Using Machine Learning to Predict Invasive Bacterial Infections in Young Febrile Infants Visiting the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population and Data Collection
2.3. Feature Selection
2.4. Machine Learning Models
2.5. Model Evaluation and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskin, M.N.; O’Rourke, E.J.; Fleisher, G.R. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J. Pediatr. 1992, 120, 22–27. [Google Scholar] [CrossRef]
- Baker, M.D.; Bell, L.M.; Avner, J.R. Outpatient management without antibiotics of fever in selected infants. N. Engl. J. Med. 1993, 329, 1437–1441. [Google Scholar] [CrossRef]
- Jaskiewicz, J.A.; McCarthy, C.A.; Richardson, A.C.; White, K.C.; Fisher, D.J.; Dagan, R.; Powell, K.R. Febrile infants at low risk for serious bacterial infection—An appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994, 94, 390–396. [Google Scholar]
- Woll, C.; Neuman, M.I.; Pruitt, C.M.; Wang, M.E.; Shapiro, E.D.; Shah, S.S.; McCulloh, R.J.; Nigrovic, L.E.; Desai, S.; DePorre, A.G.; et al. Epidemiology and Etiology of Invasive Bacterial Infection in Infants ≤ 60 Days Old Treated in Emergency Departments. J. Pediatr. 2018, 200, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Cheng, J.; Alpern, E.R.; Thurm, C.; Schroeder, L.; Black, K.; Ellison, A.M.; Stone, K.; Alessandrini, E.A. Management of febrile neonates in US pediatric emergency departments. Pediatrics 2014, 133, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Meehan, W.P.; Fleegler, E.; Bachur, R.G. Adherence to guidelines for managing the well-appearing febrile infant: Assessment using a case-based, interactive survey. Pediatr. Emerg. Care 2010, 26, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, N.N.; Keuning, M.; Hol, J.; Pajkrt, D.; Plötz, F.B. Fever Without an Apparent Source in Young Infants: A Multicenter Retrospective Evaluation of Adherence to the Dutch Guidelines. Pediatr. Infect. Dis. J. 2020, 39, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, W. In Search of an Ideal Protocol to Distinguish Risk For Serious Bacterial Infection in Febrile Young Infants. J. Pediatr. 2021, 231, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Mintegi, S.; Bressan, S.; Da Dalt, L.; Gervaix, A.; Lacroix, L. Validation of the “Step-by-Step” Approach in the Management of Young Febrile Infants. Pediatrics 2016, 138, e20154381. [Google Scholar] [CrossRef] [PubMed]
- Byington, C.L.; Reynolds, C.C.; Korgenski, K.; Sheng, X.; Valentine, K.J.; Nelson, R.E.; Daly, J.A.; Osguthorpe, R.J.; James, B.; Savitz, L.; et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics 2012, 130, e16–e24. [Google Scholar] [CrossRef] [PubMed]
