Biological Control of Mosquito Vectors: Past, Present, and Future
Abstract
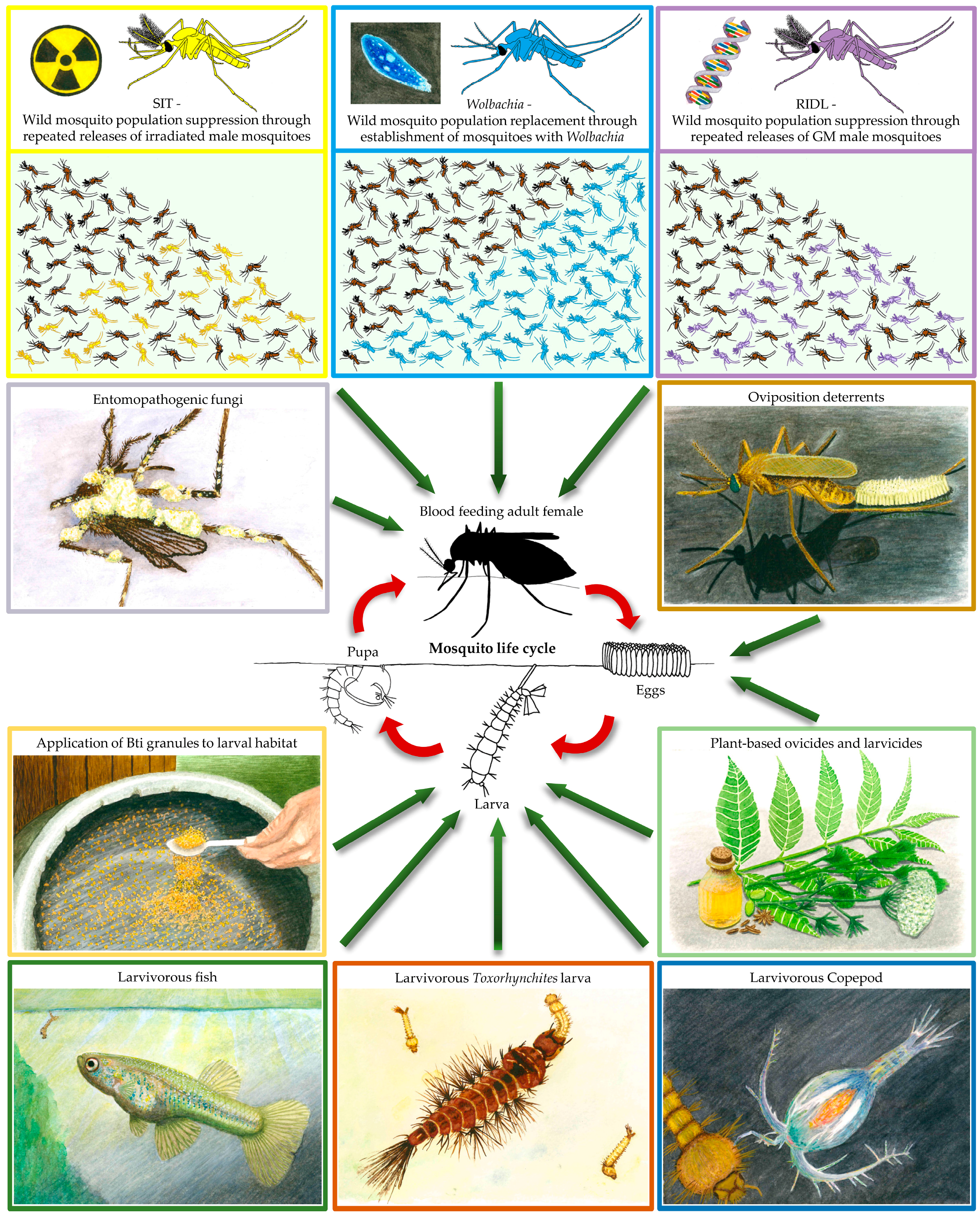
:1. Introduction
2. Using Biocontrol to Kill Mosquitoes
2.1. Plant-Borne Mosquitocides, Repellents, and Oviposition Deterrents
2.2. Mosquito Predators
2.3. Bti and Entomopathogenic Fungi
3. Releasing Mosquitoes for Disease Control
3.1. Wolbachia Endosymbiotic Bacteria
3.2. The Sterile Insect Technique
3.3. Genetically Modified Mosquitoes
4. Behavioural Knowledge: A Tool to Enhance Mosquito Control Programs?
4.1. Behavioural Quantification Helps SIT
4.2. Sound Traps
4.3. The “Lure and Kill” Technique
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol. Res. 2006, 99, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.; Abdel-Ghaffar, F.; Al-Rasheid, K.; Mehlhorn, H. Nature helps: From research to products against blood-sucking arthropods. Parasitol. Res. 2009, 105, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Strode, C.; Donegan, S.; Garner, P.; Enayati, A.A.; Hemingway, J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: Systematic review and meta-analysis. PLoS Med. 2014, 11, e1001619. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gokce, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Mehlorn, H. Encyclopedia of Parasitology, 4th ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Ranson, H.; Magill, A.; Kolaczinski, J.; Fornadel, C.; Gimnig, J.; Coetzee, M.; Simard, F.; Roch, D.K.; Hinzoumbe, C.K.; et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Yakob, L.; Walker, T. Zika virus outbreak in the Americas: The need for novel mosquito control methods. Lancet Glob. Health 2016, 4, e148–e149. [Google Scholar] [CrossRef]
- Jeffries, C.L.; Walker, T. The potential use of Wolbachia-based mosquito biocontrol strategies for Japanese encephalitis. PLoS Negl. Trop. Dis. 2015, 9, e0003576. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.; Cyranoski, D. Anti-parasite drugs sweep Nobel prize in medicine 2015. Nature 2015, 526, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Subramaniam, J.; Mahesh Kumar, P.; Suresh, U.; Roni, M.; et al. Sargassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Dinesh, D.; Kumar, P.M.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; et al. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: High antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ. Sci. Pollut. Res. Int. 2016, 23, 7543–7558. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, D.; Murugan, K.; Madhiyazhagan, P.; Panneerselvam, C.; Kumar, P.M.; Nicoletti, M.; Jiang, W.; Benelli, G.; Chandramohan, B.; Suresh, U. Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from aloe vera extracts: Towards an effective tool against the malaria vector Anopheles stephensi? Parasitol. Res. 2015, 114, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Suresh, U.; Murugan, K.; Benelli, G.; Nicoletti, M.; Barnard, D.R.; Panneerselvam, C.; Kumar, P.M.; Subramaniam, J.; Dinesh, D.; Chandramohan, B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Louca, V.; Lucas, M.C.; Green, C.; Majambere, S.; Fillinger, U.; Lindsay, S.W. Role of fish as predators of mosquito larvae on the floodplain of the Gambia river. J. Med. Entomol. 2009, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hwang, J.S. Larvicidal efficiency of aquatic predators: A perspective for mosquito biocontrol. Zool. Stud. 2006, 45, 447–466. [Google Scholar]
- Griffin, L.F.; Knight, J.M. A review of the role of fish as biological control agents of disease vector mosquitoes in mangrove forests: Reducing human health risks while reducing environmental risk. Wetl. Ecol. Manag. 2012, 20, 243–252. [Google Scholar] [CrossRef]
- Chandra, G.; Bhattacharjee, I.; Chatterjee, S.N.; Ghosh, A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008, 127, 13–27. [Google Scholar] [PubMed]
- Kamareddine, L. The biological control of the malaria vector. Toxins 2012, 4, 748–767. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Kumar, P.M.; Dinesh, D.; Chandramohan, B.; Suresh, U.; Nicoletti, M.; et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: Towards an integrative approach? Environ. Sci. Pollut. Res. Int. 2015, 22, 20067–20083. [Google Scholar] [CrossRef] [PubMed]
