Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Overview
2.2. DNA Extraction and PCR Amplification
2.3. Index PCR and Illumina Sequencing
2.4. Data Analysis
3. Results
3.1. Overall Data Analysis
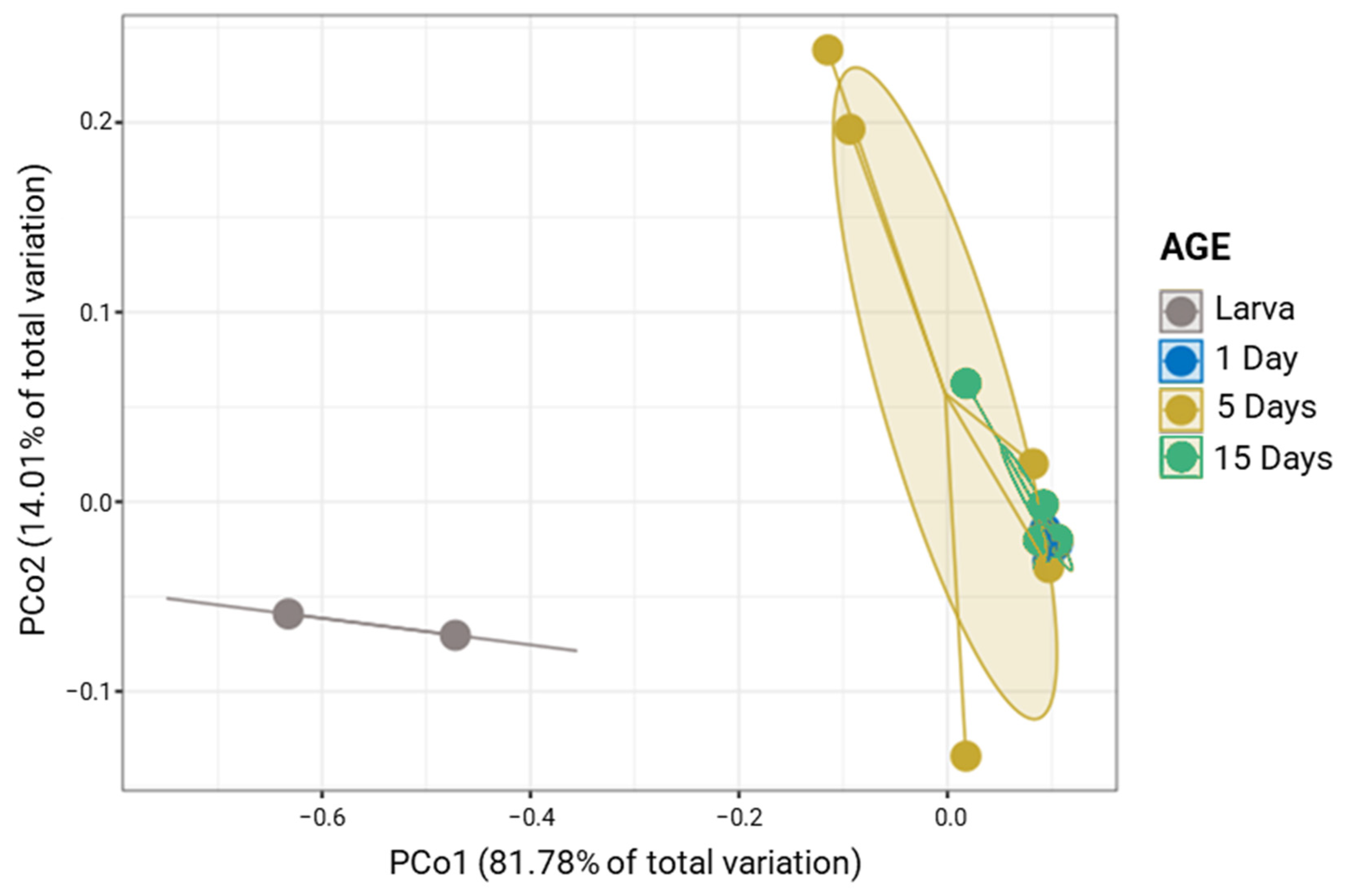
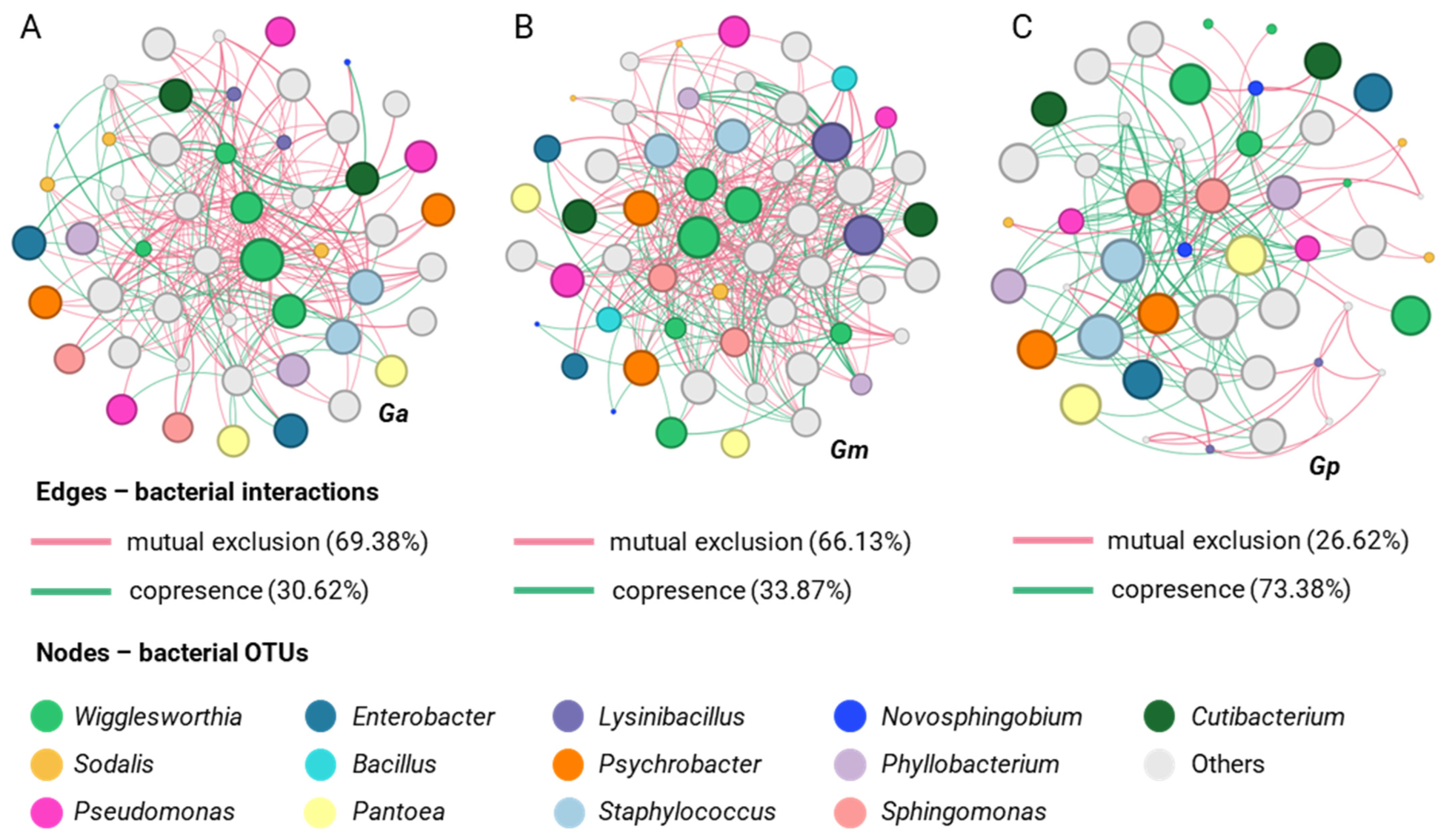
3.2. Laboratory Populations Display a Species-Specific Bacterial Profile
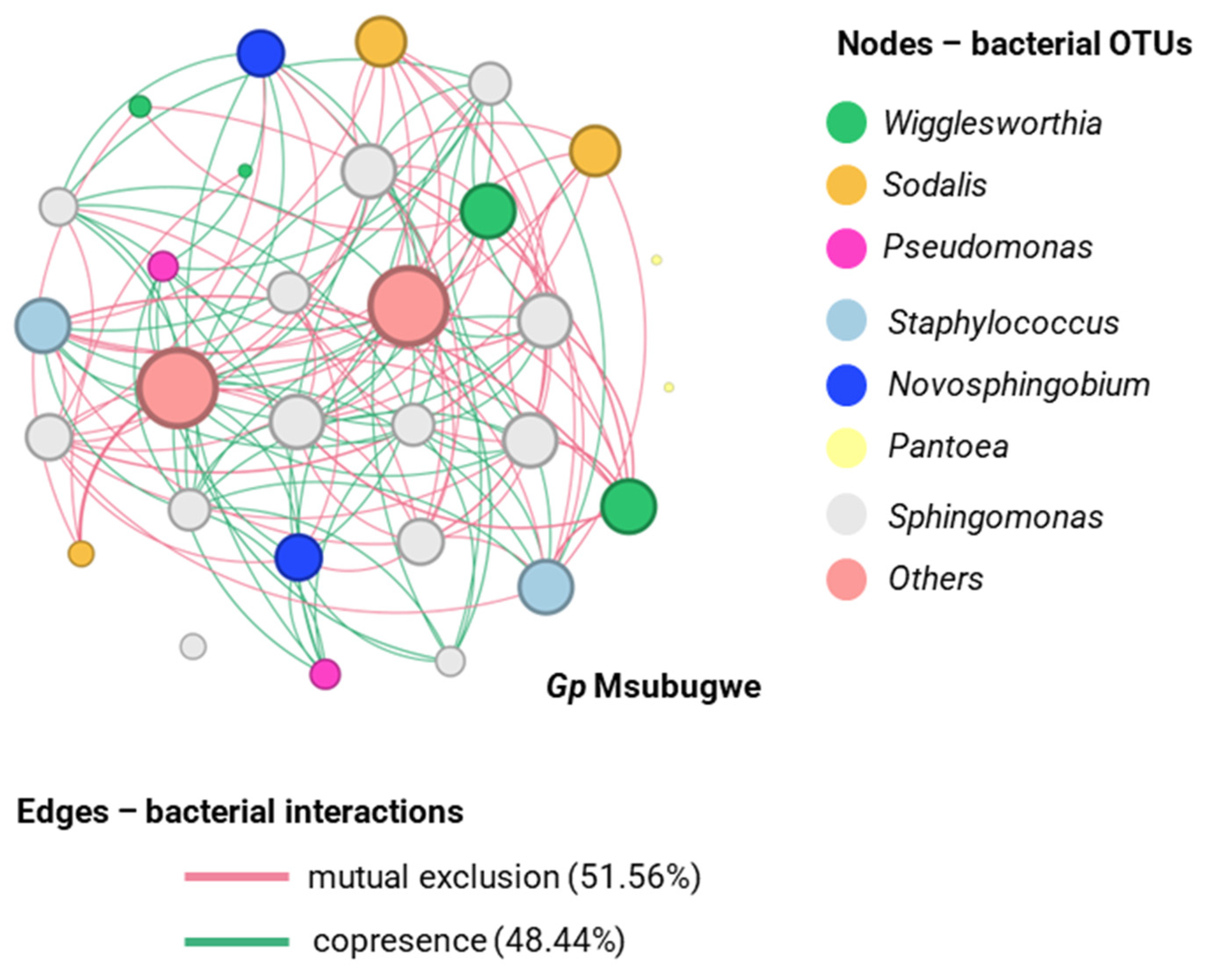
3.3. Natural Populations Display Species- and Location-Specific Bacterial Profiles
3.4. Laboratory Populations Contain Multiple Wigglesworthia and Sodalis OTUs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, D.J.; Hay, S.I.; Packer, M.J. Predicting the Distribution of Tsetse Flies in West Africa Using Temporal Fourier Processed Meteorological Satellite Data. Ann. Trop. Med. Parasitol. 1996, 90, 225–241. [Google Scholar] [CrossRef]
- Farrar, J.; Hotez, P.J.; Junghanss, T.; Lalloo, D.G.; White, N.J.; Manson, P. Manson’s Tropical Diseases, 23rd ed.; Kang, G., Ed.; Elsevier/Saunders: Philadelphia, Pennsylvania, 2013; ISBN 978-0-7020-5306-1. [Google Scholar]
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.A.; Fèvre, E.M.; Mattioli, R.C.; Jannin, J.G. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLoS Negl. Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef]
- Dargie, J. PAAT Tsetse and Trypanosomosis Information-INDEX-Volume 33, 34-Parts 1-2 (2010), 1-2 (2011)-Numbers 15196-16036; FAO: Rome, Italy, 2012; ISBN 978-92-5-107451-0. [Google Scholar]
- Raether, W.; Seidenath, H. The Activity of Fexinidazole (HOE 239) against Experimental Infections with Trypanosoma Cruzi, Trichomonads and Entamoeba Histolytica. Ann. Trop. Med. Parasitol. 1983, 77, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kande Betu Ku Mesu, V.; Mutombo Kalonji, W.; Bardonneau, C.; Valverde Mordt, O.; Ngolo Tete, D.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mahenzi Mbembo, H.; et al. Oral Fexinidazole for Stage 1 or Early Stage 2 African Trypanosoma Brucei Gambiense Trypanosomiasis: A Prospective, Multicentre, Open-Label, Cohort Study. Lancet Glob. Health 2021, 9, e999–e1008. [Google Scholar] [CrossRef] [PubMed]