- Garra, G.; Cunningham, S.J.; Crain, E.F. Reappraisal of criteria used to predict serious bacterial illness in febrile infants less than 8 weeks of age. Acad. Emerg. Med. 2005, 12, 921–925. [Google Scholar] [CrossRef]
- Hui, C.; Neto, G.; Tsertsvadze, A.; Yazdi, F.; Tricco, A.C.; Tsouros, S.; Skidmore, B.; Daniel, R. Diagnosis and management of febrile infants (0–3 months). Evid. Rep. Technol. Assess. 2012, 205, 1–297. [Google Scholar]
- Shah, A.P.; Cobb, B.T.; Lower, D.R.; Shaikh, N.; Rasmussen, J.; Hoberman, A.; Wald, E.R.; Rosendorff, A.; Hickey, R.W. Enhanced versus automated urinalysis for screening of urinary tract infections in children in the emergency department. Pediatr. Infect. Dis. J. 2014, 33, 272–275. [Google Scholar] [CrossRef]
- Pruitt, C.M.; Neuman, M.I.; Shah, S.S.; Shabanova, V.; Woll, C.; Wang, M.E.; Alpern, E.R.; Williams, D.J.; Sartori, L.; Desai, S. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2019, 204, 177–182.e171. [Google Scholar] [CrossRef]
- Talbert, A.W.; Mwaniki, M.; Mwarumba, S.; Newton, C.R.; Berkley, J.A. Invasive bacterial infections in neonates and young infants born outside hospital admitted to a rural hospital in Kenya. Pediatr. Infect. Dis. J. 2010, 29, 945. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.T.; Mahajan, P.; Bonsu, B.K.; Bennett, J.E.; Levine, D.A.; Alpern, E.R.; Nigrovic, L.E.; Atabaki, S.M.; Cohen, D.M.; VanBuren, J.M. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017, 171, e172927. [Google Scholar] [CrossRef]
- Chiu, I.-M.; Huang, L.-C.; Chen, I.-L.; Tang, K.-S.; Huang, Y.-H. Diagnostic values of C-reactive protein and complete blood cell to identify invasive bacterial infection in young febrile infants. Pediatr. Neonatol. 2019, 60, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Bressan, S.; Gomez, B.; Mintegi, S.; Da Dalt, L.; Blazquez, D.; Olaciregui, I.; de la Torre, M.; Palacios, M.; Berlese, P.; Ruano, A. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr. Infect. Dis. J. 2012, 31, 1239–1244. [Google Scholar] [CrossRef]
- Mintegi, S.; Bressan, S.; Gomez, B.; Da Dalt, L.; Blázquez, D.; Olaciregui, I.; de la Torre, M.; Palacios, M.; Berlese, P.; Benito, J. Accuracy of a sequential approach to identify young febrile infants at low risk for invasive bacterial infection. Emerg. Med. J. 2014, 31, e19–e24. [Google Scholar] [CrossRef]
- Aronson, P.L.; Shabanova, V.; Shapiro, E.D.; Wang, M.E.; Nigrovic, L.E.; Pruitt, C.M.; DePorre, A.G.; Leazer, R.C.; Desai, S.; Sartori, L.F.; et al. A Prediction Model to Identify Febrile Infants ≤ 60 Days at Low Risk of Invasive Bacterial Infection. Pediatrics 2019, 144, e20183604. [Google Scholar] [CrossRef]
- Delahanty, R.J.; Alvarez, J.; Flynn, L.M.; Sherwin, R.L.; Jones, S.S. Development and evaluation of a machine learning model for the early identification of patients at risk for sepsis. Ann. Emerg. Med. 2019, 73, 334–344. [Google Scholar] [CrossRef]