- Brodman, R.; Dorton, R. The effectiveness of pond-breeding salamanders as agents of larval mosquito control. J. Freshw. Ecol. 2006, 21, 467–474. [Google Scholar] [CrossRef]
- Bowatte, G.; Perera, P.; Senevirathne, G.; Meegaskumbura, S.; Meegaskumbura, M. Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol. Control 2013, 67, 469–474. [Google Scholar] [CrossRef]
- Schaper, S. Evaluation of Costa Rican copepods (Crustacea: Eudecapoda) for larval Aedes aegypti control with special reference to Mesocyclops thermocyclopoides. J. Am. Mosq. Control Assoc. 1999, 15, 510–519. [Google Scholar] [PubMed]
- Vu, S.N.; Nguyen, T.Y.; Kay, B.H.; Marten, G.G.; Reid, J.W. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am. J. Trop. Med. Hyg. 1998, 59, 657–660. [Google Scholar] [PubMed]
- Singh, R.K.; Dhiman, R.C.; Singh, S.P. Laboratory studies on the predatory potential of dragon-fly Nymphs on mosquito larvae. J. Commun. Dis. 2003, 35, 96–101. [Google Scholar] [PubMed]
- Bailey, P.C.E. The effect of water temperature on the functional-response of the water stick insect Ranatra dispar (Heteroptera, nepidae). Aust. J. Ecol. 1989, 14, 381–386. [Google Scholar] [CrossRef]
- Venkatesan, P.; Jeyachandra, C.M. Estimation of mosquito predation by the water bug Diplonychus indicus Venkatesan and Rao. Indian J. Exp. Biol. 1985, 23, 227–229. [Google Scholar] [PubMed]
- Cloarec, A. Factors influencing the choice of predatory tactics in a water bug, Diplonychus indicus Venk. & Rao (Heteroptera, Belostomatidae). Anim. Behav. 1990, 40, 262–271. [Google Scholar]
- Shaalan, E.A.; Canyon, D.V.; Muller, R.; Younes, M.W.; Abdel-Wahab, H.; Mansour, A.H. A mosquito predator survey in Townsville, Australia, and an assessment of Diplonychus sp. and Anisops sp. predatorial capacity against Culex annulirostris mosquito immatures. J. Vector Ecol. 2007, 32, 16–21. [Google Scholar] [CrossRef]
- Steffan, W.A.; Evenhuis, N.L. Biology of Toxorhynchites. Annu. Rev. Entomol. 1981, 26, 159–181. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Dame, D.A.; Bailey, D.L. Effect of weekly releases of Toxorhynchites amboinensis (Doleschall) on Aedes aegypti (L.) (Diptera: Culicidae) in New Orleans, Louisiana. J. Econ. Entomol. 1985, 78, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.E. Notes on the use of fresh water fish as consumers of mosquito larvae in containers used in the home. Am. J. Public Health 1922, 12, 193–194. [Google Scholar] [CrossRef]
- Harrington, R.W.; Harrington, E.S. Effects on fishes and their forage organisms of impounding a Florida salt-marsh to prevent breeding by salt-marsh mosquitos. Bull. Mar. Sci. 1982, 32, 523–531. [Google Scholar]
- Van Dam, A.R.; Walton, W.E. Comparison of mosquito control provided by the Arroyo chub (Gila orcutti) and the mosquitofish (Gambusia affinis). J. Am. Mosq. Control Assoc. 2007, 23, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Prasad, R.N. Evaluation of mosquito fish Gambusia affinis in the control of mosquito breeding in rice fields. Indian J. Malariol. 1991, 28, 171–177. [Google Scholar] [PubMed]
- Walton, W.E. Larvivorous fish including Gambusia. J. Am. Mosq. Control. Assoc. 2007, 23, 184–220. [Google Scholar] [CrossRef]
- Ohba, S.Y.; Kawada, H.; Dida, G.O.; Juma, D.; Sonye, G.; Minakawa, N.; Takagi, M. Predators of Anopheles gambiae sensu lato (Diptera: Culicidae) larvae in Wetlands, Western Kenya: Confirmation by polymerase chain reaction method. J. Med. Entomol. 2010, 47, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Chobu, M.; Nkwengulila, G.; Mahande, A.M.; Mwang’onde, B.J.; Kweka, E.J. Direct and indirect effect of predators on Anopheles gambiae sensu stricto. Acta Trop. 2015, 142, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kweka, E.J.; Zhou, G.; Gilbreath, T.M., 3rd; Afrane, Y.; Nyindo, M.; Githeko, A.K.; Yan, G. Predation efficiency of Anopheles gambiae larvae by aquatic predators in Western Kenya Highlands. Parasit. Vectors 2011. [Google Scholar] [CrossRef] [PubMed]
