3 weeks | Mild | ||
| [51] | 1 | Joint pain | 9 | 16% | NA | Mild |
| Joint swelling | 6 | 10% | NA | Mild | ||
| Arthralgia | 8 | 14% | NA | Mild | ||
| Joint stiffness | 2 | 3% | NA | Mild | ||
| [54] | 7 | Arthralgia | 2 | 30% | NA | Mild |
| Back pain | 1 | 14% | NA | Mild | ||
| Increased infection susceptibility | 1 | 14% | NA | Mild | ||
| [56] | 0.5 | Joint pain and swelling | 4 | 20% | 3 days | Mild |
| [37] | 1.5 | Joint swelling | 8 | 23% | NA | Mild |
| Arthralgia | 13 | 37% | NA | Mild | ||
| Crepitations | 5 | 14% | NA | Mild |
| Cell Type(s) | Treatment Approach | Dose (×106 Cells) | Regimen (Interval) | Phase | Follow-Up (Years) | N | Age (years) | Defect Size (cm2) | OA Grade | Main Outcome Results | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PROM/Function | MRI/Arthroscopy | |||||||||||
| Autologous BM-MSCs | Intra-articular injection | 40 | One dose | Pilot | 1 | 12 | 44–54 | NA | II–III | WOMAC a,b: 19.4–8.3 | MRI (PCI) a: 19.5–15.4 | [19] |
| Autologous BM-MSCs | Intra-articular injection | 40 | One dose | I/II | 1 | 15 | 33–64 | NA | II–III | WOMAC a,b: 25–10 | MRI T2 scores a: 59.64–51.14 | [20] |
| Autologous BM-MSCs | Intra-articular injection | 13 | One dose | NA | 2 | 56 | 24–54 | 1.5–9.3 | IV | IKDC a: 33.9–85 (HTO + MSCs) with additional improvement of 7.65 compared to HTO | MRI (MOCART) c: 43.21 (HTO) and 62.32 (HTO + MSCs) with p < 0.001 | [21] |
| Autologous BM-MSCs | Intra-articular injection | 30 | Two doses (1 month) | I/II | 1–2 | 13 | 34–63 | NA | II–III | KOOS (symptoms) a: 67.3–88.7 | MRI a: cartilage thickness: from 2.15–2.16 to 2.38–2.5 (mm) | [22] |
| Autologous BM-MSCs | Intra-articular injection | 8–9 | One dose | NA | 5 | 3 | 54–65 | NA | II–III | VAS a: from 80, 85, and 90 to 45, 8, and 45, respectively | NA | [23] |
| Autologous BM-MSCs | Intra-articular injection (+ HA) | 10 or 100 | One dose | I/II | 4 | 27 | 54–69 | NA | II–IV | WOMAC a,b: 27–27 (control), 37–17 (10 × 106), and 29–16.5 (100 × 106) | NA | [24] |
| Autologous BM-MSCs | Intra-articular injection | 1, 10, or 50 | One dose | I/IIa | 1 | 12 | 40–65 | NA | III–IV | NA | MRI: no changes for T2 scores and WORMSs | [25] |
| Autologous BM-MSCs | Intra-articular injection (+ PRP) | 40 | One dose | I | 1 | 47 | 42–71 | NA | I–IV | KOOS a,b: 36.9–54.4 (corticosteroid), 30.3–54.2 (BM-MSCs), and 37.3–59.9 (BM-MSCs + PRP) | NA | [26] |
| Autologous BM-MSCs | Intra-articular injection | 20 | One dose | I/II | 0.5 | 61 | 43–70 | NA | II–III | WOMAC a,b: 62.61–91.73 (BM-MSCs), and 69.93–72.96 (control) | NA | [30] |
| Autologous BMAC | Intra-articular injection | 0.034 | One dose | NA | 0.5 | 25 | 42–68 | NA | II–IV | VAS a: 3.1–1.5 (BMAC) and 2.9–0.8 (saline) with p = 0.44 | NA | [32] |
| Autologous BMAC | Intra-articular injection | 0.034 | One dose | NA | 0.5–1 | 25 | 42–68 | NA | I–III | VAS c: 1.2 (BMAC) and 0.7 (placebo) with p = 0.98 | MRI (T2 scores) c: 2.4 (BMAC) and 2.5 (placebo) with p = 0.27 | [33] |
| Autologous BMAC | Subchondral bone or intra-articular injections | 0.114 | One dose | NA | 2 | 60 | 48–72 | NA | I–IV | VAS a: 4–1 (subchondral) and 3.5–3.5 (intra-articular) | Defect sizes (cm2) a: from 0.4–5.2 to 1.4–2.9 (subchondral) and no regression (intra-articular) | [34] |
