Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review Methods
2.2. Feasibility Assessment Methods
2.3. Statistical Analysis Methods
2.4. Timepoints for Outcome Assessment
- Tirbanibulin 1% (TIRBA1%): 57 days after start of treatment
- 5-fluorouracil 5% (5FU5%) and 5-fluorouracil 4% (5FU4%) [39]: 4 weeks post-treatment
- 5-fluorouracil 0.5% plus salicylic acid 10% (5FU0.5% + SA) [40]: 4 weeks post-treatment
- PDT with aminolevulinic acid (ALA_PDT) sensitizer [41]: 12 weeks post-treatment
- PDT with methyl aminolevulinate (MAL_PDT) sensitizer [42]: 12 weeks post-treatment
- Cryosurgery (CRYO): 12 weeks post-treatment
- Diclofenac sodium 3% (DICLO3%) [43]: 90 to 120 days following start of treatment (30 to 60 days from end of treatment)
- Imiquimod 5% (IMQ5%) [44]: 8 to 12 weeks following end of treatment
- Imiquimod 3.75% (IMQ3.75%) [45]: 8 to 12 weeks following end of treatment
- Ingenol mebutate 0.015% (IM0.015%) [46]: 57 days after start of treatment
2.5. Sensitivity Analyses of Complete Clearance
2.5.1. Sensitivity Analysis: Single Course Data Only
2.5.2. Sensivity Analysis: ≤25 cm2 Assessment Area Only
2.5.3. Sensitivity Analysis: Single Placebo Node
2.5.4. Sensitivity Analysis: Studies Assessing Outcomes at ≥8 Weeks after Treatment
3. Results
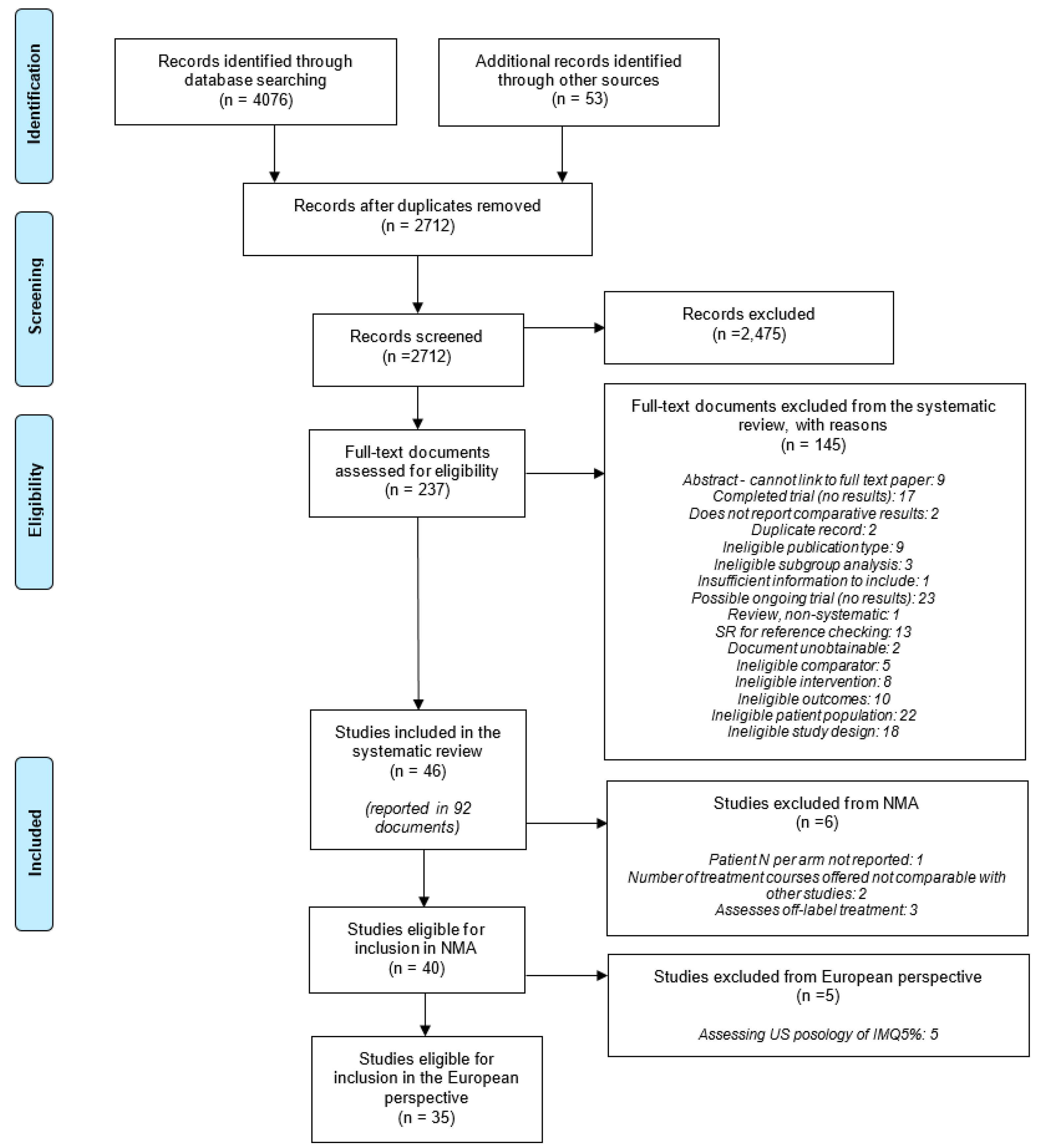
3.1. Results of the Literature Searches and Screening
3.2. Results of the Feasibility Assessment
3.3. Qualitative Synthesis: Europe
3.3.1. Lesion Count Reduction
3.3.2. Discontinuation due to AEs or LSRs
3.3.3. Incidence of Severe LSRs
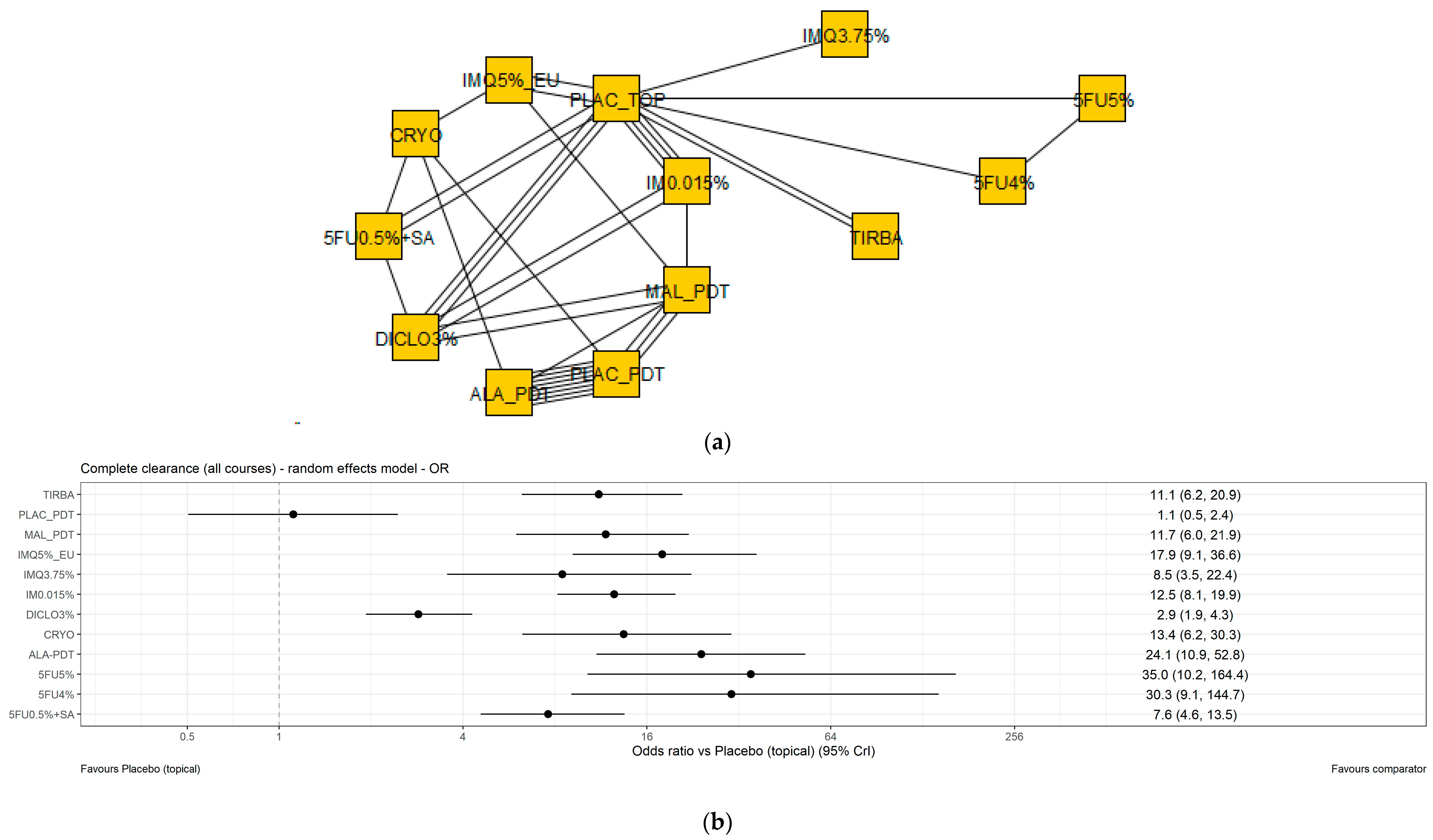
3.4. Results of the NMA of Complete Clearance
3.4.1. Base Case Analysis
3.4.2. Sensitivity Analysis: Single Course Data Only
3.4.3. Sensitivity Analysis: Studies Assessing a Treatment Area of ≤25 cm2 Only
3.4.4. Sensitivity Analysis: Single Placebo Node
3.4.5. Sensitivity Analysis: Studies Assessing Efficacy ≥8 Weeks after Treatment Only
3.4.6. Assessment of Inconsistency
4. Discussion