- Jennings, F.W.; Urquhart, G.M. The Use of the 2 Substituted 5-Nitroimidazole, Fexinidazole (Hoe 239) in the Treatment of chronicT. Brucei Infections in Mice. Z. Parasitenkd. 1983, 69, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazué, G.; Bray, M.A.; Pécoul, B. Fexinidazole—A New Oral Nitroimidazole Drug Candidate Entering Clinical Development for the Treatment of Sleeping Sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.-P.F.; et al. Oral Fexinidazole for Late-Stage African Trypanosoma Brucei Gambiense Trypanosomiasis: A Pivotal Multicentre, Randomised, Non-Inferiority Trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Tarral, A.; Blesson, S.; Mordt, O.V.; Torreele, E.; Sassella, D.; Bray, M.A.; Hovsepian, L.; Evène, E.; Gualano, V.; Felices, M.; et al. Determination of an Optimal Dosing Regimen for Fexinidazole, a Novel Oral Drug for the Treatment of Human African Trypanosomiasis: First-in-Human Studies. Clin. Pharmacokinet. 2014, 53, 565–580. [Google Scholar] [CrossRef]
- Pollastri, M.P. Fexinidazole: A New Drug for African Sleeping Sickness on the Horizon. Trends Parasitol. 2018, 34, 178–179. [Google Scholar] [CrossRef]
- Aksoy, S. Sleeping Sickness Elimination in Sight: Time to Celebrate and Reflect, but Not Relax. PLoS Negl. Trop. Dis. 2011, 5, e1008. [Google Scholar] [CrossRef]
- Welburn, S.C.; Fèvre, E.M.; Coleman, P.G.; Odiit, M.; Maudlin, I. Sleeping Sickness: A Tale of Two Diseases. Trends Parasitol. 2001, 17, 19–24. [Google Scholar] [CrossRef]
- Wang, J.; Weiss, B.L.; Aksoy, S. Tsetse Fly Microbiota: Form and Function. Front. Cell. Infect. Microbiol. 2013, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, A.M.M.; Bergoin, M.; Parker, A.G.; Maniania, N.K.; Vlak, J.M.; Bourtzis, K.; Boucias, D.G.; Aksoy, S. Improving Sterile Insect Technique (SIT) for Tsetse Flies through Research on Their Symbionts and Pathogens. J. Invertebr. Pathol. 2013, 112, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, M.J.B.; Saleh, K.M.; Ali, M.Y.; Abdulla, A.M.; Zhu, Z.-R.; Juma, K.G.; Dyck, V.A.; Msangi, A.R.; Mkonyi, P.A.; Feldmann, H.U. Glossina Austeni (Diptera: Glossinidae) Eradicated on the Island of Unguja, Zanzibar, Using the Sterile Insect Technique. J. Econ. Entomol. 2000, 93, 123–135. [Google Scholar] [CrossRef]
