The Ocular Glymphatic System—Current Understanding and Future Perspectives
Abstract
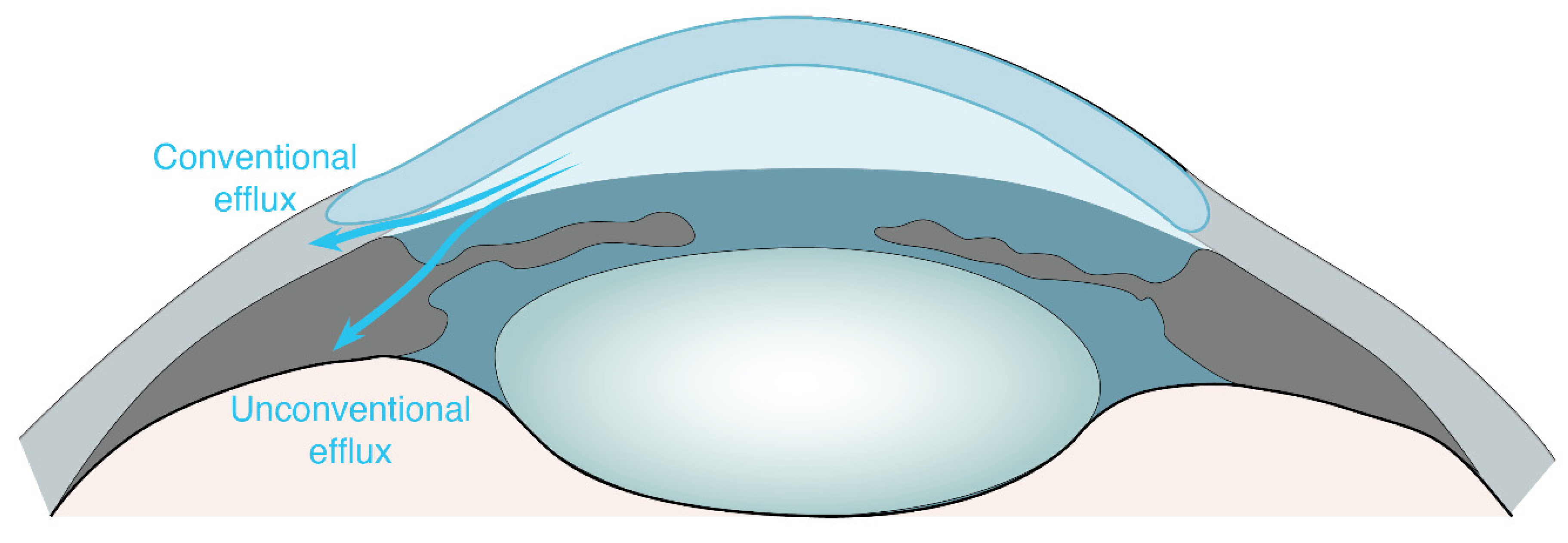
:1. Ocular Non-Glymphatic Fluid Drainage Routes
2. Ocular Glymphatic Transport
3. Ocular Glymphatic Transport in Aging and Ocular Diseases
4. Choice of Tracer to Study Ocular Glymphatic Transport
5. Importance of the Analytical Imaging Technique
6. Importance of the Correct Injection Paradigm
7. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP4 | Aquaporin-4 |
| CSF | Cerebrospinal fluid |
| ICP | Intracranial pressure |
| IOP | Intraocular pressure |
| ISF | Interstitial fluid |
| MRI | Magnetic resonance imaging |
| PVS | Perivascular spaces |
| RPE | Retinal pigment epithelium |
| SAS | Subarachnoid space |
References
- Johnson, M.; McLaren, J.W.; Overby, D.R. Unconventional Aqueous Humor Outflow: A Review. Exp. Eye Res. 2017, 158, 94–111. [Google Scholar] [CrossRef]
- Goel, M. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B.; Gagrani, M.; Ghate, D. Current Methods and New Approaches to Assess Aqueous Humor Dynamics. Expert Rev. Ophthalmol. 2021, 16, 139–160. [Google Scholar] [CrossRef]
- Park, J.-H.; Chung, H.W.; Yoon, E.G.; Ji, M.J.; Yoo, C.; Kim, Y.Y. Morphological Changes in the Trabecular Meshwork and Schlemm’s Canal after Treatment with Topical Intraocular Pressure-Lowering Agents. Sci. Rep. 2021, 11, 18169. [Google Scholar] [CrossRef] [PubMed]
- Bill, A.; Phillips, C.I. Uveoscleral Drainage of Aqueous Humour in Human Eyes. Exp. Eye Res. 1971, 12, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bill, A. The Aqueous Humor Drainage Mechanism in the Cynomolgus Monkey (Macaca Irus) with Evidence for Unconventional Routes. Investig. Ophthalmol. Vis. Sci. 1965, 4, 911–919. [Google Scholar]
- Alm, A.; Nilsson, S.F.E. Uveoscleral Outflow—A Review. Exp. Eye Res. 2009, 88, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.F.E. The Uveoscleral Outflow Routes. Eye 1997, 11, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B.; Gregerson, D.S.; Pederson, J.E. Uveoscleral Outflow Using Different-Sized Fluorescent Tracers in Normal and Inflamed Eyes. Exp. Eye Res. 1987, 45, 525–532. [Google Scholar] [CrossRef]
- Yücel, Y.H.; Johnston, M.G.; Ly, T.; Patel, M.; Drake, B.; Gümüş, E.; Fraenkl, S.A.; Moore, S.; Tobbia, D.; Armstrong, D.; et al. Identification of Lymphatics in the Ciliary Body of the Human Eye: A Novel “Uveolymphatic” Outflow Pathway. Exp. Eye Res. 2009, 89, 810–819. [Google Scholar] [CrossRef]
- Kim, M.; Johnston, M.G.; Gupta, N.; Moore, S.; Yücel, Y.H. A Model to Measure Lymphatic Drainage from the Eye. Exp. Eye Res. 2011, 93, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, A.; Thakur, S.; Nongpiur, M.E.; Schmetterer, L.; Hong, Y.-K.; Huang, A.S.; Wong, T.T. Aqueous Outflow Channels and Its Lymphatic Association: A Review. Surv. Ophthalmol. 2022, 67, 659–674. [Google Scholar] [CrossRef]
- Civan, M.M. Chapter 1 Formation of the Aqueous Humor: Transport Components and Their Integration. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2008; Volume 62, pp. 1–45. ISBN 1063-5823. [Google Scholar]