Limitations and Assumptions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.K.; Paquet, M.; Villanueva, E.; Brintnell, W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012, 12, CD004415. [Google Scholar] [CrossRef] [PubMed]
- de Berker, D.; McGregor, J.M.; Mohd Mustapa, M.F.; Exton, L.S.; Hughes, B.R. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis. Br. J. Dermatol. 2017, 176, 20–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Flohil, S.C.; van der Leest, R.J.; Dowlatshahi, E.A.; Hofman, A.; de Vries, E.; Nijsten, T. Prevalence of actinic keratosis and its risk factors in the general population: The rotterdam study. J. Investig. Dermatol. 2013, 133, 1971–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, I.; Augustin, M.; Spehr, C.; Reusch, M.; Kornek, T. Prevalence and risk factors of actinic keratoses in Germany—Analysis of multisource data. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 309–313. [Google Scholar] [CrossRef]
- OPEN VIE. Tirbanibulin as a Topical Treatment for Actinic Keratosis: Global Value Dossier [Not in the Public Domain]; OPEN VIE: Barcelona, Spain, 2021. [Google Scholar]
- Werner, R.; Stockfleth, E.; Connolly, S.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.; Jacobs, A.; Kerl, H.; Lim, H.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Chetty, P.; Choi, F.; Mitchell, T. Primary care review of actinic keratosis and its therapeutic options: A global perspective. Dermatol. Ther. 2015, 5, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.; McMichael, T.; Smith, W.; Armstrong, B. Solar Ultraviolet Radiation: Global Burden of Disease from Solar Ultraviolet Radiation; Environmental Burden of Disease, Series; no., 13; Prüss-Üstün, A., Zeeb, H., Mathers, C., Repacholi, M., Eds.; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Worldometer. Population of Europe [Webpage]. 2020. Available online: https://www.worldometers.info/world-population/europe-population/ (accessed on 1 October 2021).
