Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer
Abstract
:1. Introduction
2. Results
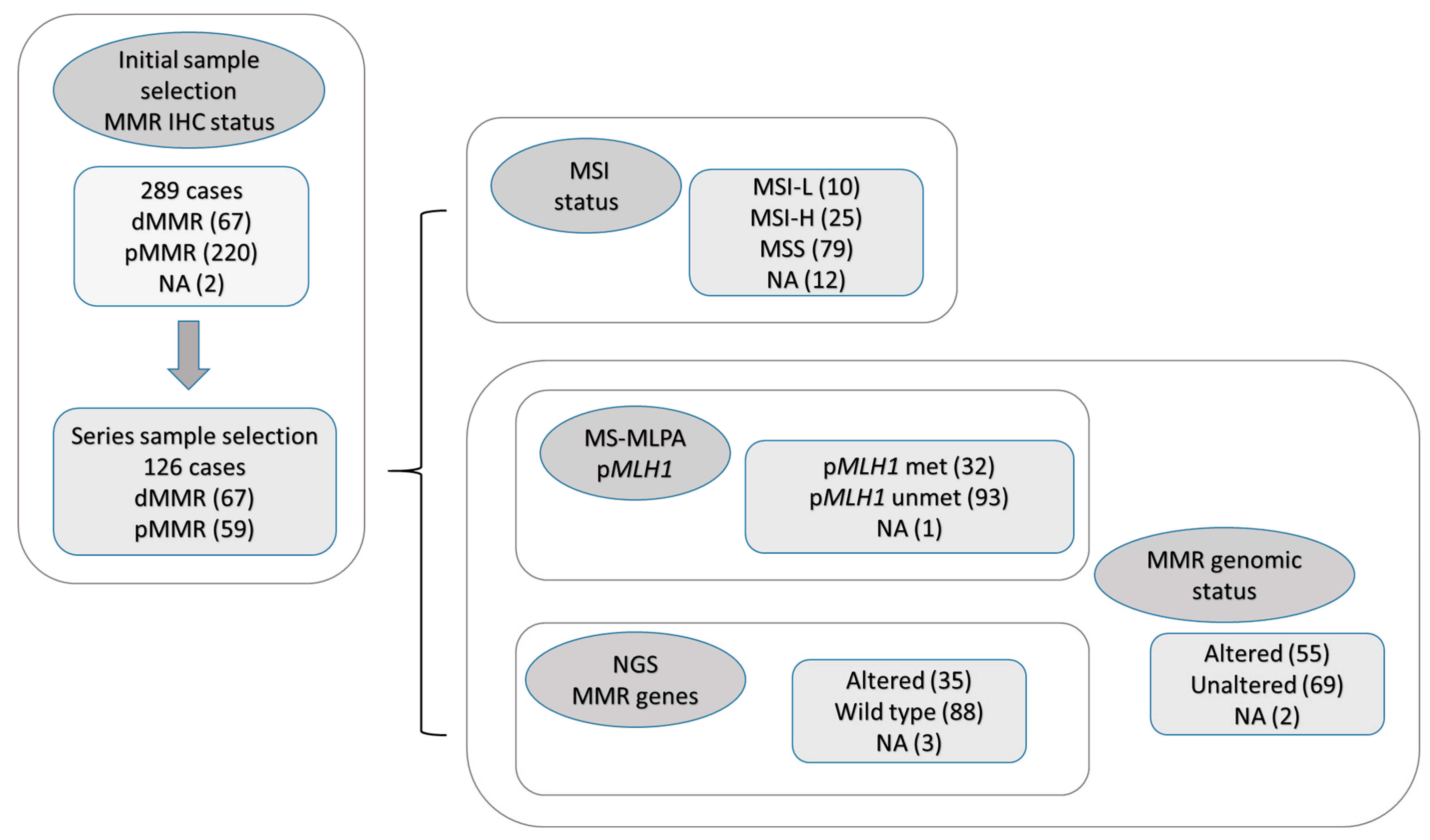
2.1. Sample Selection and Initial Screening
2.2. Descriptive Results
2.2.1. MMR Loss of Expression Based on IHC
2.2.2. MSI Status Based on MS PCR
2.2.3. MMR Genomic Status
2.3. Comparisons
2.3.1. IHC: Consideration of Two Markers and Inclusion of MSH3, versus Four Markers
2.3.2. MMR Genomic Status Compared with IHC Results
2.3.3. MS PCR-Based Status Compared with IHC Results
2.3.4. MSI PCR-Based Status Compared with MMR Genomic Status
2.3.5. Overall Comparisons
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Immunohistochemistry
4.3. DNA Extraction
4.4. MSI Testing
4.5. Next-Generation Sequencing
4.6. MLH1 Promoter Hypermethylation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997, 57, 808–811. [Google Scholar] [PubMed]
- Mojtahed, A.; Schrijver, I.; Ford, J.M.; Longacre, T.A.; Pai, R.K. A two-antibody mismatch repair protein immunohistochemistry screening approach for colorectal carcinomas, skin sebaceous tumors, and gynecologic tract carcinomas. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2011, 24, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Risinger, J.I.; Glaab, W.E.; Tindall, K.R.; Barrett, J.C.; Kunkel, T.A. Functional overlap in mismatch repair by human MSH3 and MSH6. Genetics 1998, 148, 1637–1646. [Google Scholar] [CrossRef]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef]
- Shah, S.N.; Hile, S.E.; Eckert, K.A. Defective mismatch repair, microsatellite mutation bias, and variability in clinical cancer phenotypes. Cancer Res. 2010, 70, 431–435. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Liu, J.; Zhu, W.; Lin, L.; Chen, L.; Shi, Y.; Lin, A.; Chen, G. Molecular classification grade 3 endometrial endometrioid carcinoma using a next-generation sequencing-based gene panel. Front. Oncol. 2022, 12, 935694. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Butzow, R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2020, 33, 1443–1452. [Google Scholar] [CrossRef]
- Nygren, A.O.; Ameziane, N.; Duarte, H.M.; Vijzelaar, R.N.; Waisfisz, Q.; Hess, C.J.; Schouten, J.P.; Errami, A. Methylation-specific MLPA (MS-MLPA): Simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005, 33, e128. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Ruschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Nagasaka, T.; Hamelin, R.; Boland, C.R. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PloS ONE 2010, 5, e9393. [Google Scholar] [CrossRef]
- Malapelle, U.; Parente, P.; Pepe, F.; De Luca, C.; Cerino, P.; Covelli, C.; Balestrieri, M.; Russo, G.; Bonfitto, A.; Pisapia, P.; et al. Impact of Pre-Analytical Factors on MSI Test Accuracy in Mucinous Colorectal Adenocarcinoma: A Multi-Assay Concordance Study. Cells 2020, 9, 2019. [Google Scholar] [CrossRef] [PubMed]