- Rupp, H.R. Adverse assessments of Gambusia affinis: An alternate view for mosquito control practitioners. J. Am. Mosq. Control Assoc. 1996, 12, 155–159; discussion 160–166. [Google Scholar] [PubMed]
- Kats, L.B.; Ferrer, R.P. Alien predators and amphibian declines: Review of two decades of science and the transition to conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Marten, G.G.; Astaiza, R.; Suarez, M.F.; Monje, C.; Reid, J.W. Natural control of larval Anopheles albimanus (Diptera: Culicidae) by the Predator mesocyclops (Copepoda: Cyclopoida). J. Med. Entomol. 1989, 26, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, S.C.; Martinez, R.; Wiltshire, S.; Clarke, D.; Prabhakar, P.; Spinks, M. Evaluation of caribbean strains of Macrocyclops and Mesocyclops (Cyclopoida: Cyclopidae) as biological control tools for the dengue vector Aedes aegypti. J. Am. Mosq. Control Assoc. 1997, 13, 18–23. [Google Scholar] [PubMed]
- Manrique-Saide, P.; Ibanez-Bernal, S.; Delfin-Gonzalez, H.; Parra Tabla, V. Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres. Med. Vet. Entomol. 1998, 12, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Mahesh Kumar, P.; Murugan, K.; Kovendan, K.; Panneerselvam, C.; Prasanna Kumar, K.; Amerasan, D.; Subramaniam, J.; Kalimuthu, K.; Nataraj, T. Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol. Res. 2012, 111, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Benelli, G.; Ayyappan, S.; Dinesh, D.; Panneerselvam, C.; Nicoletti, M.; Hwang, J.S.; Kumar, P.M.; Subramaniam, J.; Suresh, U. Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol. Res. 2015, 114, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Anbu, P.; Murugan, K.; Madhiyazhagan, P.; Dinesh, D.; Subramaniam, J.; Panneerselvam, C.; Suresh, U.; Alarfaj, A.A.; Munusamy, M.A.; Higuchi, A.; et al. Green-synthesised nanoparticles from Melia azedarach seeds and the cyclopoid crustacean Cyclops vernalis: An eco-friendly route to control the malaria vector Anopheles stephensi? Nat. Prod. Res. 2015, 1–8. [Google Scholar]
- Chandramohan, B.; Murugan, K.; Kovendan, K.; Panneerselvam, C.; Mahesh Kumar, P.; Madhiyazhagan, P.; Dinesh, D.; Suresh, U.; Subramaniam, J.; Amaresan, D.; et al. Nanoparticles in the fight against parasites. Parasitol. Res. Monogr. 2016, 8, 173–190. [Google Scholar]
- Soumare, M.K.; Cilek, J.E. The effectiveness of Mesocyclops longisetus (Copepoda) for the control of container-inhabiting mosquitoes in residential environments. J. Am. Mosq. Control Assoc. 2011, 27, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Chitra, T.; Murugan, K.; Naresh Kumar, A.; Madhiyazhagan, P.; Nataraj, T.; Indumathi, D.; Hwang, J.S. Laboratory and field efficacy of Pedalium murex and predatory copepod Mesocyclops longisetus on rural malaria vector Anopheles culicifacies. Asian Pac. J. Trop. Dis. 2013, 3, 111–118. [Google Scholar] [CrossRef]
- Vu, S.N.; Nguyen, T.Y.; Tran, V.P.; Truong, U.N.; Le, Q.M.; Le, V.L.; Le, T.N.; Bektas, A.; Briscombe, A.; Aaskov, J.G.; et al. Elimination of dengue by community programs using Mesocyclops (Copepoda) against Aedes aegypti in Central Vietnam. Am. J. Trop. Med. Hyg. 2005, 72, 67–73. [Google Scholar] [PubMed]
- Kay, B.H.; Tuyet Hanh, T.T.; Le, N.H.; Quy, T.M.; Nam, V.S.; Hang, P.V.; Yen, N.T.; Hill, P.S.; Vos, T.; Ryan, P.A. Sustainability and cost of a community-based strategy against Aedes aegypti in Northern and Central Vietnam. Am. J. Trop. Med. Hyg. 2010, 82, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Sinh Nam, V.; Thi Yen, N.; Minh Duc, H.; Cong Tu, T.; Trong Thang, V.; Hoang Le, N.; Hoang San, L.; Le Loan, L.; Que Huong, V.T.; Kim Khanh, L.H.; et al. Community-based control of Aedes aegypti by using Mesocyclops in Southern Vietnam. Am. J. Trop. Med. Hyg. 2012, 86, 850–859. [Google Scholar] [PubMed]
- Hales, S.; van Panhuis, W. A new strategy for dengue control. Lancet 2005, 365, 551–552. [Google Scholar] [CrossRef]