- Smith, D.W.; Lee, C.-J.; Gardiner, B.S. No Flow through the Vitreous Humor: How Strong Is the Evidence? Prog. Retin. Eye Res. 2020, 78, 100845. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Bok, D. A Specific Receptor for Retinol Binding Protein as Detected by the Binding of Human and Bovine Retinol Binding Protein to Pigment Epithelial Cells. Am. J. Ophthalmol. 1976, 81, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The Dynamic Nature of Bruch’s Membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dvoriashyna, M.; Foss, A.J.E.; Gaffney, E.A.; Repetto, R. Fluid and Solute Transport across the Retinal Pigment Epithelium: A Theoretical Model. J. R. Soc. Interface 2020, 17, 20190735. [Google Scholar] [CrossRef]
- Marmor, M.F. Mechanisms of Fluid Accumulation in Retinal Edema. Doc. Ophthalmol. 1999, 97, 239–249. [Google Scholar] [CrossRef]
- Törnquist, P.; Alm, A.; Bill, A. Permeability of Ocular Vessels and Transport across the Blood-Retinal-Barrier. Eye 1990, 4, 303–309. [Google Scholar] [CrossRef]
- Damasceno, R.W.F.; Barbosa, J.A.P.; Cortez, L.R.C.; Belfort, R., Jr. Orbital Lymphatic Vessels: Immunohistochemical Detection in the Lacrimal Gland, Optic Nerve, Fat Tissue, and Extrinsic Oculomotor Muscles. Arq. Bras. Oftalmol. 2021, 84, 209–213. [Google Scholar] [CrossRef]
- Killer, H.E.; Laeng, H.R.; Groscurth, P. Lymphatic Capillaries in the Meninges of the Human Optic Nerve. J. Neuroophthalmol. 1999, 19, 222–228. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Förstera, B.; Peng, S.; Shi, M.; Ladrón-de-Guevara, A.; et al. An Ocular Glymphatic Clearance System Removes β-Amyloid from the Rodent Eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, S.; Lee, J.H.; Dong, H.; Mourgkos, G.; Terwilliger, G.; Kraus, A.; Geraldo, L.H.; Poulet, M.; Fischer, S.; et al. Compartmentalized Ocular Lymphatic System Mediates Eye–Brain Immunity. Nature 2024, 658, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular Spaces in the Brain: Anatomy, Physiology and Pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-Dependent Glymphatic Solute Transport in the Rodent Brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef] [PubMed]
- Murdock, M.H.; Yang, C.-Y.; Sun, N.; Pao, P.-C.; Blanco-Duque, C.; Kahn, M.C.; Kim, T.; Lavoie, N.S.; Victor, M.B.; Islam, M.R.; et al. Multisensory Gamma Stimulation Promotes Glymphatic Clearance of Amyloid. Nature 2024, 627, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired Glymphatic Function and Clearance of Tau in an Alzheimer’s Disease Model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Anderson, B.; Saltzman, H.A. Retinal Oxygen Utilization Measured by Hyperbaric Blackout. Arch. Ophthalmol. 1964, 72, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Medrano, C.J.; Fox, D.A. Oxygen Consumption in the Rat Outer and Inner Retina: Light- and Pharmacologically-Induced Inhibition. Exp. Eye Res. 1995, 61, 273–284. [Google Scholar] [CrossRef]
- Denniston, A.K.; Keane, P.A. Paravascular Pathways in the Eye: Is There an “Ocular Glymphatic System”? Investig. Ophthalmol. Vis. Sci. 2015, 56, 3955–3956. [Google Scholar] [CrossRef]