- Libera, L.; Sahnane, N.; Pepe, F.; Pisapia, P.; De Luca, C.; Russo, G.; Parente, P.; Covelli, C.; Chiaravalli, A.M.; Sessa, F.; et al. Critical aspects of microsatellite instability testing in endometrial cancer: A comparison study. Hum. Pathol. 2022, 128, 134–140. [Google Scholar] [CrossRef]
- Brunetti, M.; Panagopoulos, I.; Vitelli, V.; Andersen, K.; Hveem, T.S.; Davidson, B.; Eriksson, A.G.Z.; Trent, P.K.B.; Heim, S.; Micci, F. Endometrial Carcinoma: Molecular Cytogenetics and Transcriptomic Profile. Cancers 2022, 14, 3536. [Google Scholar] [CrossRef]
- Esteller, M.; Levine, R.; Baylin, S.B.; Ellenson, L.H.; Herman, J.G. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 1998, 17, 2413–2417. [Google Scholar] [CrossRef]
- Post, E.G.; Rosenthal, M.D.; Pennock, A.T.; Rauh, M.J. Prevalence and Consequences of Sport Specialization among Little League Baseball Players. Sports Health 2021, 13, 223–229. [Google Scholar] [CrossRef]
- Hendriks, Y.M.; de Jong, A.E.; Morreau, H.; Tops, C.M.; Vasen, H.F.; Wijnen, J.T.; Breuning, M.H.; Brocker-Vriends, A.H. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): A guide for clinicians. CA Cancer J. Clin. 2006, 56, 213–225. [Google Scholar] [CrossRef]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novak, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability-High Advanced Endometrial Cancer: Results from the KEYNOTE-158 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Oaknin, A.; Pothuri, B.; Gilbert, L.; Sabatier, R.; Brown, J.; Ghamande, S.; Mathews, C.; O’Malley, D.M.; Kristeleit, R.; Boni, V.; et al. Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study. Clin. Cancer Res. 2023, CCR-22. [Google Scholar] [CrossRef]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef]
- Mills, A.M.; Liou, S.; Ford, J.M.; Berek, J.S.; Pai, R.K.; Longacre, T.A. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am. J. Surg. Pathol. 2014, 38, 1501–1509. [Google Scholar] [CrossRef]
- Shia, J.; Tang, L.H.; Vakiani, E.; Guillem, J.G.; Stadler, Z.K.; Soslow, R.A.; Katabi, N.; Weiser, M.R.; Paty, P.B.; Temple, L.K.; et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: A 2-antibody panel may be as predictive as a 4-antibody panel. Am. J. Surg. Pathol. 2009, 33, 1639–1645. [Google Scholar] [CrossRef]
- Aiyer, K.T.S.; Doeleman, T.; Ryan, N.A.; Nielsen, M.; Crosbie, E.J.; Smit, V.; Morreau, H.; Goeman, J.J.; Bosse, T. Validity of a two-antibody testing algorithm for mismatch repair deficiency testing in cancer; a systematic literature review and meta-analysis. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2022, 35, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Risinger, J.I.; Umar, A.; Boyd, J.; Berchuck, A.; Kunkel, T.A.; Barrett, J.C. Mutation of MSH3 in endometrial cancer and evidence for its functional role in heteroduplex repair. Nat. Genet. 