Genome-Wide Identification of TLP Gene Family in Populus trichocarpa and Functional Characterization of PtTLP6, Preferentially Expressed in Phloem
Abstract
1. Introduction
2. Results
2.1. Identification and Phylogenetic Analysis of TLP Gene Family in P. trichocarpa
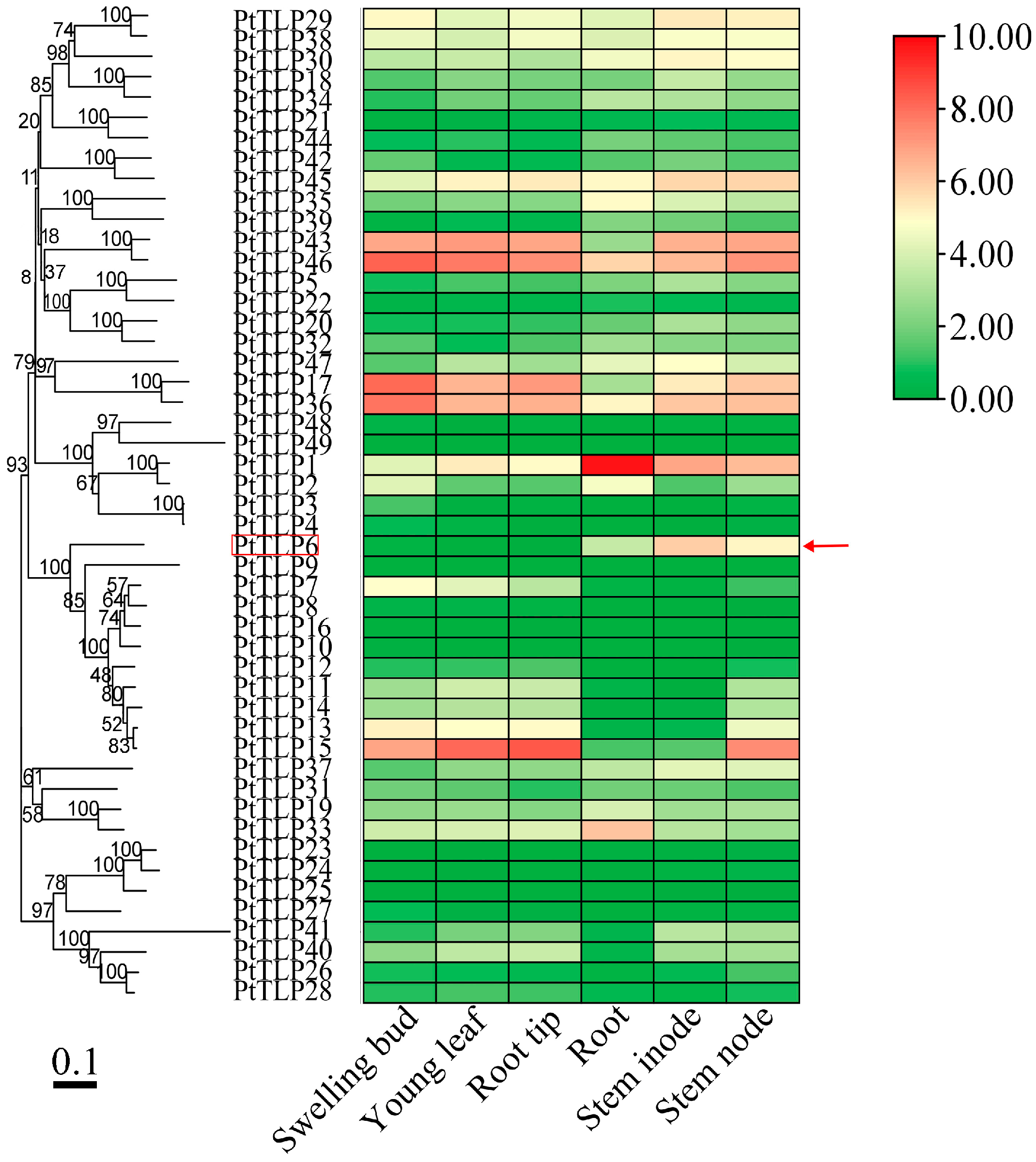
2.2. Gene Structures, Conserved Motifs, and Expression Patterns in PtTLP Genes
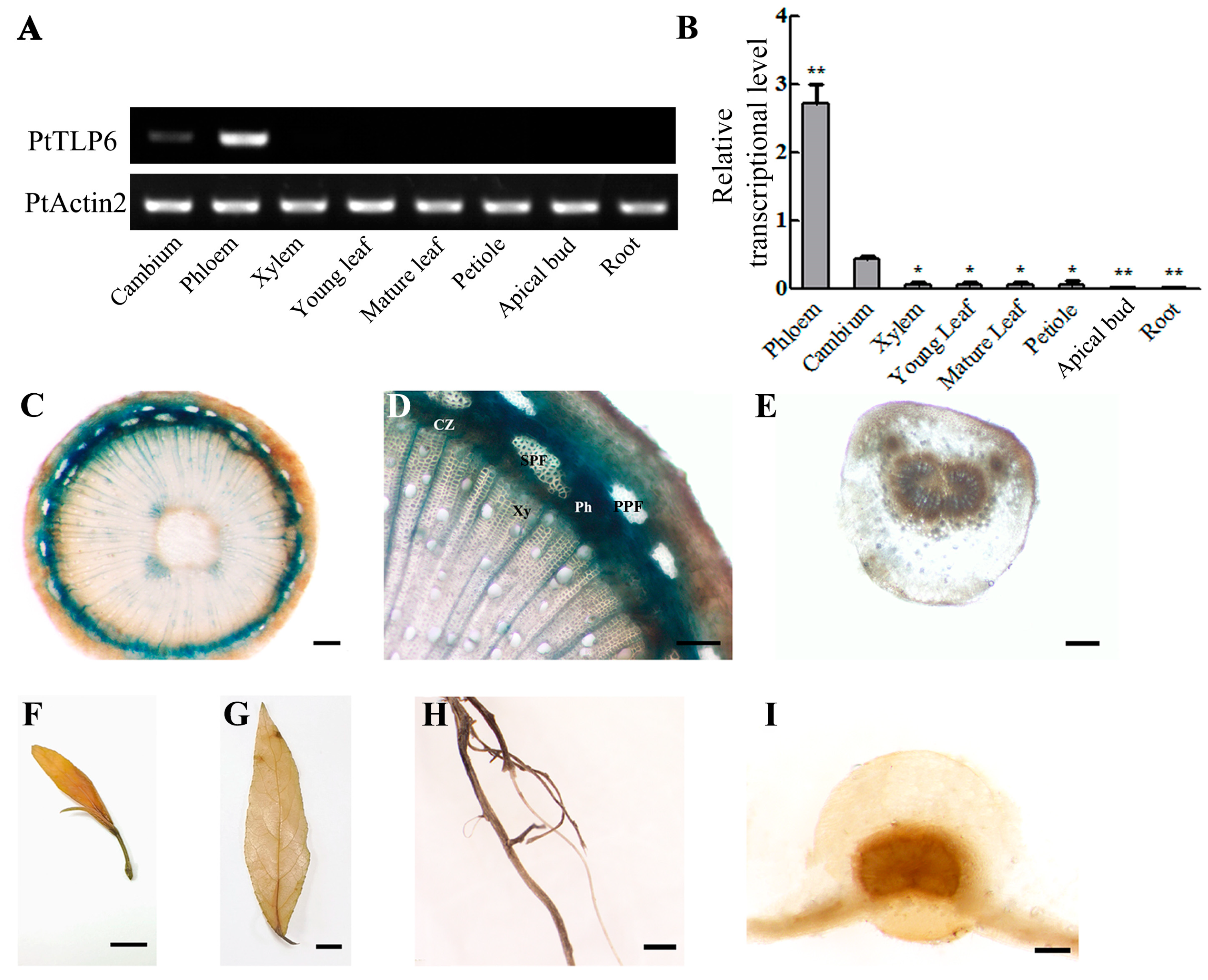
2.3. PtTLP6 Highly and Preferentially Expressed in Populus Phloem
2.4. Overexpression of PtTLP6 Gene in Arabidopsis Leads to Early Flowering
2.5. Expression Levels of Flowering Regulating Genes Were Not Altered in PtTLP6-OE Arabidopsis
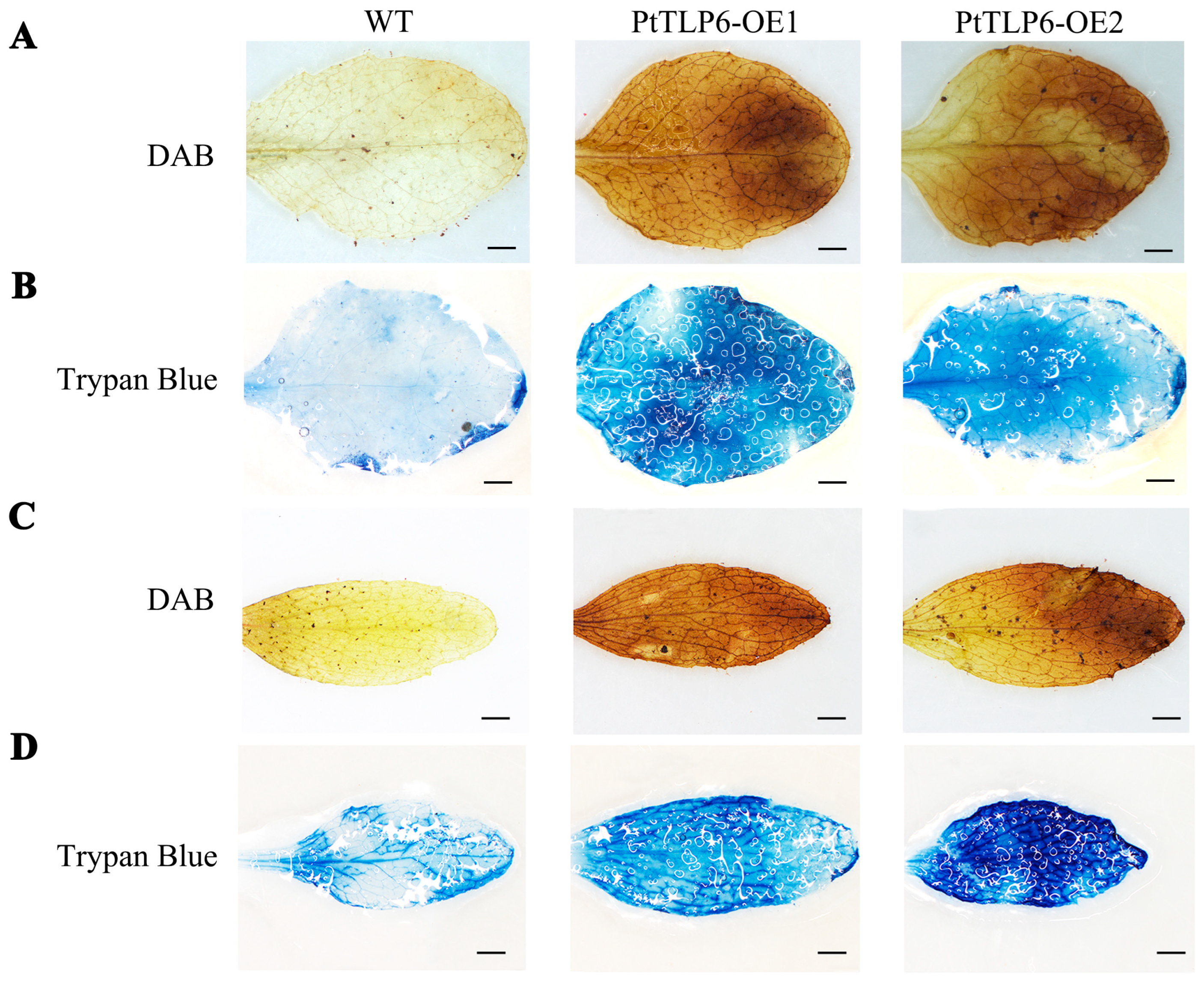
2.6. Overexpression of PtTLP6 Induces ROS Burst in Arabidopsis
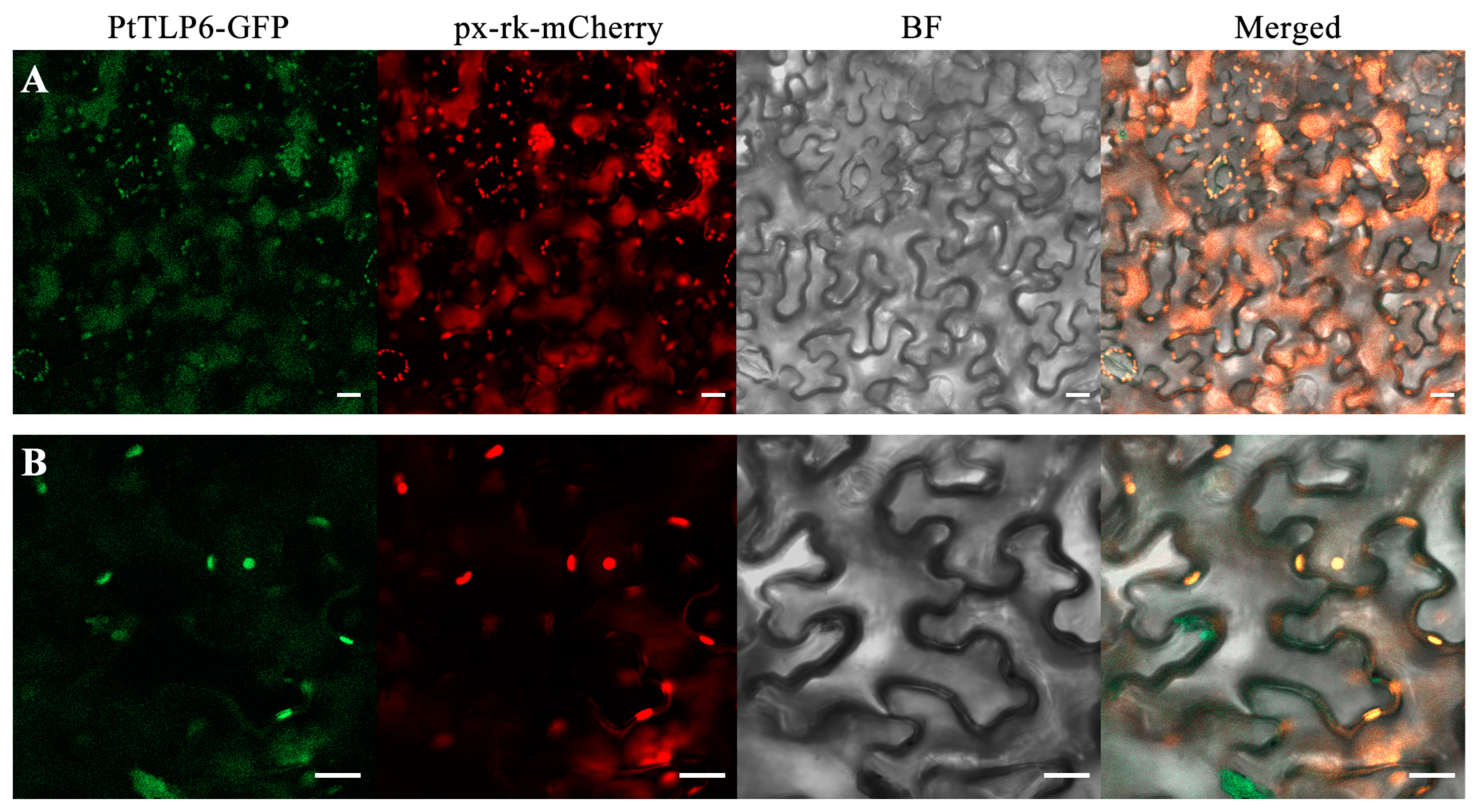
2.7. PtTLP6 Is Localized in Peroxisomes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Identification of the TLP Genes in P. trichocarpa
4.3. Chromosomal Duplication Analyses and Gene and Protein Structure Analysis
4.4. Microarray Data Analysis and Extraction of RNA, RT-PCR, and RT-qPCR
4.5. Vector Construction
4.6. Genetic Transformation
4.7. GUS Staining
4.8. Subcellular Localization
4.9. Protein Extraction and Western Blot Analysis
4.10. Histochemical Determination of H2O2
4.11. Trypan Blue Staining
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selitrennikoff, C.P. Antifungal proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Brandazza, A.; Angeli, S.; Tegoni, M.; Cambillau, C.; Pelosi, P. Plant stress proteins of the thaumatin-like family discovered in animals. FEBS Lett. 2004, 572, 3–7. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Cui, Y.; Qiao, K.; Zhu, L.; Fan, S.; Ma, Q. Comprehensive genome-wide analysis of thaumatin-like gene family in four cotton species and functional identification of GhTLP19 involved in regulating tolerance to Verticillium dahlia and drought. Front. Plant Sci. 2020, 11, 575015. [Google Scholar] [CrossRef]
