European Real-World Assessment of the Clinical Validity of a CE-IVD Panel for Ultra-Fast Next-Generation Sequencing in Solid Tumors
Abstract
:1. Introduction
2. Results
2.1. Phase I: ODxET Performance Validation with Pre-Characterized TFS R&D Samples
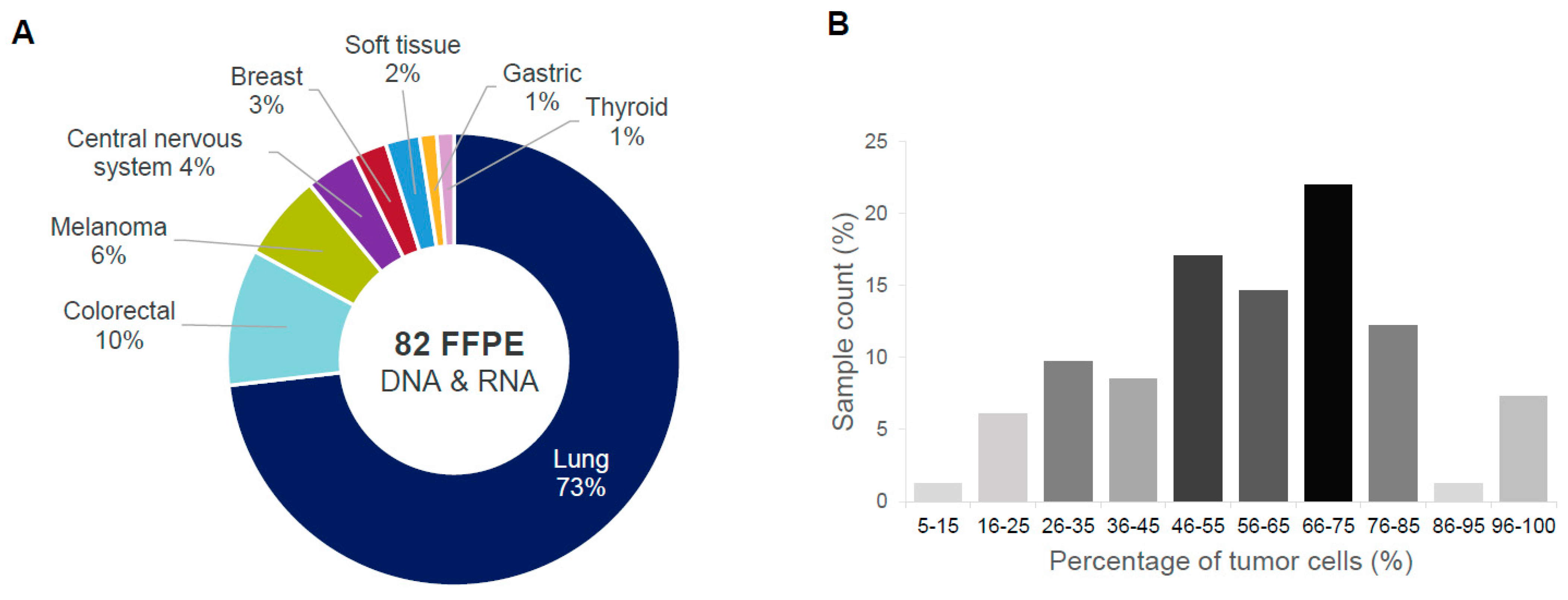
2.2. Phase II: ODxET Performance Evaluation in Six Academic Centers
2.3. Detection of SNVs and Indels
2.4. Assessment of Copy Number Variation
2.5. Detection of Fusions and Splice Variants
2.6. Analytical Performance of ODxET across Study Centers
3. Discussion
4. Materials and Methods
4.1. Academic Clinical and Research Centers
4.2. Clinical Samples
4.2.1. Study Phase I: Pre-Characterized Samples
4.2.2. Study Phase II: Biobank Clinical Samples
4.3. Genomic Profiling by Next-Generation Sequencing
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, S.K.; Ariyasu, R.; Uchibori, K.; Hayashi, R.; Chan, H.T.; Chin, Y.M.; Akita, T.; Harutani, Y.; Kiritani, A.; Tsugitomi, R.; et al. Rapid genomic profiling of circulating tumor DNA in non-small cell lung cancer using Oncomine Precision Assay with Genexus integrated sequencer. Transl. Lung Cancer Res. 2022, 11, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non-Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Beharry, A.; Diep, J.; Perdrizet, K.; Iafolla, M.A.J.; Raskin, W.; Dudani, S.; Brett, M.A.; Starova, B.; Olsen, B.; et al. Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Curr. Oncol. 2022, 29, 1326–1334. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klumpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef]
- Coghlin, C.L.; Smith, L.J.; Bakar, S.; Stewart, K.N.; Devereux, G.S.; Nicolson, M.C.; Kerr, K.M. Quantitative analysis of tumor in bronchial biopsy specimens. J. Thorac. Oncol. 2010, 5, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Tanishima, S.; Fujii, K.; Mori, R.; Okada, C.; Yanagita, E.; Shibata, Y.; Matsuoka, R.; Amano, T.; Yamada, T.; et al. Clinical impact of a cancer genomic profiling test using an in-house comprehensive targeted sequencing system. Cancer Sci. 2020, 111, 3926–3937. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Kou, T.; Kanai, M.; Yamamoto, Y.; Kamada, M.; Nakatsui, M.; Sakuma, T.; Mochizuki, H.; Hiroshima, A.; Sugiyama, A.; Nakamura, E.; et al. Clinical sequencing using a next-generation sequencing-based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017, 108, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Mileham, K.F.; Schenkel, C.; Bruinooge, S.S.; Freeman-Daily, J.; Basu Roy, U.; Moore, A.; Smith, R.A.; Garrett-Mayer, E.; Rosenthal, L.; Garon, E.B.; et al. Defining comprehensive biomarker-related testing and treatment practices for advanced non-small-cell lung cancer: Results of a survey of U.S. oncologists. Cancer Med. 2022, 11, 530–538. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef]
- mdi Europa. In Vitro Diagnostics EU Directive IVDR—In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746. Available online: https://mdi-europa.com/ivdr-in-vitro-diagnostic-medical-devices-regulation-eu-2017-746/ (accessed on 5 September 2023).