- Sterile Insect Technique-Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.; Hendrichs, J.; Robinson, A. (Eds.) Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Augustinos, A.A.; Kyritsis, G.A.; Papadopoulos, N.T.; Abd-Alla, A.M.M.; Cáceres, C.; Bourtzis, K. Exploitation of the Medfly Gut Microbiota for the Enhancement of Sterile Insect Technique: Use of Enterobacter Sp. in Larval Diet-Based Probiotic Applications. PLoS ONE 2015, 10, e0136459. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Cáceres, C.; Bourtzis, K. Medfly Gut Microbiota and Enhancement of the Sterile Insect Technique: Similarities and Differences of Klebsiella Oxytoca and Enterobacter Sp. AA26 Probiotics during the Larval and Adult Stages of the VIENNA 8D53+ Genetic Sexing Strain. Front. Microbiol. 2017, 8, 2064. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Lees, R.S.; Hendrichs, J.; Vreysen, M.J.B. More than One Rabbit out of the Hat: Radiation, Transgenic and Symbiont-Based Approaches for Sustainable Management of Mosquito and Tsetse Fly Populations. Acta Trop. 2016, 157, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S. Wigglesworthia Gen. Nov. and Wigglesworthia Glossinidia Sp. Nov., Taxa Consisting of the Mycetocyte-Associated, Primary Endosymbionts of Tsetse Flies. Int. J. Syst. Bacteriol. 1995, 45, 848–851. [Google Scholar] [CrossRef]
- Weiss, B.; Aksoy, S. Microbiome Influences on Insect Host Vector Competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.L.; Wang, J.; Aksoy, S. Tsetse Immune System Maturation Requires the Presence of Obligate Symbionts in Larvae. PLoS Biol. 2011, 9, e1000619. [Google Scholar] [CrossRef]
- Weiss, B.L.; Maltz, M.; Aksoy, S. Obligate Symbionts Activate Immune System Development in the Tsetse Fly. J. Immunol. 2012, 188, 3395–3403. [Google Scholar] [CrossRef]
- Farikou, O.; Njiokou, F.; Mbida Mbida, J.A.; Njitchouang, G.R.; Djeunga, H.N.; Asonganyi, T.; Simarro, P.P.; Cuny, G.; Geiger, A. Tripartite Interactions between Tsetse Flies, Sodalis Glossinidius and Trypanosomes—An Epidemiological Approach in Two Historical Human African Trypanosomiasis Foci in Cameroon. Infect. Genet. Evol. 2010, 10, 115–121. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Hochstrasser, M. The Biochemistry of Cytoplasmic Incompatibility Caused by Endosymbiotic Bacteria. Genes 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Doudoumis, V.; Blow, F.; Saridaki, A.; Augustinos, A.; Dyer, N.A.; Goodhead, I.; Solano, P.; Rayaisse, J.-B.; Takac, P.; Mekonnen, S.; et al. Challenging the Wigglesworthia, Sodalis, Wolbachia Symbiosis Dogma in Tsetse Flies: Spiroplasma Is Present in Both Laboratory and Natural Populations. Sci. Rep. 2017, 7, 4699. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Fardeau, M.-L.; Njiokou, F.; Ollivier, B. Glossina Spp. Gut Bacterial Flora and Their Putative Role in Fly-Hosted Trypanosome Development. Front. Cell. Infect. Microbiol. 2013, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.; Telleria, E.L.; Echodu, R.; Wu, Y.; Okedi, L.M.; Weiss, B.L.; Aksoy, S.; Caccone, A. Analysis of Multiple Tsetse Fly Populations in Uganda Reveals Limited Diversity and Species-Specific Gut Microbiota. Appl. Env. Microbiol. 2014, 80, 4301–4312. [Google Scholar] [CrossRef]
- Geiger, A.; Fardeau, M.-L.; Njiokou, F.; Joseph, M.; Asonganyi, T.; Ollivier, B.; Cuny, G. Bacterial Diversity Associated with Populations of Glossina Spp. from Cameroon and Distribution within the Campo Sleeping Sickness Focus. Microb. Ecol. 2011, 62, 632–643. [Google Scholar] [CrossRef]
- Geiger, A.; Fardeau, M.-L.; Grebaut, P.; Vatunga, G.; Josénando, T.; Herder, S.; Cuny, G.; Truc, P.; Ollivier, B. First Isolation of Enterobacter, Enterococcus, and Acinetobacter Spp. as Inhabitants of the Tsetse Fly (Glossina Palpalis Palpalis) Midgut. Infect. Genet. Evol. 2009, 9, 1364–1370. [Google Scholar] [CrossRef]
- Geiger, A.; Fardeau, M.-L.; Falsen, E.; Ollivier, B.; Cuny, G. Serratia Glossinae Sp. Nov., Isolated from the Midgut of the Tsetse Fly Glossina Palpalis Gambiensis. Int. J. Syst. Evol. Microbiol. 2010, 60, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.M.; Lehane, M.J. The Tsetse Fly Glossina Fuscipes Fuscipes (Diptera: Glossina) Harbours a Surprising Diversity of Bacteria Other than Symbionts. Antonie Van Leeuwenhoek 2011, 99, 711–720. [Google Scholar] [CrossRef]