- Rawlins, S.C.; Clark, G.G.; Martinez, R. Effects of single introduction of Toxorhynchites moctezuma upon Aedes aegypti on a Caribbean Island. J. Am. Mosq. Control. Assoc. 1991, 7, 7–10. [Google Scholar] [PubMed]
- Aditya, G.; Ash, A.; Saha, G.K. Predatory activity of Rhantus sikkimensis and larvae of Toxorhynchites splendens on mosquito larvae in Darjeeling, India. J. Vector Borne Dis. 2006, 43, 66–72. [Google Scholar] [PubMed]
- Kumar, P.M.; Murugan, K.; Madhiyazhagan, P.; Kovendan, K.; Amerasan, D.; Chandramohan, B.; Dinesh, D.; Suresh, U.; Nicoletti, M.; Alsalhi, M.S.; et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol. Res. 2016, 115, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.P.; Christopher, M.S.M.; Selvaraj, A.M.; Pandian, T.J. Studies on predation of the mosquito Culex fatigans by Ranatigrina tadpoles. Hydrobiologia 1983, 106, 59–63. [Google Scholar] [CrossRef]
- Raghavendra, K.; Sharma, P.; Dash, A.P. Biological control of mosquito populations through frogs: Opportunities & constrains. Indian J. Med. Res. 2008, 128, 22–25. [Google Scholar] [PubMed]
- Murugan, K.; Priyanka, V.; Dinesh, D.; Madhiyazhagan, P.; Panneerselvam, C.; Subramaniam, J.; Suresh, U.; Chandramohan, B.; Roni, M.; Nicoletti, M.; et al. Predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector, Aedes aegypti, in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol. Res. 2015, 114, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Weterings, R. Tadpoles of three common anuran species from Thailand do not prey on mosquito larvae. J. Vector Ecol. 2015, 40, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Lin, S.M.; Tseng, L.C.; Murugan, K.; Hwang, J.S. Bio-efficacy potential of Seaweed gracilaria firma with copepod, Megacyclops formosanus for the control larvae of dengue vector Aedes aegypti. Hydrobiologia 2014, 741, 113–123. [Google Scholar] [CrossRef]
- Murugan, K.; Venus, J.S.; Panneerselvam, C.; Bedini, S.; Conti, B.; Nicoletti, M.; Sarkar, S.K.; Hwang, J.S.; Subramaniam, J.; Madhiyazhagan, P.; et al. Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: Do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environ. Sci. Pollut. Res. Int. 2015, 22, 17053–17064. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, B.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Chandirasekar, R.; Dinesh, D.; Kumar, P.M.; Kovendan, K.; Suresh, U.; Subramaniam, J.; et al. Characterization and mosquitocidal potential of neem cake-synthesized silver nanoparticles: Genotoxicity and impact on predation efficiency of mosquito natural enemies. Parasitol. Res. 2016, 115, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Kumar, P.J.; Panneerselvam, C.; Subramaniam, J.; Madhiyazhagan, P.; Suresh, U.; Nicoletti, M.; Alarfaj, A.A.; Munusamy, M.A.; et al. Datura metel-synthesized silver nanoparticles magnify predation of dragonfly nymphs against the malaria vector Anopheles stephensi. Parasitol. Res. 2015, 114, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Nataraj, D.; Madhiyazhagan, P.; Sujitha, V.; Chandramohan, B.; Panneerselvam, C.; Dinesh, D.; Chandirasekar, R.; Kovendan, K.; Suresh, U.; et al. Carbon and silver nanoparticles in the fight against the filariasis vector Culex quinquefasciatus: Genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol. Res. 2016, 115, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Labeeba, M.A.; Panneerselvam, C.; Dinesh, D.; Suresh, U.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.S.; Wang, L.; Nicoletti, M.; et al. Aristolochia indica green-synthesized silver nanoparticles: A sustainable control tool against the malaria vector Anopheles stephensi? Res. Vet. Sci. 2015, 102, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Becker, N. Microbial control of mosquitoes: Management of the upper rhine mosquito population as a model programme. Parasitol. Today 1997, 13, 485–487. [Google Scholar] [CrossRef]
- Lacey, L.A. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 2007, 23, 133–163. [Google Scholar] [CrossRef]
- Novak, R.J.; Gubler, D.J.; Underwood, D. Evaluation of slow-release formulations of temephos (Abate) and Bacillus thuringiensis var. israelensis for the control of Aedes aegypti in Puerto Rico. J. Am. Mosq. Control Assoc. 1985, 1, 449–453. [Google Scholar] [PubMed]