- Shafaf, N.; Malek, H. Applications of machine learning approaches in emergency medicine; a review article. Arch. Acad. Emerg. Med. 2019, 7, 34. [Google Scholar] [PubMed]
- Taylor, R.A.; Pare, J.R.; Venkatesh, A.K.; Mowafi, H.; Melnick, E.R.; Fleischman, W.; Hall, M.K. Prediction of in-hospital mortality in emergency department patients with sepsis: A local big data–driven, machine learning approach. Acad. Emerg. Med. 2016, 23, 269–278. [Google Scholar] [CrossRef]
- Horng, S.; Sontag, D.A.; Halpern, Y.; Jernite, Y.; Shapiro, N.I.; Nathanson, L.A. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS ONE 2017, 12, e0174708. [Google Scholar] [CrossRef] [PubMed]
- Feudtner, C.; Feinstein, J.A.; Zhong, W.; Hall, M.; Dai, D. Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014, 14, 199. [Google Scholar] [CrossRef]
- Malhi, A.; Gao, R.X. PCA-based feature selection scheme for machine defect classification. IEEE Trans. Instrum. Meas. 2004, 53, 1517–1525. [Google Scholar] [CrossRef]
- Song, F.; Guo, Z.; Mei, D. Feature selection using principal component analysis. In Proceedings of the 2010 International Conference on System Science, Engineering Design and Manufacturing Informatization, Yichang, China, 12–14 November 2010; pp. 27–30. [Google Scholar]
- Tanaka, K.; Kurita, T.; Meyer, F.; Berthouze, L.; Kawabe, T. Stepwise feature selection by cross validation for EEG-based Brain Computer Interface. In Proceedings of the 2006 IEEE International Joint Conference on Neural Network Proceedings, Vancouver, BC, Canada, 16–21 July 2006; pp. 4672–4677. [Google Scholar]
- Bastanlar, Y.; Ozuysal, M. Introduction to machine learning. Methods Mol. Biol. 2014, 1107, 105–128. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. arXiv 2016, arXiv:1603.02754. [Google Scholar]
- Elkan, C. The foundations of cost-sensitive learning. In Proceedings of the International Joint Conference on Artificial Intelligence, Stockholm, Sweden, 13–19 July 2018; pp. 973–978. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ramgopal, S.; Horvat, C.M.; Yanamala, N.; Alpern, E.R. Machine learning to predict serious bacterial infections in young febrile infants. Pediatrics 2020, 146, e20194096. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhao, D.; Zaiane, O. An optimized cost-sensitive SVM for imbalanced data learning. In Proceedings of the Pacific-Asia Conference on Knowledge Discovery and Data Mining, Singapore, 11–14 May 2020; pp. 280–292. [Google Scholar]
- Xia, Y.; Liu, C.; Liu, N. Cost-sensitive boosted tree for loan evaluation in peer-to-peer lending. Electron. Commer. Res. Appl. 2017, 24, 30–49. [Google Scholar] [CrossRef]