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 91–97. [Google Scholar] [CrossRef]
- Athanasakis, K.; Boubouchairopoulou, N.; Tarantilis, F.; Tsiantou, V.; Kontodimas, S.; Kyriopoulos, J. Cost-effectiveness of Ingenol Mebutate Gel for the Treatment of Actinic Keratosis in Greece. Clin. Ther. 2017, 39, 993–1002. [Google Scholar] [CrossRef]
- Tennvall, G.R.; Norlin, J.; Malmberg, I.; Erlendsson, A.M.; Hædersdal, M. Health related quality of life in patients with actinic keratosis—An observational study of patients treated in dermatology specialist care in Denmark. Health Qual. Life Outcomes 2015, 13, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulko, C. Cutaneous Squamous Cell Carcinoma: Expensive to Treat. MedPage Today. 2020. Available online: https://www.medpagetoday.com/resource-centers/contemporary-approaches-non-melanoma-skin-cancer/cutaneous-squamous-cell-carcinoma-expensive-treat/2747 (accessed on 1 October 2021).
- British National Formulary (BNF). Photodamage [Webpage]; National Institute for Health and Care Excellence (NICE): London, UK; Manchester, UK, 2021; Available online: https://bnf.nice.org.uk/treatment-summary/photodamage.html (accessed on 1 October 2021).
- Stockfleth, E. The importance of treating the field in actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl 2), 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Health and Care Excellence. Actinic keratosis: Ingenol Mebutate gel: Evidence Summary [ESN14]; NICE: Manchester, UK, 2013; Available online: https://www.nice.org.uk/advice/esnm14/chapter/Overview (accessed on 21 October 2020).
- Heppt, M.V.; Leiter, U.; Steeb, T.; Amaral, T.; Bauer, A.; Becker, J.C.; Breitbart, E.; Breuninger, H.; Diepgen, T.; Dirschka, T.; et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma—short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J. Dtsch. Dermatol. Ges. 2020, 18, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Summary of Product Characteristics: Klisyri 10 mg/g ointment [Webpage]; European Medicines Agency: Amsterdam, The Netherlands, 2021; Last updated August 2021; Available online: https://www.ema.europa.eu/en/documents/product-information/klisyri-epar-product-information_en.pdf (accessed on 20 January 2022).
- Vegter, S.; Tolley, K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS ONE. 2014, 9, e96829. [Google Scholar] [CrossRef] [Green Version]
- Steeb, T.; Wessely, A.; Petzold, A.; Brinker, T.J.; Schmitz, L.; Leiter, U.; Garbe, C.; Schöffski, O.; Berking, C.; Heppt, M.V.; et al. Evaluation of Long-term Clearance Rates of Interventions for Actinic Keratosis: A Systematic Review and Network Meta-analysis. JAMA Dermatol. 2021, 157, 1066–1077. [Google Scholar] [CrossRef]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The natural history of actinic keratosis: A systematic review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; The Cochrane Collaboration: London, UK, 2019; Available online: https://www.cochrane-handbook.org (accessed on 21 October 2020).
- Centre for Reviews and Dissemination. PROSPERO [online database]; CRD: York, UK, 2015; Last updated 29 June 2015; Available online: http://www.crd.york.ac.uk/PROSPERO/ (accessed on 21 October 2020).
- European Medicines Agency. Risks of Picato for Actinic Keratosis Outweigh Benefits; European Medicines Agency: Amsterdam, The Netherlands, 2020; Available online: https://www.ema.europa.eu/en/medicines/human/referrals/picato (accessed on 30 September 2021).
- Jansen, M.; Kessels, J.; Merks, I.; Nelemans, P.; Kelleners-Smeets, N.; Mosterd, K.; Essers, B. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br. J. Dermatol. 2020, 21, 21. [Google Scholar]
- Jansen, M.H.; Kessels, J.P.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.; Van Pelt, H.P.; Quaedvlieg, P.J.; Essers, B.A.; Steijlen, P.M.; Kelleners-Smeets, N.W.; et al. Randomized trial of four treatment approaches for actinic keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Ageing Pharmaceutical Benefits Advisory Committee (PBAC). Appendix 4: Heterogeneity of treatment effect across studies. In Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee; Pharmaceutical Benefits Advisory Committee (PBAC): Canberra, Australia, 2016. Available online: https://pbac.pbs.gov.au/appendixes/appendix-4-heterogeneity-of-treatment-effect-across-studies.html#Table-a4-1 (accessed on 27 August 2021).