| Autologous BMAC | Subchondral bone injections | 0.156 | One dose | NA | 2–10 | 140 | 65–90 | NA | II–IV | VAS a: 3.5–1.5 (BMAC), and 3.4–2.5 (TKA) | Cartilage volume increase of 2.3% at 2-year follow-up | [35] |
| Autologous AD-MSCs | Intra-articular injection | 10, 50, or 100 | One dose | I/II | 0.5 | 18 | 54–72 | 2–6 | III–IV | WOMAC a,b: 54.2–32.8 (100 × 106) and no improvement (10 and 50 × 106) | Decrease of 40–51% (MRI) and 64% (arthroscopy) in hyaline cartilage defect size | [39] |
| Autologous AD-MSCs | Intra-articular injection | 2, 10, or 50 | One dose | I | 0.5 | 18 | 57–74 | NA | III–IV | WOMAC a,b: 60.7–27.6 (2 × 106), 47.2–24.3 (10 × 106), and 38.8–16.2 (50 × 106) | Possible cartilage improvement in three of six patients | [40] |
| Autologous AD-MSCs | Intra-articular injection | 100 | One dose | IIb | 0.5 | 12 | 55–69 | 0.4–7 | II–IV | WOMAC a,b: 60.0–26.7 | Defect sizes(cm2) a: 3.12–3.15 (MSC) and 3.20–3.56 (control) | [41] |
| Autologous AD-MSCs | Intra-articular injection | 100 | One or two doses (6 months) | NA | 1 | 30 | 44–65 | NA | II–III | WOMAC a,b: 59.0–60.0 (control), 59.6–84.0 (one dose), and 54.4–87.3 (two doses) | MRI (MOAKS): progression of cartilage loss in 67% (control), 30% (one dose), and 11% (two doses) of the participants | [42] |
| Autologous AD-MSCs (SVF) | Intra-articular injection | 15 or 30 | One dose | NA | 0.5–1 | 37 | 41–74 | NA | II–III | WOMAC a,b: 47.1–13.2 (30 × 106), 56.2–21.8 (15 × 106), and 49.3–41.9 (placebo) | No significant difference in cartilage thickness between MSC and control groups | [43] |
| Autologous AD-MSCs | Intra-articular injection | 10, 20, or 50 | Two doses (12 months) | I/IIa | 2 | 18 | 40–70 | NA | II–III | WOMAC a,b: 25.8–8.0 (10 × 106), 49.0–12.4 (20 × 106), and 31.2–12.4 (50 × 106) | MRI a: 23–125 mm3 (cartilage volume) | [44] |
| Autologous AD-MSCs (Re-Join®) | Intra-articular injection | 50 | One dose | IIb | 1 | 52 | 45–64 | NA | I–III | WOMAC a,b: 30.83–21.35 (Re-Join®) and 34.17–27.25 (HA) with p < 0.0005 | MRI: apparent overall increase in cartilage thickness | [45] |
| Autologous AD-MSCs (Re-Join®) | Intra-articular injection | 50 | One dose | IIa | 2 | 30 | 52–70 | 1–8 | III | WOMAC a,b: 40.2–37.3 (MF), 40.9–31.0 (MF + HA), and 45.8–29.0 (MF + HA + Re-Join®) | ICRS-II a: 28.1–27.4 (MF), 27.7–43.2 (MF + HA), and 32.0–55.9 (MF + HA + Re-Join®) | [46] |
| Autologous AD-MSCs | AMI | Not applicable | Not applicable | NA | 2 | 80 | 32–46 | 3–7 | III–IV | KOOS (symptoms) c: 32.3 (group 1) and 27.8 (group 2) with p = 0.005 | MRI (MOCART) c: 62.4 (group 1) and 51.8 (group 2) with p = 0.033 | [48] |
| Autologous AD-MSCs | AMI | Not applicable | Not applicable | NA | 1–2 | 70 | 42–68 | 2.1–9.5 | NA | KOOS (symptoms) c: 67.3 (MSC) and 73.6 (MSC-AC) with p < 0.001 | Higher Kanamiya grades in the MSC-AC group | [49] |
| Allogeneic BM-MSCs | Intra-articular injection | 40 | One dose | I/II | 1 | 30 | 36–73 | NA | II–IV | WOMAC a,b: 45–41 | MRI (PCI) a: 14–9.5 (MSCs) and 15.5–12.5 (HA) with p < 0.05 at 1 year | [28] |
| Allogeneic BM-MSCs (Stempeucel®) | Intra-articular injection | 25, 50, 75, or 150 | One dose | II | 1 | 60 | 47–67 | NA | II–III | WOMAC a,b: 1315.8–717.8 (25 × 106), 1498.4–359.9 (50 × 106), and 1239.6–233.8 (control) | MRI (WORMS) a: 67.0–66.1 (25 M), 78.8–78.0 (50 M), and 76.5–74.9 (control) | [29] |