- Armengol, G.; Hernandez, J.; Velez, J.G.; Orduz, S. Long-lasting effects of a Bacillus thuringiensis serovar israelensis experimental tablet formulation for Aedes aegypti (Diptera: Culicidae) control. J. Econ. Entomol. 2006, 99, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Rapley, L.P.; Benjamin, S. Bacillus thuringiensis var. israelensis (Bti) provides residual control of Aedes aegypti in small containers. Am. J. Trop. Med. Hyg. 2010, 82, 1053–1059. [Google Scholar] [PubMed]
- Lam, P.H.; Boon, C.S.; Yng, N.Y.; Benjamin, S. Aedes albopictus control with spray application of Bacillus thuringiensis israelensis, strain AM 65–52. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1071–1081. [Google Scholar] [PubMed]
- Georghiou, G.P.; Wirth, M.C. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 1997, 63, 1095–1101. [Google Scholar] [PubMed]
- Gómez-Dantés, H.; Willoquet, J.R. Dengue in the Americas: Challenges for prevention and control. Cad. Saude Publica 2009, 25, S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.J.; Knols, B.G.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004. [Google Scholar] [CrossRef]
- Knols, B.G.; Bukhari, T.; Farenhorst, M. Entomopathogenic fungi as the next-generation control agents against malaria mosquitoes. Future Microbiol. 2010, 5, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Blanford, S.; Chan, B.H.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal pathogen reduces potential for malaria transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.R.; Carolino, A.T.; Paula, C.O.; Samuels, R.I. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasit. Vectors 2011. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.R.; Carolino, A.T.; Silva, C.P.; Samuels, R.I. Susceptibility of adult female Aedes aegypti (Diptera: Culicidae) to the entomopathogenic fungus Metarhizium anisopliae is modified following blood feeding. Parasit. Vectors 2011. [Google Scholar] [CrossRef] [PubMed]
- Darbro, J.M.; Johnson, P.H.; Thomas, M.B.; Ritchie, S.A.; Kay, B.H.; Ryan, P.A. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am. J. Trop. Med. Hyg. 2012, 86, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.J.; Takken, W.; Knols, B.G. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007, 102, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mnyone, L.L.; Kirby, M.J.; Lwetoijera, D.W.; Mpingwa, M.W.; Simfukwe, E.T.; Knols, B.G.; Takken, W.; Russell, T.L. Tools for delivering entomopathogenic fungi to malaria mosquitoes: Effects of delivery surfaces on fungal efficacy and persistence. Malar. J. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbro, J.M.; Thomas, M.B. Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am. J. Trop. Med. Hyg. 2009, 80, 992–997. [Google Scholar] [PubMed]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Segata, N.; Pompon, J.; Marcenac, P.; Shaw, W.R.; Dabire, R.K.; Diabate, A.; Levashina, E.A.; Catteruccia, F. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Brelsfoard, C.L.; Dobson, S.L. Wolbachia effects on host fitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Plichart, C.; Sang, A.C.; Brelsfoard, C.L.; Bossin, H.C.; Dobson, S.L. Open release of male mosquitoes infected with a Wolbachia biopesticide: Field performance and infection containment. PLoS Negl. Trop. Dis. 2012, 6, e1797. [Google Scholar] [CrossRef] [PubMed]
- Brelsfoard, C.L.; St. Clair, W.; Dobson, S.L. Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasit. Vectors 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lees, R.S.; Xi, Z.; Gilles, J.R.; Bourtzis, K. Combining the sterile insect technique with Wolbachia-based approaches: II—A safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS ONE 2015, 10, e0135194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lees, R.S.; Xi, Z.; Bourtzis, K.; Gilles, J.R. Combining the sterile insect technique with the incompatible insect technique: Iii-robust mating competitiveness of irradiated triple Wolbachia-infected Aedes albopictus males under semi-field conditions. PLoS ONE 2016, 11, e0151864. [Google Scholar] [CrossRef] [PubMed]