- Weiss, B.L.; Wang, J.; Maltz, M.A.; Wu, Y.; Aksoy, S. Trypanosome Infection Establishment in the Tsetse Fly Gut Is Influenced by Microbiome-Regulated Host Immune Barriers. PLoS Pathog. 2013, 9, e1003318. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Santos-Garcia, D.; Dionyssopoulou, E.; Moreira, M.; Papapanagiotou, A.; Scarvelakis, M.; Doudoumis, V.; Ramos, S.; Aguiar, A.F.; Borges, P.A.V.; et al. Detection and Characterization of Wolbachia Infections in Natural Populations of Aphids: Is the Hidden Diversity Fully Unraveled? PLoS ONE 2011, 6, e28695. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.L.; Bowen, H. PEG Precipitation for Selective Removal of Small DNA Fragments. Focus 2003, 25, 18. [Google Scholar]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. UNCROSS2: Identification of Cross-Talk in 16S rRNA OTU Tables. bioRxiv 2018, 400762. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles Instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.4–4.2. 2017. Available online: https://CRAN.R-project.org/package=vegan. (accessed on 25 October 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 978-0-470-38737-5. [Google Scholar]
- Chen, J.; Bittinger, K.; Charlson, E.S.; Hoffmann, C.; Lewis, J.; Wu, G.D.; Collman, R.G.; Bushman, F.D.; Li, H. Associating Microbiome Composition with Environmental Covariates Using Generalized UniFrac Distances. Bioinformatics 2012, 28, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.R. An Evaluation of the Relative Robustness of Techniques for Ecological Ordination. Vegetatio 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Steele, J.A.; Countway, P.D.; Xia, L.; Vigil, P.D.; Beman, J.M.; Kim, D.Y.; Chow, C.-E.T.; Sachdeva, R.; Jones, A.C.; Schwalbach, M.S.; et al. Marine Bacterial, Archaeal and Protistan Association Networks Reveal Ecological Linkages. ISME J. 2011, 5, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic Molecular Ecological Network of Soil Microbial Communities in Response to Elevated CO2. mBio 2011, 2, e00122-11. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. CoNet App: Inference of Biological Association Networks Using Cytoscape. F1000Res 2016, 5, 1519. [Google Scholar] [CrossRef] [PubMed]