- Liu, Q.; Sui, X.; Wang, Y.; Zhu, M.; Zhou, Y.; Gao, F. Genome-wide analyses of thaumatin-like protein family genes reveal the involvement in the response to low-temperature stress in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 2209. [Google Scholar] [CrossRef]
- Kuwabara, C.; Takezawa, D.; Shimada, T.; Hamada, T.; Fujikawa, S.; Arakawa, K. Abscisic acid- and cold-induced thaumatin-like protein in winter wheat has an antifungal activity against snow mould, Microdochium nivale. Physiol. Plant 2002, 115, 101–110. [Google Scholar] [CrossRef]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric beta-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef]
- Kitajima, S.; Sato, F. Plant pathogenesis-related proteins: Molecular mechanisms of gene expression and protein function. J. Biochem. 1999, 125, 1–8. [Google Scholar] [CrossRef]
- Cao, J.; Lv, Y.; Hou, Z.; Li, X.; Ding, L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regul. 2016, 79, 299–307. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Choat, B.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Drought tolerance traits do not vary across sites differing in water availability in Banksia serrata (Proteaceae). Funct. Plant Biol. 2019, 46, 624–633. [Google Scholar] [CrossRef]
- Jayasankar, S.; Li, Z.; Gray, D.J. Constitutive expression of Vitis vinifera thaumatin-like protein after in vitro selection and its role in anthracnose resistance. Funct. Plant Biol. 2003, 30, 1105–1115. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, H.; Zhang, X.; Guo, C.; Wang, M.; Wang, Y.; Wang, X. Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 2017, 7, 4269. [Google Scholar] [CrossRef]
- Misra, R.C.; Sandeep Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef]