- Min, K.T.; Benzer, S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 1997, 94, 10792–10796. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Walker, T.; O’Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Dean, J.L.; Khoo, C.; Dobson, S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005, 35, 903–910. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wmel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.; Hoang Nle, T.; Chau, N.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Kien, D.T.; Clapham, H.; Aguas, R.; Trung, V.T.; Chau, T.N.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Walker, E.C.; Uribe Yepes, A.; Dario Velez, I.; Christensen, B.M.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, G.; Joshi, D.; Dong, Y.; Lu, P.; Zhou, G.; Pan, X.; Xu, Y.; Dimopoulos, G.; Xi, Z. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 2013, 340, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef] [PubMed]
- Dutra, H.L.; Rocha, M.N.; Dias, F.B.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 2016, 19, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, C.L.; Walker, T. Biocontrol strategies for arboviral diseases and the potential influence of resident strains in mosquitoes. Curr. Trop. Med. Rep. 2016, 3, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, M.J.; Saleh, K.; Mramba, F.; Parker, A.; Feldmann, U.; Dyck, V.A.; Msangi, A.; Bouyer, J. Sterile insects to enhance agricultural development: The case of sustainable tsetse eradication on Unguja Island, Zanzibar, using an area-wide integrated pest management approach. PLoS Negl. Trop. Dis. 2014, 8, e2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, G.J.; Perea, E.Z.; Killeen, G.F.; Stancil, J.D.; Clark, S.J.; Morrison, A.C. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc. Natl. Acad. Sci. USA 2009, 106, 11530–11534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, R.S.; Knols, B.; Bellini, R.; Benedict, M.Q.; Bheecarry, A.; Bossin, H.C.; Chadee, D.D.; Charlwood, J.; Dabire, R.K.; Djogbenou, L.; et al. Review: Improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Trop. 2014, 132, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Caputo, B.; Ienco, A.; Cianci, D.; Pombi, M.; Petrarca, V.; Baseggio, A.; Devine, G.J.; della Torre, A. The “auto-dissemination” approach: A novel concept to fight Aedes albopictus in urban areas. PLoS Negl. Trop. Dis. 2012, 6, e1793. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Lefrancois, T. Boosting the sterile insect technique to control mosquitoes. Trends Parasitol. 2014, 30, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Chandre, F.; Gilles, J.; Baldet, T. Alternative vector control methods to manage the Zika virus outbreak: More haste, less speed. Lancet Glob. Health 2016, 4, e364. [Google Scholar] [CrossRef]
- Hirunkanokpun, S.; Carlson, J.O.; Kittayapong, P. Evaluation of mosquito densoviruses for controlling Aedes aegypti (Diptera: Culicidae): Variation in efficiency due to virus strain and geographic origin of mosquitoes. Am. J. Trop. Med. Hyg. 2008, 78, 784–790. [Google Scholar] [PubMed]
- De Wise Valdez, M.R.; Suchman, E.L.; Carlson, J.O.; Black, W.C. A large scale laboratory cage trial of Aedes densonucleosis virus (AeDNV). J. Med. Entomol 2010, 47, 392–399. [Google Scholar] [CrossRef]
- Burivong, P.; Pattanakitsakul, S.N.; Thongrungkiat, S.; Malasit, P.; Flegel, T.W. Markedly reduced severity of dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AaLDNV). Virology 2004, 329, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Shao, D.; Huang, X.; Li, J.; Chen, H.; Zhang, Q.; Zhang, J. The pathogenicity of mosquito densovirus (C6/36DNV) and its interaction with dengue virus type II in Aedes albopictus. Am. J. Trop. Med. Hyg. 2006, 75, 1118–1126. [Google Scholar] [PubMed]