1996, 14, 102–105. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Talhouk, A.; Li-Chang, H.H.; Leung, S.; Huntsman, D.G.; Gilks, C.B.; McAlpine, J.N. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol. Oncol. 2015, 137, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Buza, N.; Ziai, J.; Hui, P. Mismatch repair deficiency testing in clinical practice. Expert. Rev. Mol. Diagn. 2016, 16, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Tafe, L.J.; Riggs, E.R.; Tsongalis, G.J. Lynch syndrome presenting as endometrial cancer. Clin. Chem. 2014, 60, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerdere, F.; Claes, K.B.; Van Dorpe, J.; Rottiers, I.; Van der Meulen, J.; Breyne, J.; Swaerts, K.; Martens, G. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci. Rep. 2021, 11, 12880. [Google Scholar] [CrossRef]
- Kuismanen, S.A.; Moisio, A.L.; Schweizer, P.; Truninger, K.; Salovaara, R.; Arola, J.; Butzow, R.; Jiricny, J.; Nystrom-Lahti, M.; Peltomaki, P. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am. J. Pathol. 2002, 160, 1953–1958. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Eisenberg, R.; Vnencak-Jones, C.L. Differences in Microsatellite Instability Profiles between Endometrioid and Colorectal Cancers: A Potential Cause for False-Negative Results? J. Mol. Diagn. JMD 2017, 19, 57–64. [Google Scholar] [CrossRef]
- Goodfellow, P.J.; Billingsley, C.C.; Lankes, H.A.; Ali, S.; Cohn, D.E.; Broaddus, R.J.; Ramirez, N.; Pritchard, C.C.; Hampel, H.; Chassen, A.S.; et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers from GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4301–4308. [Google Scholar] [CrossRef]
- Ferguson, S.E.; Aronson, M.; Pollett, A.; Eiriksson, L.R.; Oza, A.M.; Gallinger, S.; Lerner-Ellis, J.; Alvandi, Z.; Bernardini, M.Q.; MacKay, H.J.; et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer 2014, 120, 3932–3939. [Google Scholar] [CrossRef]
- Gatius, S.; Velasco, A.; Varela, M.; Cuatrecasas, M.; Jares, P.; Setaffy, L.; Bonhomme, B.; Santon, A.; Lindemann, K.; Croce, S.; et al. Comparison of the Idylla MSI assay with the Promega MSI Analysis System and immunohistochemistry on formalin-fixed paraffin-embedded tissue of endometrial carcinoma: Results from an international, multicenter study. Virchows Arch. Int. J. Pathol. 2022, 480, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Raut, C.P.; Rodriguez-Bigas, M.A. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis. Markers 2004, 20, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bruegl, A.S.; Kernberg, A.; Broaddus, R.R. Importance of PCR-based Tumor Testing in the Evaluation of Lynch Syndrome-associated Endometrial Cancer. Adv. Anat. Pathol. 2017, 24, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ollikainen, M.; Abdel-Rahman, W.M.; Moisio, A.L.; Lindroos, A.; Kariola, R.; Jarvela, I.; Poyhonen, M.; Butzow, R.; Peltomaki, P. Molecular analysis of familial endometrial carcinoma: A manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.S.; Blum, A.; Walts, A.; Alsabeh, R.; Tran, H.; Koeffler, H.P.; Karlan, B.Y. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol. Oncol. 2010, 116, 516–521. [Google Scholar] [CrossRef]