- Dias, S.; Welton, N.J.; Sutton, A.J.; Ades, A.E. Technical Support Document 2: A General Linear Modelling Framework for Pair-wise and Network Meta-Analysis of Randomised Controlled Trials. Sheffield: 2011 (updated September 2016). Available online: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%202Sep2016v2.pdf (accessed on 7 December 2020).
- Turner, R.M.; Jackson, D.; Wei, Y.; Thompson, S.G.; Higgins, J.P. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat. Med. 2015, 34, 984–998. [Google Scholar] [CrossRef] [Green Version]
- Decision Support Unit. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. Sheffield: 2014. Available online: http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.final_.15April2014.pdf (accessed on 7 December 2020).
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), Vienna, Austria, 20–22 March 2003. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 7 December 2020).
- Plummer, M. rjags: Bayesian graphical models using MCMC. R Package Version 2016, 4. [Google Scholar]
- Python Software Foundation. Python Language Reference, Version 3; Python Software Foundation: Beaverton, OR, USA, 2018; Available online: http://www.python.org (accessed on 23 March 2021).
- PyStan Developers. PyStan Release v3.0.0. rcRead the Docs. 2019. Available online: https://pystan.readthedocs.io/en/latest/ (accessed on 23 March 2021).
- Stan Development Team. Stan Modeling Language Users Guide and Reference Manual, 2.25. 2020. Available online: https://mc-stan.org (accessed on 23 March 2021).
- Datapharm. Summary of Product Characteristics: Efudix 5% Cream; Datapharm: Leatherhead, UK, 2020. Available online: https://www.medicines.org.uk/emc/product/9260/smpc (accessed on 4 April 2021).
- Datapharm. Summary of Product Characteristics: Actikerall 5mg/g + 100mg/g Cutaneous Solution; Datapharm: Leatherhead, UK, 2016; Available online: https://www.medicines.org.uk/emc/product/4621/smpc (accessed on 4 April 2021).
- European Medicines Agency. Summary of Product Characteristics: Ameluz 78 mg/g gel; European Medicines Agency: Amsterdam, The Netherlands, 2016; Available online: https://www.ema.europa.eu/en/documents/product-information/ameluz-epar-product-information_en.pdf (accessed on 20 January 2022).
- Datapharm. Summary of Product Characteristics: Metvix 160 mg/g Cream; Datapharm: Leatherhead, UK, 2021; Available online: https://www.medicines.org.uk/emc/product/6777/smpc (accessed on 4 April 2021).
- Datapharm. Summary of Product Characteristics: Solaraze 3% Gel; Datapharm: Leatherhead, UK, 2007; Available online: https://www.medicines.org.uk/emc/product/6385 (accessed on 4 April 2021).
- European Medicines Agency. Summary of Product Characteristics: Aldara 5% Cream; European Medicines Agency: Amsterdam, The Netherlands, 2008; Available online: https://www.ema.europa.eu/en/documents/product-information/aldara-epar-product-information_en.pdf (accessed on 20 January 2022).
- European Medicines Agency. Summary of Product Characteristics: Zyclara 3.75% Cream; European Medicines Agency: Amsterdam, The Netherlands, 2017; Available online: https://www.ema.europa.eu/en/documents/product-information/zyclara-epar-product-information_en.pdf (accessed on 20 January 2022).
- Datapharm. Summary of Product Characteristics: Picato 150 mcg/g Gel; Datapharm: Leatherhead, UK, 2019; Available online: https://www.medicines.org.uk/emc/product/2888/smpc (accessed on 4 April 2021).