- Yo, C.-H.; Hsieh, P.-S.; Lee, S.-H.; Wu, J.-Y.; Chang, S.-S.; Tasi, K.-C.; Lee, C.-C. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without source: A systematic review and meta-analysis. Ann. Emerg. Med. 2012, 60, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Michelson, K.A.; Neuman, M.I.; Pruitt, C.M.; Desai, S.; Wang, M.E.; DePorre, A.G.; Leazer, R.C.; Sartori, L.F.; Marble, R.D.; Rooholamini, S.N.; et al. Height of fever and invasive bacterial infection. Arch. Dis. Child. 2020, Aug, 20. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.R.; Shen, M.W.; Biondi, E.A.; Bendel-Stenzel, M.; Chen, C.N.; French, J.; Lee, V.; Evans, R.C.; Jerardi, K.E.; Mischler, M. Bacteraemic urinary tract infection: Management and outcomes in young infants. Arch. Dis. Child. 2016, 101, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schnadower, D.; Kuppermann, N.; Macias, C.G.; Freedman, S.B.; Baskin, M.N.; Ishimine, P.; Scribner, C.; Okada, P.; Beach, H.; Bulloch, B.; et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics 2010, 126, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Benito, H.; Mozún, R.; Trujillo, J.E.; Merino, P.A.; Group for the Study of Febrile Infant of the RISeuP-SPERG Network. Febrile young infants with altered urinalysis at low risk for invasive bacterial infection. a Spanish Pediatric Emergency Research Network’s Study. Pediatr. Infect. Dis. J. 2015, 34, 17–21. [Google Scholar] [CrossRef]
- Standage, S.W.; Wong, H.R. Biomarkers for pediatric sepsis and septic shock. Expert Rev. Anti-Infect. Ther. 2011, 9, 71–79. [Google Scholar] [CrossRef]
- Scott, H.F.; Donoghue, A.J.; Gaieski, D.F.; Marchese, R.F.; Mistry, R.D. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad. Emerg. Med. 2012, 19, 1276–1280. [Google Scholar] [CrossRef]
| Predictor | Points a |
|---|---|
| Age < 21 days old | 1 |
| Highest temperature in the ED 38.0–38.4 °C | 2 |
| Highest temperature in the ED ≥ 38.4 °C | 4 |
| Abnormal urinalysis result b | 3 |
| ANC ≥ 5185 cells per µL | 2 |
| Northern Hospital Mean (SD)/n(%) | Middle West Hospital Mean (SD)/n(%) | Southern Hospital Mean (SD)/n(%) | p-Value | |
|---|---|---|---|---|
| Total number of patients | 2653 | 168 | 1390 | |
| Age, days-old | 32 (18.0) | 31 (17.5) | 32 (18.1) | 0.504 |
| Male | 1552 (58.5) | 91 (54.2) | 819 (58.9) | 0.497 |
| IBI | 82 (3.1) | 3 (1.8) | 41 (2.9) | 0.625 |
| Bacteremia | 76 (2.9) | 2 (1.2) | 39 (2.8) | 0.439 |
| Bacterial Meningitis | 14 (0.5) | 1 (0.6) | 3 (0.2) | 0.333 |
| With IBI (n = 126) | Without IBI (n = 4085) | p-Value | |
|---|---|---|---|
| Age, d, median (IQR) | 31 (20–43) | 36 (23–50) | 0.001 |
| Male sex, n (%) | 78 (61.9) | 2384 (58.4) | 0.463 |
| Vital signs | |||
| Triage temperature, median (IQR) | 38.4 (37.9–38.9) | 37.7 (37.1–38.3) | <0.001 |
| Highest ED temperature, median (IQR) | 38.6 (38.0–39.1) | 37.8 (37.2–38.4) | <0.001 |