- Doudoumis, V.; Augustinos, A.; Saridaki, A.; Parker, A.; Abd-Alla, A.M.M.; Bourtzis, K.; Tsiamis, G. Different Laboratory Populations Similar Bacterial Profile? The Case of Glossina Palpalis Gambiensis. BMC Microbiol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera Littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef] [PubMed]
- Hroncova, Z.; Havlik, J.; Killer, J.; Doskocil, I.; Tyl, J.; Kamler, M.; Titera, D.; Hakl, J.; Mrazek, J.; Bunesova, V.; et al. Variation in Honey Bee Gut Microbial Diversity Affected by Ontogenetic Stage, Age and Geographic Location. PLoS ONE 2015, 10, e0118707. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Augustinos, A.; Bourtzis, K.; Willems, A.; De Meyer, M. Impact of Sample Preservation and Manipulation on Insect Gut Microbiome Profiling. A Test Case with Fruit Flies (Diptera, Tephritidae). Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Binetruy, F.; Dupraz, M.; Buysse, M.; Duron, O. Surface Sterilization Methods Impact Measures of Internal Microbial Diversity in Ticks. Parasites Vectors 2019, 12, 268. [Google Scholar] [CrossRef]
- Fernández-Ruiz, N.; Pinecki-Socias, S.; Estrada-Peña, A.; Wu-Chuang, A.; Maitre, A.; Obregón, D.; Cabezas-Cruz, A.; de Blas, I.; Nijhof, A.M. Decontamination Protocols Affect the Internal Microbiota of Ticks. Parasites Vectors 2023, 16, 189. [Google Scholar] [CrossRef]
- Hammer, T.J.; Dickerson, J.C.; Fierer, N. Evidence-Based Recommendations on Storing and Handling Specimens for Analyses of Insect Microbiota. PeerJ 2015, 3, e1190. [Google Scholar] [CrossRef] [PubMed]
- Rio, R.V.M.; Wu, Y.; Filardo, G.; Aksoy, S. Dynamics of Multiple Symbiont Density Regulation during Host Development: Tsetse Fly and Its Microbial Flora. Proc. R. Soc. B 2006, 273, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Tsakeng, C.U.B.; Tanekou, T.T.M.; Soffack, S.F.; Tirados, I.; Noutchih, C.; Njiokou, F.; Bigoga, J.D.; Wondji, C.S. Assessing the Tsetse Fly Microbiome Composition and the Potential Association of Some Bacteria Taxa with Trypanosome Establishment. Microorganisms 2022, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Balmand, S.; Lohs, C.; Aksoy, S.; Heddi, A. Tissue Distribution and Transmission Routes for the Tsetse Fly Endosymbionts. J. Invertebr. Pathol. 2013, 112, S116–S122. [Google Scholar] [CrossRef] [PubMed]