- Ezzedine, K.; Painchault, C.; Brignone, M. Systematic literature review and network meta-analysis of the efficacy and acceptability of interventions in actinic keratoses. Acta Derm. Venereol. 2021, 101, 1–8. Available online: https://www.medicaljournals.se/acta/content/abstract/10.2340/00015555-3690 (accessed on 1 April 2021). [CrossRef] [PubMed]
- Gupta, A.K.; Paquet, M. Network meta-analysis of the outcome ‘participant complete clearance’ in nonimmunosuppressed participants of eight interventions for actinic keratosis: A follow-up on a Cochrane review. Br. J. Dermatol. 2013, 169, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.D.; McCullough, J.L.; Ross, P. Cell proliferation in normal epidermis. J. Investig. Dermatol. 1984, 82, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K. New Method of Measurement of Epidermal Turnover in Humans. Cosmetics 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Yap, L.M.; Marks, R.; Shumack, S. Short-course therapy with imiquimod 5% cream for solar keratoses: A randomized controlled trial. Australas J. Dermatol. 2003, 44, 250–255. [Google Scholar] [CrossRef]
- Reinhold, U.; Dirschka, T.; Ostendorf, R.; Aschoff, R.; Berking, C.; Philipp-Dormston, W.G.; Hahn, S.; Lau, K.; Jäger, A.; Schmitz, B.; et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz(R)) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED(R) lamp. Br. J. Dermatol. 2016, 175, 696–705. [Google Scholar]
- Athenex. Clinical Study Report: [KX01-AK-003] A Phase 3, Double-Blind, Vehicle-Controlled, Randomized, Parallel Group, Multicenter, Efficacy and Safety Study of KX2-391 Ointment 1% in Adult Subjects with Actinic Keratosis on the Face or Scalp; Athenex Inc.: Cranford, NJ, USA, 2019; pp. 1–619. [Google Scholar]
- Athenex. Clinical Study Report: [KX01-AK-004] A Phase 3, Double-Blind, Vehicle-Controlled, Randomized, Parallel Group, Multicenter, Efficacy and Safety Study of KX2-391 Ointment 1% in Adult Subjects with Actinic Keratosis on the Face or Scalp; Athenex Inc.: Cranford, NJ, USA, 2019; pp. 1–620. [Google Scholar]
- Szeimies, R.-M.; Gerritsen, M.-J.P.; Gupta, G.; Ortonne, J.P.; Serresi, S.; Bichel, J.; Lee, J.H.; Fox, T.L.; Alomar, A. Imiquimod 5% cream for the treatment of actinic keratosis: Results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J. Am. Acad. Dermatol. 2004, 51, 547–555. [Google Scholar] [CrossRef]
- Lebwohl, M.; Dinehart, S.; Whiting, D.; Lee, P.K.; Tawfik, N.; Jorizzo, J.; Lee, J.H.; Fox, T.L. Imiquimod 5% cream for the treatment of actinic keratosis: Results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J. Am. Acad. Dermatol. 2004, 50, 714–721. [Google Scholar] [CrossRef]
- Korman, N.; Moy, R.; Ling, M.; Matheson, R.; Smith, S.; McKane, S.; Lee, J.H. Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: Results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch. Dermatol. 2005, 141, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Hanke, C.W.; Beer, K.R.; Stockfleth, E.; Wu, J.; Rosen, T.; Levy, S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J. Am. Acad. Dermatol. 2010, 62, 573–581. [Google Scholar] [CrossRef]
- Swanson, N.; Abramovits, W.; Berman, B.; Kulp, J.; Rigel, D.S.; Levy, S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J. Am. Acad. Dermatol. 2010, 62, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Jorizzo, J.; Dinehart, S.; Matheson, R.; Moore, J.K.; Ling, M.; Fox, T.L.; McRae, S.; Fielder, S.; Lee, J.H. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J. Am. Acad. Dermatol. 2007, 57, 265–268. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, A.; Bichel, J.; McRae, S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br. J. Dermatol. 2007, 157, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; von Kiedrowski, R.; Dominicus, R.; Ryan, J.; Ellery, A.; Falqués, M.; Azeredo, R.R. Efficacy and safety of 5-fluorouracil 0.5%/salicylic acid 10% in the field-directed treatment of actinic keratosis: A phase III, randomized, double-blind, vehicle-controlled trial. Dermatol. Ther. 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohil, M.A. Efficacy, Safety, and Tolerability of 4% 5-Fluorouracil Cream in a Novel Patented Aqueous Cream Containing Peanut Oil Once Daily Compared With 5% 5-Fluorouracil Cream Twice Daily: Meeting the Challenge in the Treatment of Actinic Keratosis. J. Drugs Dermatol. 2016, 15, 1218–1224. [Google Scholar] [PubMed]
- Pariser, D.M.; Lowe, N.J.; Stewart, D.M.; Jarratt, M.T.; Lucky, A.W.; Pariser, R.J.; Yamauchi, P.S. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: Results of a prospective randomized multicenter trial. J. Am. Acad. Dermatol. 2003, 48, 227–232. [Google Scholar] [CrossRef]
- Peplin. A Multicenter Study to Evaluate the Safety and Efficacy of PEP005 Topical Gel When Used to Treat Actinic Keratoses on the Head (Face or Scalp). Identifier: NCT00700063. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2008. Available online: https://ClinicalTrials.gov/show/NCT00700063 (accessed on 8 July 2020).
- Peplin. A Multi-Center Study to Evaluate the Efficacy and Safety of pep005 (Ingenol Mebutate) Gel, When Used to Treat Actinic Keratoses on the Head (Face or Scalp). Identifier: NCT00915551. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2009. Available online: https://ClinicalTrials.gov/show/NCT00915551 (accessed on 8 July 2020).
- Peplin. A Multi-Center Study to Evaluate the Efficacy and Safety of pep005 (Ingenol Mebutate) Gel, When Used to Treat Actinic Keratoses on the Head (Face or Scalp). Identifier: NCT00916006. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2009. Available online: https://ClinicalTrials.gov/show/NCT00916006 (accessed on 8 July 2020).
- Gage Development Company. Study Comparing GDC 695 and Diclofenac Sodium Gel, 3% in Subjects with Actinic Keratoses. Identifier: NCT02952898. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2016. Available online: https://ClinicalTrials.gov/show/NCT02952898 (accessed on 8 July 2020).
- Actavis Inc. An Equivalence Study of Generic Ingenol Mebutate Gel 0.015% and Picato Gel 0.015% in Subjects with Actinic keratosis. Identifier: NCT03200912. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2016. Available online: https://ClinicalTrials.gov/show/NCT03200912 (accessed on 8 July 2020).
- Dermapharm, A.G. Multicenter, Randomised, Double-Blind Clinical Trial on the Efficacy and Safety of Medicinal Products Containing Diclofenac in Patients with Actinic Keratosis. Identifier: EUCTR2014-001621-33-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2014; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001621-33/DE/ (accessed on 8 July 2020).