- Ruz-Caracuel, I.; Lopez-Janeiro, A.; Heredia-Soto, V.; Ramon-Patino, J.L.; Yebenes, L.; Berjon, A.; Hernandez, A.; Gallego, A.; Ruiz, P.; Redondo, A.; et al. Clinicopathological features and prognostic significance of CTNNB1 mutation in low-grade, early-stage endometrial endometrioid carcinoma. Virchows Arch. Int. J. Pathol. 2021, 479, 1167–1176. [Google Scholar] [CrossRef]
- Mills, A.M.; Longacre, T.A. Lynch Syndrome Screening in the Gynecologic Tract: Current State of the Art. Am. J. Surg. Pathol. 2016, 40, e35–e44. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef] [PubMed]

| (a) | |||||
| MMR Genomic Status | |||||
| Altered | No | Total | |||
| IHC | dMMR | 46 | 21 | 67 | |
| pMMR | 9 | 48 | 57 | ||
| Total | 55 | 69 | 124 | ||
| (b) | |||||
| PCR | |||||
| MSI-H | MSI-L | MSS | Total | ||
| IHC | dMMR | 22 | 7 | 34 | 63 |
| pMMR | 3 | 3 | 45 | 51 | |
| Total | 25 | 10 | 79 | 114 | |
| (c) | |||||
| PCR | |||||
| MSI-H | MSI-L | MSS | Total | ||
| MMR genomic status | Altered | 24 | 7 | 18 | 49 |
| No | 1 | 3 | 59 | 63 | |
| Total | 25 | 10 | 77 | 112 | |
| Reference Variable | Response Variable | Sensibility (%) | Specificity (%) | Total Proportion of Correct Classifications (%) |
|---|---|---|---|---|
| IHC | Genomic status | 83.6 | 69.6 | 75.8 |
| PCR (MSS + MSI-L/MSI-H) | 88.0 | 53.9 | 61.4 | |
| PCR (MSS/MSI-L + MSI-H) | 82.9 | 57.0 | 64.9 | |
| Genomic status | IHC | 68.7 | 84.2 | 75.8 |
| PCR (MSS + MSI-L/MSI-H) | 96.0 | 71.3 | 76.8 | |
| PCR (MSS/MSI-L + MSI-H) | 88.6 | 76.6 | 80.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiola, M.; Heredia-Soto, V.; Ruz-Caracuel, I.; Baillo, A.; Ramon-Patino, J.L.; Escudero, F.J.; Miguel, M.; Pelaez-Garcia, A.; Hernandez, A.; Feliu, J.; et al. Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 14468. https://doi.org/10.3390/ijms241914468
Mendiola M, Heredia-Soto V, Ruz-Caracuel I, Baillo A, Ramon-Patino JL, Escudero FJ, Miguel M, Pelaez-Garcia A, Hernandez A, Feliu J, et al. Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(19):14468. https://doi.org/10.3390/ijms241914468
Chicago/Turabian StyleMendiola, Marta, Victoria Heredia-Soto, Ignacio Ruz-Caracuel, Amparo Baillo, Jorge Luis Ramon-Patino, Francisco Javier Escudero, Maria Miguel, Alberto Pelaez-Garcia, Alicia Hernandez, Jaime Feliu, and et al. 2023. "Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer" International Journal of Molecular Sciences 24, no. 19: 14468. https://doi.org/10.3390/ijms241914468