- Rout, E.; Nanda, S.; Joshi, R.K. Molecular characterization and heterologous expression of a pathogen induced PR5 gene from garlic (Allium sativum L.) conferring enhanced resistance to necrotrophic fungi. Eur. J. Plant Pathol. 2016, 144, 345–360. [Google Scholar] [CrossRef]
- Munis, M.F.; Tu, L.; Deng, F.; Tan, J.; Xu, L.; Xu, S.; Long, L.; Zhang, X. A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco. Biochem. Biophys. Res. Commun. 2010, 393, 38–44. [Google Scholar] [CrossRef]
- Neale, A.D.; Wahleithner, J.A.; Lund, M.; Bonnett, H.T.; Kelly, A.; Meeks-Wagner, D.R.; Peacock, W.J.; Dennis, E.S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell 1990, 2, 673–684. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, A.K.; Xiang, F.; Park, C.M. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: Insights into their roles in salt response of seed germination. Plant Cell Physiol. 2008, 49, 334–344. [Google Scholar] [CrossRef]
- Ogata, C.M.; Gordon, P.F.; de Vos, A.M.; Kim, S.H. Crystal structure of a sweet tasting protein thaumatin I, at 1.65 A resolution. J. Mol. Biol. 1992, 228, 893–908. [Google Scholar] [CrossRef]
- Batalia, M.A.; Monzingo, A.F.; Ernst, S.; Roberts, W.; Robertus, J.D. The crystal structure of the antifungal protein zeamatin, a member of the thaumatin-like, PR-5 protein family. Nat. Struct. Biol. 1996, 3, 19–23. [Google Scholar] [CrossRef]
- Koiwa, H.; Kato, H.; Nakatsu, T.; Oda, J.; Yamada, Y.; Sato, F. Crystal structure of tobacco PR-5d protein at 1.8 A resolution reveals a conserved acidic cleft structure in antifungal thaumatin-like proteins. J. Mol. Biol. 1999, 286, 1137–1145. [Google Scholar] [CrossRef]
- Min, K.; Ha, S.C.; Hasegawa, P.M.; Bressan, R.A.; Yun, D.J.; Kim, K.K. Crystal structure of osmotin, a plant antifungal protein. Proteins 2004, 54, 170–173. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakrabarti, C. Crystal structure analysis of NP24-I: A thaumatin-like protein. Planta 2008, 228, 883–890. [Google Scholar] [CrossRef]