- Almirall, S.A. Double-blind, Randomized, Vehicle- and Comparator-Controlled, Multicenter Trial to Evaluate the Efficacy and safety of LAS41007 in the Treatment of Actinic Keratosis. Identifier: EUCTR2010-022244-20-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2010; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-022244-20/DE/ (accessed on 8 July 2020).
- Pariser, D.; Loss, R.; Jarratt, M.; Abramovits, W.; Spencer, J.; Geronemus, R.; Bailin, P.; Bruce, S. Topical methyl-aminolevulinate photodynamic therapy using red light-emitting diode light for treatment of multiple actinic keratoses: A randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 2008, 59, 569–576. [Google Scholar] [CrossRef]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br. J. Dermatol. 2012, 166, 137–146. [Google Scholar] [CrossRef]
- Pariser, D.M.; Houlihan, A.; Ferdon, M.B.; Berg, J.E. Group P-AI. Randomized vehicle-controlled study of short drug incubation aminolevulinic acid photodynamic therapy for actinic keratoses of the face or scalp. Dermatol. Surg. 2016, 42, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Stockfleth, E.; Harwood, C.; Guillén, C.S.; Larsson, T.; Østerdal, M.; Skov, T. Phase IV head-to-head randomized controlled trial comparing ingenol mebutate 0.015% gel with diclofenac sodium 3% gel for the treatment of actinic keratosis on the face or scalp. Br. J. Dermatol. 2018, 178, 433–442. [Google Scholar] [CrossRef]
- MEDA Pharma GmbH & Co. KG. Long-Term Effects of Aldara® 5% Cream and Solaraze® 3% gel in the Treatment of Actinic keratoses on the face or scalp (LEIDA). Identifier: EUCTR2007-004884-24-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2015; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-004884-24/DE (accessed on 8 July 2020).
- Freeman, M.; Vinciullo, C.; Francis, D.; Spelman, L.; Nguyen, R.; Fergin, P.; Thai, K.-E.; Murrell, D.; Weightman, W.; Anderson, C.; et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: A prospective, randomized study. J. Dermatol. Treat. 2003, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Stockfleth, E.; Popp, G.; Borrosch, F.; Brüning, H.; Dominicus, R.; Mensing, H.; Reinhold, U.; Reich, K.; Moor, A.; et al. Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: Results of two randomized controlled phase III studies. Br. J. Dermatol. 2009, 160, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Almirall Hermal GmbH. Study on the efficacy of Verrumal(R) compared to placebo and Solaraze(R) in the Treatment of Actinic Keratosis Grade I to II. Identifier: EUCTR2007-003889-18-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2007; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-003889-18/DE/ (accessed on 8 July 2020).
- Taro Pharmaceuticals USA. Bioequivalence Study of Two Imiquimod Cream 5%. Identifier: NCT00828568. In ClinicalTrials.gov; US National Library of Medicine: Bethesda, MD, USA, 2008. Available online: https://ClinicalTrials.gov/show/NCT00828568 (accessed on 8 July 2020).
- Biofrontera Bioscience GmbH. A Randomized Placebo-Controlled Clinical Trial of Topical Photodynamic Therapy with a Nanoemulsion Formulation of 5-Aminolevulinic Acid for the Treatment of Actinic Keratosis. Identifier: EUCTR2006-000314-20-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2006; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2006-000314-20/DE/ (accessed on 8 July 2020).
- Szeimies, R.-M.; Radny, P.; Sebastian, M.; Borrosch, F.; Dirschka, T.; Krähn-Senftleben, G.; Reich, K.; Pabst, G.; Voss, D.; Foguet, M.; et al. Photodynamic Therapy with BF-200 ALA for the Treatment of Actinic Keratosis: Results of a Prospective, Randomized, Double-Blind, Placebo-Controlled Phase III Study. Br. J. Dermatol. 2010, 163, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E.; Werschler, P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J. Drugs Dermatol. 2007, 6, 144–147. [Google Scholar]
- Arisi, M.; Zane, C.; Polonioli, M.; Tomasi, C.; Moggio, E.; Cozzi, C.; Soglia, S.; Caravello, S.; Calzavara-Pinton, I.; Venturini, M.; et al. Effects of MAL-PDT, ingenol mebutate and diclofenac plus hyaluronate gel monitored by high-frequency ultrasound and digital dermoscopy in actinic keratosis- a randomized trial. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1225–1232. [Google Scholar] [CrossRef]
- Piacquadio, D.J.; Chen, D.M.; Farber, H.F.; Fowler, J.F., Jr.; Glazer, S.D.; Goodman, J.J.; Hruza, L.L.; Jeffes, E.W.B.; Ling, M.R.; Phillips, T.J.; et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: Investigator-blinded, phase 3, multicenter trials. Arch. Dermatol. 2004, 140, 41–46. [Google Scholar] [CrossRef]
- Foley, P.; Merlin, K.; Cumming, S.; Campbell, J.; Crouch, R.; Harrison, S.; Cahill, J. A comparison of cryotherapy and imiquimod for treatment of actinic keratoses: Lesion clearance, safety, and skin quality outcomes. J. Drugs Dermatol. 2011, 10, 1432–1438. [Google Scholar]
- Samorano, L.; Torezan, L.; Sanches, J.A. Evaluation of the tolerability and safety of a 0.015% ingenol mebutate gel compared to 5% 5-fluorouracil cream for the treatment of facial actinic keratosis: A prospective randomized trial. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1822–1827. [Google Scholar] [CrossRef]
- Köse, O.; Koc, E.; Erbil, A.H.; Caliskan, E.; Kurumlu, Z. Comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% imiquimod cream in the treatment of actinic keratosis. J. Dermatol. Treat. 2008, 19, 159–163. [Google Scholar] [CrossRef]
- Simon, J.C.; Dominicus, R.; Karl, L.; Rodriguez, R.; Willers, C.; Dirschka, T. A prospective randomized exploratory study comparing the efficacy of once-daily topical 0.5% 5-fluorouracil in combination with 10.0% salicylic acid (5-FU/SA) vs. cryosurgery for the treatment of hyperkeratotic actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 881–889. [Google Scholar] [CrossRef]
- MEDA Pharma GmbH & Co. KG. Long-term effects of Aldara® 5% cream and Solaraze® 3% gel in the treatment of actinic keratoses on the face or scalp with respect to the risk of progression to in-situ and invasive squamous cell carcinoma (LEIDA 2). Identifier: EUCTR2010-022054-16-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2010; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-022054-16/DE/ (accessed on 8 July 2020).