- Mosimann, A.L.; Bordignon, J.; Mazzarotto, G.C.; Motta, M.C.; Hoffmann, F.; Santos, C.N. Genetic and biological characterization of a densovirus isolate that affects dengue virus infection. Mem. Inst. Oswaldo Cruz 2011, 106, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.; Barde, P.V.; Gokhale, M.D.; Singh, D.K.; Mourya, D.T. Evidence of co-infection of chikungunya and densonucleosis viruses in C6/36 cell lines and laboratory infected Aedes aegypti (L.) mosquitoes. Parasit. Vectors 2010. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Oviedo, M.N.; Lacroix, R.; Naish, N.; Morrison, N.I.; et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Delatte, H.; Hue, T.; Reiter, P. Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J. Med. Entomol. 2009, 46, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef] [PubMed]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Schliekelman, P.; Ellner, S.; Gould, F. Pest control by genetic manipulation of sex ratio. J. Econ. Entomol. 2005, 98, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.S. Sex-ratio manipulation in relation to insect pest control. Annu. Rev. Genet. 1983, 17, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.S.; Black, W.C.T. Mosquito genomes: Structure, organization, and evolution. Adv. Genet. 1999, 41, 1–33. [Google Scholar] [PubMed]
- Benelli, G.; Mehlhorn, H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016, 115, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Lees, R.S.; Gilles, J.R.L.; Hendrichs, J.; Vreysen, M.J.B.; Bourtzis, K. Back to the future: The sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef]
- Oliva, C.F.; Vreysen, M.J.; Dupe, S.; Lees, R.S.; Gilles, J.R.; Gouagna, L.C.; Chhem, R. Current status and future challenges for controlling malaria with the sterile insect technique: Technical and social perspectives. Acta Trop. 2013, 1321, S130–S139. [Google Scholar] [CrossRef] [PubMed]
- Helinski, M.E.; Harrington, L.C. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J. Med. Entomol. 2011, 48, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ponlawat, A.; Harrington, L.C. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 422–426. [Google Scholar] [CrossRef]
- Cator, L.J.; Arthur, B.J.; Ponlawat, A.; Harrington, L.C. Behavioral observations and sound recordings of free-flight mating swarms of Ae. aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2011, 48, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. The best time to have sex: Mating behaviour and effect of daylight time on male sexual competitiveness in the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 887–894. [Google Scholar] [CrossRef] [PubMed]
- South, S.H.; Arnqvist, G. Evidence of monandry in a mosquito (Sabethes cyaneus) with elaborate ornaments in both sexes. J. Insect Behav. 2008, 21, 451–459. [Google Scholar] [CrossRef]
- Zsemlye, J.L.; Hancock, R.G.; Foster, W.A. Analysis of a complex vertical copulatory-courtship display in the yellow fever vector Sabethes chloropterus. Med. Vet. Entomol. 2005, 19, 276–285. [Google Scholar] [CrossRef] [PubMed]
- South, S.H.; Steiner, D.; Arnqvist, G. Male mating costs in a polygynous mosquito with ornaments expressed in both sexes. Proc. R. Soc. B Biol. Sci. 2009, 276, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.K.; Hancock, R.G.; Foster, W.A. Epigamic display and unique mating position in Wyeomyia arthrostigma (Diptera: Culicidae). J. Insect Behav. 1996, 9, 739–753. [Google Scholar] [CrossRef]
- Balestrino, F.; Medici, A.; Candini, G.; Carrieri, M.; Maccagnani, B.; Calvitti, M.; Maini, S.; Bellini, R. Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J. Med. Entomol. 2010, 47, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Wiwatanaratanabutr, I.; Allan, S.; Linthicum, K.; Kittayapong, P. Strain-specific differences in mating, oviposition, and host-seeking behavior between Wolbachia-infected and uninfected Aedes albopictus. J. Am. Mosq. Control Assoc. 2010, 26, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Gilles, J.; Merancienne, D.; Lemperiere, G.; Fontenille, D. Sexual performance of male mosquito Aedes albopictus. Med. Vet. Entomol. 