- Shatters, R.G., Jr.; Boykin, L.M.; Lapointe, S.L.; Hunter, W.B.; Weathersbee, A.A., 3rd. Phylogenetic and structural relationships of the PR5 gene family reveal an ancient multigene family conserved in plants and select animal taxa. J. Mol. Evol. 2006, 63, 12–29. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C.; et al. The genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, e38. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, R.; Jiang, S.Y.; Kumar, N.; Venkatesh, P.N.; Ramachandran, S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008, 49, 865–879. [Google Scholar] [CrossRef]
- Lee, T.H.; Tang, H.; Wang, X.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2013, 41, 1152–1158. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Zamani, A.; Ekramoddoullah, A.K. Expression profiling of a complex thaumatin-like protein family in western white pine. Planta 2010, 231, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Sreedasyam, A.; Plott, C.; Hossain, M.S.; Lovell, J.T.; Grimwood, J.; Jenkins, J.W.; Daum, C.; Barry, K.; Carlson, J.; Shu, S.; et al. JGI Plant Gene Atlas: An updateable transcriptome resource to improve functional gene descriptions across the plant kingdom. Nucleic Acids Res. 2023, 51, 8383–8401. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell. Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550–550.e2. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.H.; Zipfel, W.R.; Webb, W.W.; Hanson, M.R. The green fluorescent protein as a marker to visualize plant mitochondria in vivo. Plant J. 1997, 11, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Dabney-Smith, C.; van Den Wijngaard, P.W.; Treece, Y.; Vredenberg, W.J.; Bruce, B.D. The C terminus of a chloroplast precursor modulates its interaction with the translocation apparatus and PIRAC. J. Biol. Chem. 1999, 274, 32351–32359. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, J.; Zhou, X.; Luan, Y.; Luan, F. Genome-wide identification, characterization and expression analysis of the TLP gene family in melon (Cucumis melo L.). Genomics 2020, 112, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhou, X.; Zhang, X.; Li, X.; Zhang, P.; He, Y. Genome-wide identification and characterization of thaumatin-like protein family genes in wheat and analysis of their responses to Fusarium head blight infection. Food Prod. Process. Nutr. 2022, 4, 24. [Google Scholar] [CrossRef]
- Zhao, J.P.; Su, X.H. Patterns of molecular evolution and predicted function in thaumatin-like proteins of Populus trichocarpa. Planta 2010, 232, 949–962. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2016, 44, D1167–D1171. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, V.; De Swaef, T.; Bauweraerts, I.; Steppe, K. Phloem transport: A review of mechanisms and controls. J. Exp. Bot. 2013, 64, 4839–4850. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Shinohara, H.; Matsubayashi, Y.; Fukuda, H. A novel pollen-pistil interaction conferring high-temperature tolerance during reproduction via CLE45 signaling. Curr. Biol. 2013, 23, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Sawikowska, A.; Lee, J.E.; Benstein, R.M.; Neumann, M.; Krajewski, P.; Schmid, M. Phloem companion cell-specific transcriptomic and epigenomic analyses identify MRF1, a regulator of flowering. Plant Cell 2019, 31, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Hardtke, C.S. Phloem development. New Phytol. 2023, 239, 852–867. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Xu, J.; Li, Z.; Jiang, C.; Zhou, Y.; Yang, Z.; Deng, M.; Lv, J.; Zhao, K. Tomato-thaumatin-like protein genes Solyc08g080660 and Solyc08g080670 confer resistance to five soil-borne diseases by enhancing β-1,3-glucanase activity. Genes 2023, 14, 1622. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shang, X.; He, Q.; Li, W.; Song, X.; Zhang, B.; Guo, W. A cell wall-localized β-1,3-glucanase promotes fiber cell elongation and secondary cell wall deposition. Plant Physiol. 2023, 194, 106–123. [Google Scholar] [CrossRef]