- Zane, C.; Facchinetti, E.; Rossi, M.; Specchia, C.; Calzavara-Pinton, P.; Calzavara-Pinton, P. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br. J. Dermatol. 2014, 170, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Serra-Guillén, C.; Nagore, E.; Hueso, L.; Traves, V.; Messeguer, F.; Sanmartín, O.; Llombart, B.; Requena, C.; Botella-Estrada, R. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: Clinical and histologic outcomes. J. Am. Acad. Dermatol. 2012, 66, e131–e137. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Nestor, M.S.; Newburger, J.; Park, H.; Swenson, N. Treatment of facial actinic keratoses with aminolevulinic acid photodynamic therapy (ALA-PDT) or ingenol mebutate 0.015% gel with and without prior treatment with ALA-PDT. J. Drugs Dermatol. 2014, 13, 1353–1356. [Google Scholar] [PubMed]
- Dermapharm, A.G. Double-blind, randomized, clinical trial to compare the efficacy and safety of diclofenc 3% gel vs. solaraze 3% gel vs. vehicle for the treatment of patients with actinic keratosis. Identifier: EUCTR2011-003317-41-DE. In EU Clinical Trials Register; European Medicines Agency: London, UK, 2011; Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-003317-41/DE/ (accessed on 8 July 2020).
- McGuinness, L.; Higgins, J. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Aldara® (imiquimod) Cream, 5%; US Food and Drug Administration: Silver Springs, MD, USA, 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020723s022lbl.pdf (accessed on 4 April 2021).
- US Food and Drug Administration. Highlights of Prescribing Information: KLISYRI (Tirbanibulin) Ointment, for Topical Use. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213189s000lbl.pdf (accessed on 4 February 2021).
- Steeb, T.; Wessely, A.; Schmitz, L.; Heppt, F.; Kirchberger, M.C.; Berking, C.; Heppt, M.V. Interventions for Actinic Keratosis in Nonscalp and Nonface Localizations: Results from a Systematic Review with Network Meta-Analysis. J. Investig. Dermatol. 2021, 141, 345–354.e8. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, N.; Cai, L.; Li, Q. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: A network meta-analysis. Dermatol. Ther. 2019, 32, e12822. [Google Scholar] [CrossRef]
- The Pharma Letter. Denmark’s LEO Pharma Initiates Picato Phase-Out. London: The Pharma Letter. 2020. Available online: https://www.thepharmaletter.com/article/denmark-s-leo-pharma-initiates-picato-phase-out (accessed on 30 September 2021).
- Shergill, B.; Zokaie, S.; Carr, A.J. Non-adherence to topical treatments for actinic keratosis. Patient Prefer. Adherence 2013, 8, 35–41. [Google Scholar]
- Erntoft, S.; Norlin, J.; Pollard, C.; Diepgen, T. Patient-reported adherence and persistence to topical treatments for actinic keratosis: A longitudinal diary study. Br. J. Dermatol. 2016, 175, 1094–1096. [Google Scholar] [CrossRef]
- Scottish Medicines Consortium (SMC). Detailed Advice: Tirbanibulin 10mg/g Ointment (Klisyri®); SMC: Glasgow, UK, 13 December 2021; Available online: https://www.scottishmedicines.org.uk/media/6538/tirbanibulin-klisyri-final-november-2021-for-website.pdf (accessed on 14 January 2022).