- De Vooght, L.; Caljon, G.; Van Hees, J.; Van Den Abbeele, J. Paternal Transmission of a Secondary Symbiont during Mating in the Viviparous Tsetse Fly. Mol. Biol. Evol. 2015, 32, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Attardo, G.M.; Lohs, C.; Heddi, A.; Alam, U.H.; Yildirim, S.; Aksoy, S. Analysis of Milk Gland Structure and Function in Glossina Morsitans: Milk Protein Production, Symbiont Populations and Fecundity. J. Insect Physiol. 2008, 54, 1236–1242. [Google Scholar] [CrossRef]
- Lazaro, J.E.H.; Nitcheu, J.; Predicala, R.Z.; Mangalindan, G.C.; Nesslany, F.; Marzin, D.; Concepcion, G.P.; Diquet, B. Heptyl Prodigiosin, a Bacterial Metabolite, Is Antimalarial in Vivo and Non-Mutagenic In Vitro. J. Nat. Toxins 2002, 11, 367–377. [Google Scholar]
- Lysyk, T.J.; Kalischuk-Tymensen, L.; Selinger, L.B.; Lancaster, R.C.; Wever, L.; Cheng, K.-J. Rearing Stable Fly Larvae (Diptera: Muscidae) on an Egg Yolk Medium. J. Med. Entomol. 1999, 36, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Malele, I.; Nyingilili, H.; Lyaruu, E.; Tauzin, M.; Bernard Ollivier, B.; Cayol, J.-L.; Fardeau, M.-L.; Geiger, A. Bacterial Diversity Obtained by Culturable Approaches in the Gut of Glossina pallidipes Population from a Non Sleeping Sickness Focus in Tanzania: Preliminary Results. BMC Microbiol. 2018, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Feder, D.; Garcia, E.S. Isolation of Serratia Marcescens in the Midgut of Rhodnius prolixus: Impact on the Establishment of the Parasite Trypanosoma Cruzi in the Vector. Exp. Parasitol. 2004, 107, 89–96. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites Vectors 2013, 6, 146. [Google Scholar] [CrossRef]
- Li, H.; Medina, F.; Vinson, S.B.; Coates, C.J. Isolation, Characterization, and Molecular Identification of Bacteria from the Red Imported Fire Ant (Solenopsis invicta) Midgut. J. Invertebr. Pathol. 2005, 89, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nazni, W.A.; Seleena, B.; Lee, H.L.; Jeffery, J.; Rogayah, T.A.R.T.; Sofian, M.A. Bacteria Fauna from the House Fly, Musca domestica (L.). Trop. Biomed. 2005, 22, 225–231. [Google Scholar] [PubMed]