- Wang, F.; Shen, S.; Cui, Z.; Yuan, S.; Qu, P.; Jia, H.; Meng, L.; Hao, X.; Liu, D.; Ma, L.; et al. Puccinia triticina effector protein Pt_21 interacts with wheat thaumatin-like protein TaTLP1 to inhibit its antifungal activity and suppress wheat apoplast immunity. Crop J. 2023, 11, 1431–1440. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, S.; Jiang, D.; Lu, J.; Huang, Z.; Liu, Z.; Zhou, H.; Zhuang, C.; Li, J. Initiation and execution of programmed cell death and regulation of reactive oxygen species in plants. Int. J. Mol. Sci. 2021, 22, 12942. [Google Scholar] [CrossRef]
- Mittler, R.; Lam, E. In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol. 1995, 108, 489–493. [Google Scholar] [CrossRef]
- Lee, J.; Chen, H.; Lee, G.; Emonet, A.; Kim, S.G.; Shim, D.; Lee, Y. MSD2-mediated ROS metabolism fine-tunes the timing of floral organ abscission in Arabidopsis. New Phytol. 2022, 235, 2466–2480. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, J.; Chen, H.; Hu, J.; Liu, P.; Zhou, B. RNA-seq analysis of apical meristem reveals integrative regulatory network of ROS and chilling potentially related to flowering in Litchi chinensis. Sci. Rep. 2017, 7, 10183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, N.; Zhang, Z.; Huang, X.; Chen, H.; Hu, Z.; Pang, X.; Liu, W.; Lu, Y. Hydrogen peroxide and nitric oxide promote reproductive growth in Litchi chinensis. Biol. Plant. 2012, 56, 321–329. [Google Scholar] [CrossRef]