2011, 25, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Jacquet, M.; Gilles, J.; Lemperiere, G.; Maquart, P.O.; Quilici, S.; Schooneman, F.; Vreysen, M.J.; Boyer, S. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: Mating vigour of sterilized males. PLoS ONE 2012, 7, e49414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, R.; Balestrino, F.; Medici, A.; Gentile, G.; Veronesi, R.; Carrieri, M. Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J. Med. Entomol. 2013, 50, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hamady, D.; Ruslan, N.B.; Ahmad, A.H.; Rawi, C.S.; Ahmad, H.; Satho, T.; Miake, F.; Zuharah, W.F.; FuKumitsu, Y.; Saad, A.R.; et al. Colonized Aedes albopictus and its sexual performance in the wild: Implications for sit technology and containment. Parasit. Vectors 2013. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Calvitti, M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med. Vet. Entomol. 2013, 27, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Madakacherry, O.; Lees, R.S.; Gilles, J.R.L. Aedes albopictus (Skuse) males in laboratory and semi-field cages: Release ratios and mating competitiveness. Acta Trop. 2014, 132, S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Damiens, D.; Vreysen, M.J.; Lemperiere, G.; Gilles, J. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE 2013, 8, e78884. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Romano, D.; Messing, R.H.; Canale, A. First report of behavioural lateralisation in mosquitoes: Right-biased kicking behaviour against males in females of the Asian tiger mosquito, Aedes albopictus. Parasitol. Res. 2015, 114, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Jaffe, K. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 2007, 23, 1–10. [Google Scholar] [CrossRef]
- Pitts, R.J.; Mozuaraitis, R.; Gauvin-Bialecki, A.; Lemperiere, G. The roles of kairomones, synomones and pheromones in the chemically-mediated behaviour of male mosquitoes. Acta Trop. 2014, 132, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.C.; Offenhauser, W., Jr. The first field tests of recorded mosquito sounds used for mosquito destruction. Am. J. Trop. Med. Hyg. 1949, 29, 811–825. [Google Scholar] [PubMed]
- Kanda, T.; Loong, K.P.; Chiang, G.L.; Cheong, W.H.; Lim, T.W. Field study on sound trapping and the development of trapping method for both sexes of Mansonia in malaysia. Trop. Biomed. 1988, 5, 37–42. [Google Scholar]
- Ikeshoji, T. Acoustic attraction of male mosquitoes in a cage. Med. Entomol. Zool. 1981, 32, 7–15. [Google Scholar] [CrossRef]
- Diabate, A.; Tripet, F. Targeting male mosquito mating behaviour for malaria control. Parasit. Vectors 2015. [Google Scholar] [CrossRef] [PubMed]
- Cator, L.J.; Harrington, L.C. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim. Behav. 2011, 82, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae: Current knowledge and potential applications for integrated pest management. J. Pest Sci. 2014, 87, 385–405. [Google Scholar] [CrossRef]
- Charlwood, J.D.; Pinto, J.; Sousa, C.A.; Ferreira, C.; do Rosario, V.E. Male size does not affect mating success (of Anopheles gambiae in Sao Tome). Med. Vet. Entomol. 2002, 16, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Diabate, A.; Yaro, A.S.; Dao, A.; Diallo, M.; Huestis, D.L.; Lehmann, T. Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol. Biol. 2011. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benelli, G.; Jeffries, C.L.; Walker, T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects 2016, 7, 52. https://doi.org/10.3390/insects7040052
Benelli G, Jeffries CL, Walker T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects. 2016; 7(4):52. https://doi.org/10.3390/insects7040052
Chicago/Turabian StyleBenelli, Giovanni, Claire L. Jeffries, and Thomas Walker. 2016. "Biological Control of Mosquito Vectors: Past, Present, and Future" Insects 7, no. 4: 52. https://doi.org/10.3390/insects7040052
APA StyleBenelli, G., Jeffries, C. L., & Walker, T. (2016). Biological Control of Mosquito Vectors: Past, Present, and Future. Insects, 7(4), 52. https://doi.org/10.3390/insects7040052