- Wostyn, P.; van Dam, D.; Audenaert, K.; Killer, H.E.; de Deyn, P.P.; de Groot, V. A New Glaucoma Hypothesis: A Role of Glymphatic System Dysfunction. Fluids Barriers CNS 2015, 12, 16. [Google Scholar] [CrossRef]
- Wostyn, P.; Killer, H.E.; De Deyn, P.P. Glymphatic Stasis at the Site of the Lamina Cribrosa as a Potential Mechanism Underlying Open-angle Glaucoma. Clin. Exp. Ophthalmol. 2017, 45, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed. Res. Int. 2017, 2017, 5123148. [Google Scholar] [CrossRef]
- Denniston, A.K.; Keane, P.A.; Aojula, A.; Sinclair, A.J.; Mollan, S.P. The Ocular Glymphatic System and Idiopathic Intracranial Hypertension: Author Response to “Hypodense Holes and the Ocular Glymphatic System”. Investig. Opthalmol. Vis. Sci. 2017, 58, 1134. [Google Scholar] [CrossRef] [PubMed]
- Denniston, A.K.; Keane, P.A. “Black Holes” and the Ocular Glymphatic System: Author Response to “The Glymphatic System: A New Player in Ocular Diseases?”. Investig. Opthalmol. Vis. Sci. 2016, 57, 5428. [Google Scholar] [CrossRef] [PubMed]
- Nuel, J.; Benoit, F. The Paths of Elimination of the Intra-Ocular Fluid of the Anterior Chamber and the Back of the Eye. Arch. D’opht 1900, 20, 11. [Google Scholar]
- Berens, C.; Posner, A. The Circulation of the Intra-Ocular Fluid: The Importance of the Optic Nerve. Trans. Am. Ophthalmol. Soc. 1932, 30, 227–244. [Google Scholar]
- Rodriguez-Peralta, L.A. Hematic and Fluid Barriers in the Optic Nerve. J. Comp. Neurol. 1966, 126, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Posterior Drainage of the Intraocular Fluid from the Vitreous. Exp. Eye Res. 1966, 5, 123-IN16. [Google Scholar] [CrossRef]
- Cantrill Herbert, L.; Pederson Jonathan, E. Experimental Retinal Detachment: VI. The Permeability of the Blood-Retinal Barrier. Arch. Ophthalmol. 1984, 102, 747. [Google Scholar] [CrossRef]
- Toris, C.B.; Pederson, J.E. Experimental Retinal Detachment: VIII. Retinochoroidal Horseradish Peroxidase Diffusion across the Blood-Retinal Barrier. Arch. Ophthalmol. 1985, 103, 266. [Google Scholar] [CrossRef] [PubMed]
- Cantrill Herbert, L.; Pederson Jonathan, E. Experimental Retinal Detachment: III. Vitreous Fluorophotometry. Arch. Ophthalmol. 1982, 100, 1810. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, I.; Yamashita, H. An Electron Microscopic Study on the Blood-Optic Nerve and Fluid-Optic Nerve Barrier. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 1975, 196, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Optic Disc Edema in Raised Intracranial Pressure. Arch. Ophthalmol. 1977, 95, 1553. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Fluids in the Anterior Part of the Optic Nerve in Health and Disease. Surv. Ophthalmol. 1978, 23, 1–25. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; De Deyn, P.P.; Killer, H.E. The Glymphatic System: A New Player in Ocular Diseases? Investig. Opthalmol. Vis. Sci. 2016, 57, 5426. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Gupta, N.; Ahari, A.; Zhou, X.; Hanna, J.; Yücel, Y.H. Evidence for Cerebrospinal Fluid Entry Into the Optic Nerve via a Glymphatic Pathway. Investig. Opthalmol. Vis. Sci. 2017, 58, 4784. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Veruki, M.L.; Torp, R.; Haug, F.