- Zimmermann, P.; Heinlein, C.; Orendi, G.; Zentgraf, U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006, 29, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Okumoto, K.; Tateishi, K.; Ikoma, Y.; Matsuda, E.; Nishimura, M.; Tsukamoto, T.; Osumi, T.; Ohashi, K.; Higuchi, O.; et al. Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: Studies with PEX5-defective CHO cell mutants. Mol. Cell. Biol. 1998, 18, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Kasuya-Arai, I.; Mori, H.; Miyazawa, S.; Osumi, T.; Hashimoto, T.; Fujiki, Y. Carboxyl-terminal consensus Ser-Lys-Leu-related tripeptide of peroxisomal proteins functions in vitro as a minimal peroxisome-targeting signal. J. Biol. Chem. 1992, 267, 14405–14411. [Google Scholar] [CrossRef] [PubMed]
- Osumi, T.; Tsukamoto, T.; Hata, S.; Yokota, S.; Miura, S.; Fujiki, Y.; Hijikata, M.; Miyazawa, S.; Hashimoto, T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem. Biophys. Res. Commun. 1991, 181, 947–954. [Google Scholar] [CrossRef]
- Letunic, I.; Copley, R.R.; Schmidt, S.; Ciccarelli, F.D.; Doerks, T.; Schultz, J.; Ponting, C.P.; Bork, P. SMART 4.0: Towards genomic data integration. Nucleic Acids Res. 2004, 32, 142–144. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Salvatore, M.; Emanuelsson, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting sequence signals in targeting peptides using deep learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhen, C.; Xu, W.; Wang, C.; Cheng, Y. Simple, rapid and efficient transformation of genotype Nisqually-1: A basic tool for the first sequenced model tree. Sci. Rep. 2017, 7, 2638. [Google Scholar] [CrossRef]
- Rolland, V. Determining the subcellular localization of fluorescently tagged proteins using protoplasts extracted from transiently transformed Nicotiana benthamiana Leaves. Methods Mol. Biol. 2018, 1770, 263–283. [Google Scholar] [CrossRef]