| Allogeneic AD-MSCs (AlloJoin®) | Intra-articular injection | 10, 20, or 50 | Two doses (3 months) | I | 1 | 22 | 49–65 | NA | II–III | WOMAC a,b: 48.00–24.29 (10 × 106), 42.13–25.63 (20 × 106), and 40.14–29.43 (50 × 106) | MRI a: 10.34–54.58 mm3 of total cartilage volume increase (low dose) | [47] |
| Allogeneic AD-MSCs | Intra-articular injection | 10, 20, or 50 | One dose | I/IIa | 1 | 18 | 40–70 | NA | II–III | WOMAC a,b: 38.83–24.33 (50 × 106), 48.83–23.17 (20 × 106), and 46.17–27.50 (10 × 106) | MRI (T1rho) a: 41.55–38.82 (high dose), 39.30–37.48 (mid-dose), and 38.91–37.94 (low dose) | [50] |
| Allogeneic AD-MSCs | Intra-articular injection | 16, 32, or 64 | One dose | I/II | 1 | 57 | 51–79 | NA | II–III | WOMAC a,b: 41.50–25.75 (HA), 42.88–22.53 (16 × 106), 46.41–18.65 (32 × 106), and 35.27–13.40 (64 × 106) | NA | [51] |
| Autologous SD-MSCs | MAMI | Not applicable | Not applicable | NA | 2 | 14 | 18–46 | 2.1–4.3 | NA | KOOS (symptoms) a,c: 66.33–89.80 (MAMI) and 67.46–83.67 (MACI) with p = 0.015 | MRI (graft infill, score 1–4)a: 2.93–3.86 (MAMI) and 2.64–3.29 (MACI) with p = 0.005 from 3 to 6 months | [52] |
| Allogeneic hUCB-MSCs | Intra-articular injection (+ HA) | 12–20 | One dose | I/II | 0.5–7 | 7 | 29–77 | 4.6–8.1 | III–IV | IKDC a: 39.1–63.2 (6 months) | MRI ΔR1 index of 1.44 (3 years) | [54] |
| Allogeneic hUCB-MSCs | Intra-articular injection (+ HA) | 10 | One dose | NA | 1 | 29 | 48–68 | NA | I–IV | WOMAC a: 22.55–13.46 (mild OA) and 27.57–16.42 (severe OA) | MRI (medial T2 map) a: 58.72–62.58 (mild OA) and 201.57–68.97 (severe OA) | [55] |
| Allogeneic PLMSCs | Intra-articular injection | 50–60 | One dose | Pilot | 0.5 | 20 | NA | NA | II–IV | KOOS (symptoms) c: 41.10 (PLMSCs) and 38.80 (control) | MRI a: Increase in chondral thickness: 2.7 to 3.5 mm | [56] |
| Autologous PBMSCs | Intra-articular injection (+ HA) | NA | NA | IIb | 2 | 120 | 23–55 | ≥3 | III–IV | IKDC a: 42.7–48.1 (control) and 43.1–65.6 (intervention) | MRI (MOCART) a: 10.9–15.6 (control) and 13.1–54.0 (intervention) | [53] |
| Allogeneic BM-MSCs | ACI + BM-MSCs | Not applicable | Not applicable | I/II | 1.5 | 35 | 22–38 | 2–5 | NA | KOOS a,b: 57.9–85.4 | MRI (T1rho) c: 43.1 (healthy control) and 47.9 (repaired cartilage) | [37] |
| Allogeneic BM-MSCs | ACI + BM-MSCs | Not applicable | Not applicable | I/II | 5 | 35 | 22–38 | 2–5 | NA | KOOS a,b: 57.9–78.9 | NA | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, D.d.C.; Araújo, L.T.d.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; Santos, R.R.d.; Barbosa, J.D.V.; Soares, M.B.P. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 9939. https://doi.org/10.3390/ijms24129939
Carneiro DdC, Araújo LTd, Santos GC, Damasceno PKF, Vieira JL, Santos RRd, Barbosa JDV, Soares MBP. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. International Journal of Molecular Sciences. 2023; 24(12):9939. https://doi.org/10.3390/ijms24129939
Chicago/Turabian StyleCarneiro, Diego de Carvalho, Lila Teixeira de Araújo, Girlaine Café Santos, Patrícia Kauanna Fonseca Damasceno, Jaqueline Leite Vieira, Ricardo Ribeiro dos Santos, Josiane Dantas Viana Barbosa, and Milena Botelho Pereira Soares. 2023. "Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage" International Journal of Molecular Sciences 24, no. 12: 9939. https://doi.org/10.3390/ijms24129939