-M.; Laake, J.H.; Nielsen, S.; Agre, P.; Ottersen, O.P. Aquaporin-4 Water Channel Protein in the Rat Retina and Optic Nerve: Polarized Expression in Müller Cells and Fibrous Astrocytes. J. Neurosci. 1998, 18, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Delle, C.; Peng, W.; Plá, V.; Giannetto, M.; Kusk, P.; Sigurdsson, B.; Sakurai, S.; Sweeney, A.; Sun, Q.; et al. Age- and Glaucoma-Induced Changes to the Ocular Glymphatic System. Neurobiol. Dis. 2023, 188, 106322. [Google Scholar] [CrossRef]
- Tong, X.J.; Akdemir, G.; Wadhwa, M.; Verkman, A.S.; Smith, A.J. Large Molecules from the Cerebrospinal Fluid Enter the Optic Nerve but Not the Retina of Mice. Fluids Barriers CNS 2024, 21, 1. [Google Scholar] [CrossRef]
- Kimball, E.; Schaub, J.; Quillen, S.; Keuthan, C.; Pease, M.E.; Korneva, A.; Quigley, H. The Role of Aquaporin-4 in Optic Nerve Head Astrocytes in Experimental Glaucoma. PLoS ONE 2021, 16, e0244123. [Google Scholar] [CrossRef] [PubMed]
- Kimball, E.C.; Quillen, S.; Pease, M.E.; Keuthan, C.; Nagalingam, A.; Zack, D.J.; Johnson, T.V.; Quigley, H.A. Aquaporin 4 Is Not Present in Normal Porcine and Human Lamina Cribrosa. PLoS ONE 2022, 17, e0268541. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Gupta, N.; Paczka-Giorgi, L.A.; Zhou, X.; Ahari, A.; Lani, R.; Hanna, J.; Yücel, Y.H. Reduced Cerebrospinal Fluid Inflow to the Optic Nerve in Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 5876. [Google Scholar] [CrossRef] [PubMed]
- Delle, C.; Wang, X.; Giannetto, M.; Newbold, E.; Peng, W.; Gomolka, R.S.; Ladrón-de-Guevara, A.; Cankar, N.; Schiøler Nielsen, E.; Kjaerby, C.; et al. Transient but Not Chronic Hyperglycemia Accelerates Ocular Glymphatic Transport. Fluids Barriers CNS 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, J.; Zhang, W.; Gong, X.; Yan, S.; Zhang, K.; Luo, Z.; Sun, J.; Jiang, Q.; Lou, M. Impairment of the Glymphatic Pathway and Putative Meningeal Lymphatic Vessels in the Aging Human. Ann. Neurol. 2020, 87, 357–369. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of Paravascular Clearance Pathways in the Aging Brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of Cerebrospinal Fluid Is Driven by Arterial Pulsations and Is Reduced in Hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef] [PubMed]
- Delle, C.; Cankar, N.; Digebjerg Holgersson, C.; Hvorup Knudsen, H.; Schiøler Nielsen, E.; Kjaerby, C.; Mori, Y.; Nedergaard, M.; Weikop, P. Long-Term High-Fat Diet Increases Glymphatic Activity in the Hypothalamus in Mice. Sci. Rep. 2023, 13, 4137. [Google Scholar] [CrossRef] [PubMed]
- von Holstein-Rathlou, S.; Petersen, N.C.; Nedergaard, M. Voluntary Running Enhances Glymphatic Influx in Awake Behaving, Young Mice. Neurosci. Lett. 2018, 662, 253–258. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian Control of Brain Glymphatic and Lymphatic Fluid Flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef]