- Cao, S.; Guo, M.; Cheng, J.; Cheng, H.; Liu, X.; Ji, H.; Liu, G.; Cheng, Y.; Yang, C. Aspartic proteases modulate programmed cell death and secondary cell wall synthesis during wood formation in poplar. J. Exp. Bot. 2022, 73, 6876–6890. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhou, M.; Cheng, Y. Lectin affinity-based glycoproteome analysis of the developing xylem in poplar. For. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. A simple, rapid, and reliable protocol to localize hydrogen peroxide in large plant organs by DAB-mediated tissue printing. Front. Plant Sci. 2014, 5, 745. [Google Scholar] [CrossRef]
- Bowling, S.A.; Clarke, J.D.; Liu, Y.; Klessig, D.F.; Dong, X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 1997, 9, 1573–1584. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Ma, X.; Xu, S.; Cheng, J.; Xu, W.; Elsheery, N.I.; Cheng, Y. Genome-Wide Identification of TLP Gene Family in Populus trichocarpa and Functional Characterization of PtTLP6, Preferentially Expressed in Phloem. Int. J. Mol. Sci. 2024, 25, 5990. https://doi.org/10.3390/ijms25115990
Guo M, Ma X, Xu S, Cheng J, Xu W, Elsheery NI, Cheng Y. Genome-Wide Identification of TLP Gene Family in Populus trichocarpa and Functional Characterization of PtTLP6, Preferentially Expressed in Phloem. International Journal of Molecular Sciences. 2024; 25(11):5990. https://doi.org/10.3390/ijms25115990
Chicago/Turabian StyleGuo, Mengjie, Xujun Ma, Shiying Xu, Jiyao Cheng, Wenjing Xu, Nabil Ibrahim Elsheery, and Yuxiang Cheng. 2024. "Genome-Wide Identification of TLP Gene Family in Populus trichocarpa and Functional Characterization of PtTLP6, Preferentially Expressed in Phloem" International Journal of Molecular Sciences 25, no. 11: 5990. https://doi.org/10.3390/ijms25115990
APA StyleGuo, M., Ma, X., Xu, S., Cheng, J., Xu, W., Elsheery, N. I., & Cheng, Y. (2024). Genome-Wide Identification of TLP Gene Family in Populus trichocarpa and Functional Characterization of PtTLP6, Preferentially Expressed in Phloem. International Journal of Molecular Sciences, 25(11), 5990. https://doi.org/10.3390/ijms25115990