- Bayle, A.; Bonastre, J.; Chaltiel, D.; Latino, N.; Rouleau, E.; Peters, S.; Galotti, M.; Bricalli, G.; Besse, B.; Giuliani, R. ESMO study on the availability and accessibility of biomolecular technologies in oncology in Europe. Ann. Oncol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Mateo, J.; Steuten, L.; Aftimos, P.; Andre, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Marmarelis, M.E.; Hwang, W.T.; Scholes, D.G.; McWilliams, T.L.; Singh, A.P.; Sun, L.; Kosteva, J.; Costello, M.R.; Cohen, R.B.; et al. Association between Availability of Molecular Genotyping Results and Overall Survival in Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2023, 7, e2300191. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Lennerz, J.; Johnson, M.L.; Gordan, L.N.; Dumanois, R.H.; Quagliata, L.; Ritterhouse, L.L.; Cappuzzo, F.; Wang, B.; Xue, M.; et al. Compromised Outcomes in Stage IV Non-Small-Cell Lung Cancer with Actionable Mutations Initially Treated without Tyrosine Kinase Inhibitors: A Retrospective Analysis of Real-World Data. JCO Oncol. Pract. 2023, OP2200611. [Google Scholar] [CrossRef]
- Bellevicine, C.; Malapelle, U.; Vigliar, E.; Pisapia, P.; Vita, G.; Troncone, G. How to prepare cytological samples for molecular testing. J. Clin. Pathol. 2017, 70, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Cainap, C.; Balacescu, O.; Cainap, S.S.; Pop, L.A. Next Generation Sequencing Technology in Lung Cancer Diagnosis. Biology 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Dalens, L.; Niogret, J.; Kaderbhai, C.G.; Boidot, R. Is There a Role for Large Exome Sequencing in the Management of Metastatic Non-Small Cell Lung Cancer: A Brief Report of Real Life. Front. Oncol. 2022, 12, 863057. [Google Scholar] [CrossRef] [PubMed]



| Study Center | Total Run Time | |
|---|---|---|
| Run #1 (h:min) | Run #2 (h:min) | |
| Basel | 18:06 | 18:03 |
| Naples | 18:22 | 18:15 |
| Nice | 18:00 | 17:56 |
| Porto | 18:16 | 18:01 |
| Rome | 18:38 | 18:34 |
| Valencia | 19:01 | 18:34 |
| Sample | Cancer Type | Variant Type | Expected Variant (Pre-Characterized) | Unit of Measure | TFS R&D | Basel | Naples | Nice | Rome | Valencia | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected | Observed | Observed | Observed | Observed | Observed | ||||||
| DNA variants | 1 | Lung | Deletion | EGFR exon 19 del | Allele frequency | 30.5% | 31.4% | ‡ | 34.6% | 32.3% | 29.8% |
| 2 | Lung | Insertion | EGFR exon 20 ins | Allele frequency | 41.4% | 34.0% | 36.9% | 32.2% | 36.7% | 35.7% | |
| 5 | Bladder | CNV | ERBB2 CNV | Copy number | 35.3 | 37.5 | 36.9 | 37.7 | 36.0 | 36.3 | |
| 6 | Small intestine | SNV | BRAF V600E | Allele frequency | 53.3% | 53.1% | 51.5% | 51.2% | 53.3% | 53.0% | |
| RNA variants | 3 | Lung | Splice variant | MET Exon 14 Skip | No. of molecules | 1787 | 1872 | 1955 | 515 | 1974 | 1889 |
| 4 | Lung | Fusion | KIF5B-RET | No. of molecules | 110 | 124 | 47 | 134 | 84 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Normanno, N.; Machado, J.C.; Pescarmona, E.; Buglioni, S.; Navarro, L.; Esposito Abate, R.; Ferro, A.; Mensink, R.; Lambiase, M.; Lespinet-Fabre, V.; et al. European Real-World Assessment of the Clinical Validity of a CE-IVD Panel for Ultra-Fast Next-Generation Sequencing in Solid Tumors. Int. J. Mol. Sci. 2023, 24, 13788. https://doi.org/10.3390/ijms241813788
Normanno N, Machado JC, Pescarmona E, Buglioni S, Navarro L, Esposito Abate R, Ferro A, Mensink R, Lambiase M, Lespinet-Fabre V, et al. European Real-World Assessment of the Clinical Validity of a CE-IVD Panel for Ultra-Fast Next-Generation Sequencing in Solid Tumors. International Journal of Molecular Sciences. 2023; 24(18):13788. https://doi.org/10.3390/ijms241813788
Chicago/Turabian StyleNormanno, Nicola, José Carlos Machado, Edoardo Pescarmona, Simonetta Buglioni, Lara Navarro, Riziero Esposito Abate, Anabela Ferro, Rob Mensink, Matilde Lambiase, Virginie Lespinet-Fabre, and et al. 2023. "European Real-World Assessment of the Clinical Validity of a CE-IVD Panel for Ultra-Fast Next-Generation Sequencing in Solid Tumors" International Journal of Molecular Sciences 24, no. 18: 13788. https://doi.org/10.3390/ijms241813788