- Holstein-Rønsbo, S.; Gan, Y.; Giannetto, M.J.; Rasmussen, M.K.; Sigurdsson, B.; Beinlich, F.R.M.; Rose, L.; Untiet, V.; Hablitz, L.M.; Kelley, D.H.; et al. Glymphatic Influx and Clearance Are Accelerated by Neurovascular Coupling. Nat. Neurosci. 2023, 26, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the Eye: The Role of Amyloid Beta in Retinal Degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Bedussi, B.; Almasian, M.; de Vos, J.; VanBavel, E.; Bakker, E.N. Paravascular Spaces at the Brain Surface: Low Resistance Pathways for Cerebrospinal Fluid Flow. J. Cereb. Blood Flow Metab. 2018, 38, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.H.; Ringstad, G.; Jørstad, Ø.K.; Moe, M.C.; Sandell, T.; Eide, P.K. The Human Visual Pathway Communicates Directly With the Subarachnoid Space. Investig. Opthalmol. Vis. Sci. 2019, 60, 2773. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.; Ponnaganti, S.; Dandu, R. Beware of Artifacts in Orbital Magnetic Resonance Imaging. Indian J. Ophthalmol. 2020, 68, 2516. [Google Scholar] [CrossRef]
- Li, S.K.; Hao, J.; Liu, H.; Lee, J. MRI Study of Subconjunctival and Intravitreal Injections. J. Pharm. Sci. 2012, 101, 2353–2363. [Google Scholar] [CrossRef]

| Tracer | Size (in kDa) | Reference | Suitability |
|---|---|---|---|
| Study of retrograde ocular glymphatic transport (intracisternal injection) | |||
| Dextran * | 2000 | [51] | Unsuitable, too large |
| 500 | [48] | Unsuitable, too large | |
| 70 (*) | [48,51] | Low experimental experience; infrequent transport was reported [48] | |
| 40 * | [48] | Suitable | |
| 10 * | [22,48,50,54,55] | Suitable; optimal for deeper/farther tissue distribution | |
| Ovalbumin * | 45 * | [48] | Suitable |
| Bovine serum albumin | 66 * | [50,55] | Suitable |
| Study of anterograde ocular glymphatic transport (intravitreal injection) | |||
| Human beta-amyloid (1–40) | 4.7 * | [22,50,55] | Suitable |
| Study of glial lamina integrity (intravitreal injection) | |||
| Dextran | 500 * | [22] | Usage of different-sized tracers aid to determine extent of barrier breakdown |
| 10 * | [22,55] | ||
| 3 * | [22] | ||
| Anterograde ocular glymphatic transport—intravitreal injection | ||
| Total volume | Flow rate | Total injection duration |
| 1 µL | 0.2 µL/min | 5 min |
| Retrograde ocular glymphatic transport—intracisternal injection | ||
| Total volume | Flow rate | Total injection duration |
| 10 µL | 1.5 µL/min | 10 min |
| 15 µL | 2 µL/min | 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delle, C.; Wang, X.; Nedergaard, M. The Ocular Glymphatic System—Current Understanding and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 5734. https://doi.org/10.3390/ijms25115734
Delle C, Wang X, Nedergaard M. The Ocular Glymphatic System—Current Understanding and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(11):5734. https://doi.org/10.3390/ijms25115734
Chicago/Turabian StyleDelle, Christine, Xiaowei Wang, and Maiken Nedergaard. 2024. "The Ocular Glymphatic System—Current Understanding and Future Perspectives" International Journal of Molecular Sciences 25, no. 11: 5734. https://doi.org/10.3390/ijms25115734
APA StyleDelle, C., Wang, X., & Nedergaard, M. (2024). The Ocular Glymphatic System—Current Understanding and Future Perspectives. International Journal of Molecular Sciences, 25(11), 5734. https://doi.org/10.3390/ijms25115734