- Heilbronner, S.; Holden, M.T.G.; Van Tonder, A.; Geoghegan, J.A.; Foster, T.J.; Parkhill, J.; Bentley, S.D. Genome Sequence of Staphylococcus Lugdunensis N920143 Allows Identification of Putative Colonization and Virulence Factors: Staphylococcus lugdunensis Genome Sequence. FEMS Microbiol. Lett. 2011, 322, 60–67. [Google Scholar] [CrossRef]









| Species | Location | Developmental Stage | Sex | Number of Samples |
|---|---|---|---|---|
| Glossina austeni | VVBD Insectary (Tanga, Tanzania) | Larva | - | 2 |
| 1 Day | Male | 3 | ||
| 5 Days | 2 | |||
| 15 Days | 3 | |||
| 1 Day | Female | 3 | ||
| 5 Days | 3 | |||
| 15 Days | 3 | |||
| Glossina morsitans morsitans | VVBD Insectary (Tanga, Tanzania) | Larva | - | 3 |
| 1 Day | Male | 3 | ||
| 15 Days | 3 | |||
| 1 Day | Female | 3 | ||
| 5 Days | 6 | |||
| 15 Days | 3 | |||
| Doma (Morogoro, Tanzania) | Adult | Male | 20 | |
| Glossina pallidipes | VVBD Insectary (Tanga, Tanzania) | Larva | - | 2 |
| 1 Day | Male | 3 | ||
| 5 Days | 3 | |||
| 15 Days | 3 | |||
| 1 Day | Female | 3 | ||
| 5 Days | 3 | |||
| 15 Days | 3 | |||
| Doma (Morogoro, Tanzania) | Adult | Male | 20 | |
| Makao (Serengeti National Park, Tanzania) | Adult | Male | 19 | |
| Msubugwe (Pangani district) | Adult | Male | 20 | |
| Glossina swynnertoni | Makao (Serengeti National Park, Tanzania) | Adult | Male | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Yamlahi, Y.; Bel Mokhtar, N.; Maurady, A.; Britel, M.R.; Batargias, C.; Mutembei, D.E.; Nyingilili, H.S.; Malulu, D.J.; Malele, I.I.; Asimakis, E.; et al. Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations. Insects 2023, 14, 840. https://doi.org/10.3390/insects14110840
El Yamlahi Y, Bel Mokhtar N, Maurady A, Britel MR, Batargias C, Mutembei DE, Nyingilili HS, Malulu DJ, Malele II, Asimakis E, et al. Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations. Insects. 2023; 14(11):840. https://doi.org/10.3390/insects14110840
Chicago/Turabian StyleEl Yamlahi, Youssef, Naima Bel Mokhtar, Amal Maurady, Mohammed R. Britel, Costas Batargias, Delphina E. Mutembei, Hamisi S. Nyingilili, Deusdedit J. Malulu, Imna I. Malele, Elias Asimakis, and et al. 2023. "Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations" Insects 14, no. 11: 840. https://doi.org/10.3390/insects14110840