| Triage HR, median (IQR) | 177 (161–189) | 159 (143–175) | <0.001 |
| Laboratory test | |||
| WBC, median (IQR) | 10.9 (6.4–14.1) | 11.2 (8.2–13.4) | 0.462 |
| Hb, median (IQR) | 11.6 (9.8–12.8) | 12.3 (10.2–14.2) | <0.001 |
| Platelet, median (IQR) | 365 (285–441) | 389 (302–458) | 0.027 |
| Neutrophil, median (IQR) | 55.7 (43.7–69.1) | 37.1 (24–49) | <0.001 |
| Band, mean | 0 (0–1.5) | 0 (0) | <0.001 |
| Eosinophil, median (IQR) | 0.8 (0–2.0) | 2 (1–4) | <0.001 |
| Lymphocyte, median (IQR) | 33.9 (22.8–45.1) | 47.5 (35.2–60.0) | <0.001 |
| ANC, median (IQR) | 6397 (2828–8795) | 4368 (2112–5546) | <0.001 |
| CRP, median (IQR) | 35.2 (2.4–47.6) | 0.8 (0–5.7) | <0.001 |
| Abnormal urine test, n (%) | 61 (48.6) | 740 (18.2) | <0.001 |
| IBI score, median (IQR) | 4 (2–6) | 2 (0–4) | <0.001 |
| IBI ≥ 2, n (%) | 110 (87.3) | 2345 (57.4) | <0.001 |
| Logistic Regression | SVM | XGBoost | |
|---|---|---|---|
| 1st | Neutrophil | CRP | Eosinophil |
| 2nd | CRP | Heart Rate | Band |
| 3rd | Lymphocyte | Neutrophil | WBC |
| 4th | Basophil | Basophil | CRP |
| 5th | Band | Band | Heart Rate |
| 6th | Platelet | ANC | |
| 7th | Age | Monocyte | |
| 8th | Temperature |
| Outcome, Mean (SD) | IBI Score | LR | SVM | XGBoost | p-Value |
|---|---|---|---|---|---|
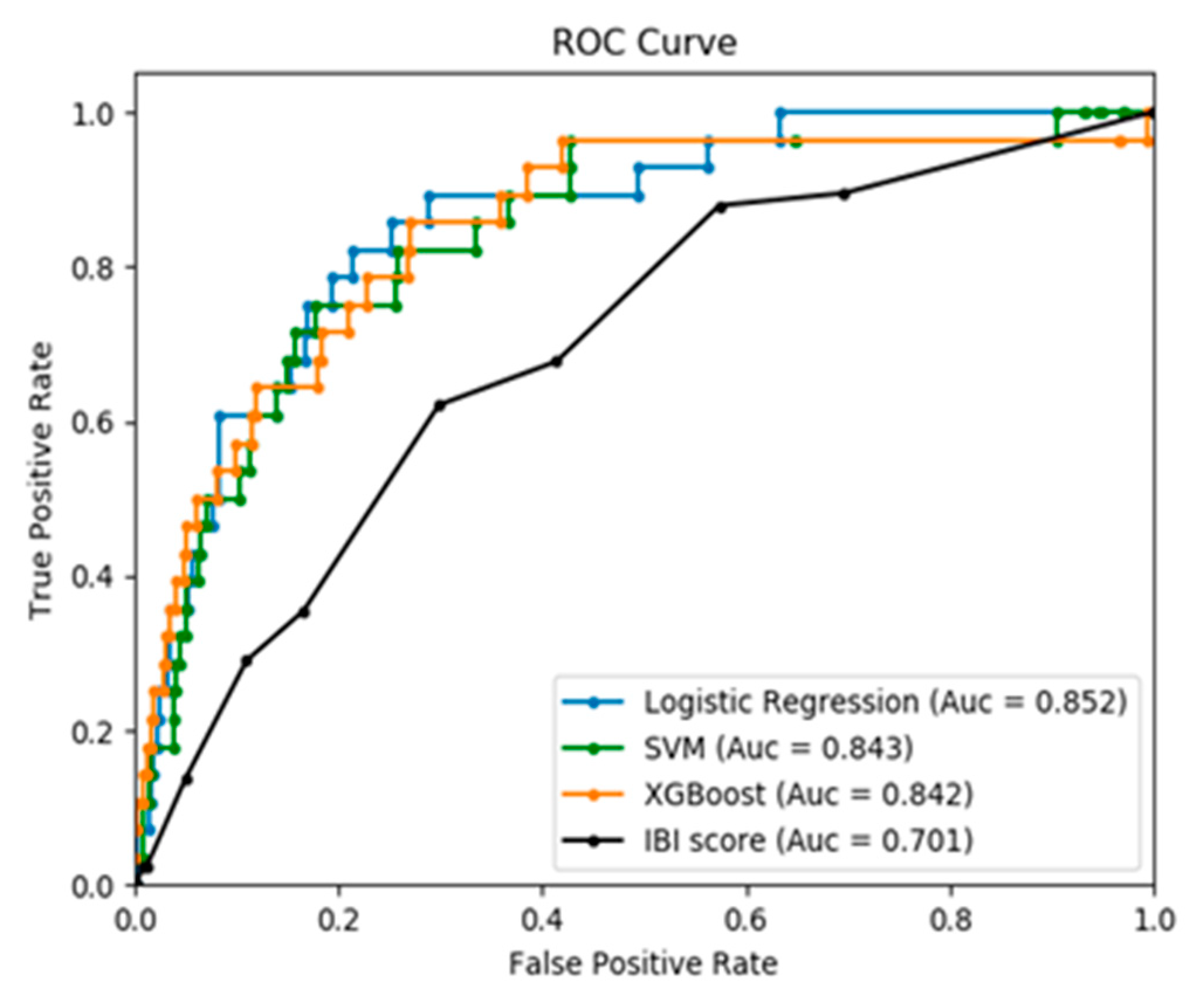
| AUROC | 0.70 (0.03) * | 0.85 (0.04) | 0.84 (0.03) | 0.84 (0.03) | <0.001 |
| IBI score ≥ 2 | |||||
| Sensitivity | 0.85 (0.06) | 0.90 (0.07) | 0.91 (0.07) | 0.90 (0.08) | 0.219 |
| Specificity | 0.43(0.01) * | 0.59 (0.02) ** | 0.60 (0.03) ** | 0.57 (0.02) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, I.-M.; Cheng, C.-Y.; Zeng, W.-H.; Huang, Y.-H.; Lin, C.-H.R. Using Machine Learning to Predict Invasive Bacterial Infections in Young Febrile Infants Visiting the Emergency Department. J. Clin. Med. 2021, 10, 1875. https://doi.org/10.3390/jcm10091875
Chiu I-M, Cheng C-Y, Zeng W-H, Huang Y-H, Lin C-HR. Using Machine Learning to Predict Invasive Bacterial Infections in Young Febrile Infants Visiting the Emergency Department. Journal of Clinical Medicine. 2021; 10(9):1875. https://doi.org/10.3390/jcm10091875
Chicago/Turabian StyleChiu, I-Min, Chi-Yung Cheng, Wun-Huei Zeng, Ying-Hsien Huang, and Chun-Hung Richard Lin. 2021. "Using Machine Learning to Predict Invasive Bacterial Infections in Young Febrile Infants Visiting the Emergency Department" Journal of Clinical Medicine 10, no. 9: 1875. https://doi.org/10.3390/jcm10091875
APA StyleChiu, I.-M., Cheng, C.-Y., Zeng, W.-H., Huang, Y.-H., & Lin, C.-H. R. (2021). Using Machine Learning to Predict Invasive Bacterial Infections in Young Febrile Infants Visiting the Emergency Department. Journal of Clinical Medicine, 10(9), 1875. https://doi.org/10.3390/jcm10091875