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Trials in adults with AK of the face and/or scalp |
|
| Interventions |
|
|
| Comparators | Any of the interventions listed above compared to each other or to placebo/vehicle | Trials with any other comparators |
| Outcomes |
Partial clearance Lesion count reduction Recurrence
|
|
| Study designs |
|
|
| Limits | Papers in languages other than English to be listed for information but no data extracted |
| Study Identifier | Population | Period over Which Discontinuations Are Assessed | Definition of Adverse Event Leading to Reported Discontinuations | Intervention Code | N Analyzed | N (%) Patients Discontinuing |
|---|---|---|---|---|---|---|
| Alomar 2007 [61] | ITT | Up to 4 weeks after end of last treatment course | LSR | IMQ5%_EU | 129 | 2 (1.6 *) |
| Up to 4 weeks after end of last treatment course | PLAC_TOP | 130 | 0 | |||
| Almirall Hermal GmbH: EUCTR-2007-003889 [79] | Safety = ITT | During 12 weeks treatment period | Local TEAE | 5FU0.5% + SA | 187 | 7 (3.7 *) |
| During 12 weeks treatment period | DICLO3% | 185 | 9 (4.9 *) | |||
| During 12 weeks treatment period | PLAC_TOP | 98 | 1 (1.0 *) | |||
| Almirall S.A., 2010: EUCTR-2010-022244-20 [71] | Safety = ITT | Up to 150 days after star of treatment (60 days after end of treatment) | TEAE | DICLO3% | 381 | 8 (2.1) |
| Up to 150 days after start of treatment (60 days after end of treatment) | PLAC_TOP | 127 | 5 (3.9) | |||
| Up to 150 days after start of treatment (60 days after end of treatment) | Cutaneous side effect (erythema, oedema, pruritus, rash, skin exfoliation) | DICLO3% | 381 | 47 (12.3) | ||
| Up to 150 days after start of treatment (60 days after end of treatment) | PLAC_TOP | 127 | 3 (2.4) | |||
| Athenex, Inc 2019a NCT03285477 [53] | ITT | Up to 57 days after start of treatment | TRAE | TIRBA | 175 | 0 |
| Up to 57 days after start of treatment | PLAC_TOP | 176 | 0 | |||
| Athenex, Inc 2019b NCT03285490 [54] | ITT | Up to 57 days after start of treatment | TRAE | TIRBA | 178 | 0 |
| Up to 57 days after start of treatment | PLAC_TOP | 173 | 0 | |||
| Chen 2003 [51] | PP | Up to 4 weeks after end of last treatment course | LSR | IMQ5%_EU | 29 | 0 |
| Up to 4 weeks after end of last treatment course | PLAC_TOP | 10 | 0 | |||
| Freeman 2003 [77] | ITT | NR | Local AE | MAL_PDT | 88 | 1 (1.1 *) |
| NR | PLAC_PDT | 23 | NR | |||
| NR | CRYO | 89 | NR | |||
| Hauschild 2009 AK 03 [78] | Safety | Up to 12 weeks after PDT session | TRAE | ALA_PDT | 69 | 1 (1.4 *) |
| Up to 12 weeks after PDT session | PLAC_PDT | 34 | NR | |||
| Hauschild 2009 AK 04 [78] | Safety | Up to 12 weeks after PDT session | TRAE | ALA_PDT | 148 | 2 (1.4 *) |
| Up to 12 weeks after PDT session | PLAC_PDT | 49 | NR | |||
| Up to 12 weeks after CRYO session | CRYO | 149 | NR | |||
| Jansen 2019 [28] | Patients who completed AE diaries | During treatment or the 2 weeks after the end of treatment | Serious TRAE | 5FU5% | 135 | 0 |
| During treatment or the 2 weeks after the end of treatment | IMQ5%_EU | 121 | 0 | |||
| During treatment or the 2 weeks after the end of treatment | MAL_PDT | 117 | 0 | |||
| During treatment or the 2 weeks after the end of treatment | IM0.015% | 140 | 0 | |||
| Piacquadio 2004 [85] | Safety | Up to 12 weeks (4 weeks after last PDT) | TRAE | ALA_PDT | 181 | 0 |
| Up to 12 weeks (4 weeks after last PDT) | PLAC_PDT | 62 | 0 | |||
| Reinhold 2016 [52] | ITT | Up to 12 weeks after last PDT session | TEAE | ALA_PDT | 55 | 0 |
| Up to 12 weeks after last PDT session | PLAC_PDT | 32 | 0 | |||
| Simon 2015 [89] | Completers | Up to 8 weeks after end of treatment | LSR | 5FU0.5% + SA | 33 | 3 (9.1) |
| Up to 11 weeks after last available CRYO session | CRYO | 33 | 0 | |||
| Stockfleth 2017 [62] | Safety = ITT | During 12 weeks treatment period | TEAE | 5FU0.5% + SA | 108 | 2 (1.9) |
| During 12 weeks treatment period | PLAC_TOP | 55 | 0 | |||
| Stockfleth 2018 [75] | Safety | Up to 56 days after start of patient’s last treatment course | TRAE | IM0.015% | 247 | 5 * (2) |
| Up to 29 days after end of treatment | DICLO3% | 234 | 14 * (6) | |||
| Swanson 2010 [59] | ITT | Up to 8 weeks after end of treatment | TRAE | IMQ3.75% | 160 | 1 (0.6 *) |
| Up to 8 weeks after end of treatment | PLAC_TOP | 159 | 1 (0.6 *) |
| Proportion of Patients Experiencing Severe LSRs: n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Identifier | Population | Timepoint of Assessment | Intervention | N Analyzed | Redness/Erythema | Flaking/Scaling/Dryness | Erosion/Ulceration | Scabbing/Crusting | Vesicles | Swelling/Oedema | Itching/Pruritus | Weeping/Exudate |
| Almirall Hermal GmbH, 2007: EUCTR-2007-003889 [79] | ITT | During 12 weeks treatment period | 5FU0.5% + SA | NR | NR | NR | NR | NR | NR | NR | 9 (4.8) | NR |
| DICLO3% | NR | NR | NR | NR | NR | NR | NR | 13 (7) | NR | |||
| PLAC_TOP | NR | NR | NR | NR | NR | NR | NR | 0 | NR | |||
| Alomar 2007 [61] | ITT | Up to 4 weeks after end of treatment | IMQ5%_EU | 129 | 40 (31) | 15 (11.6) | 14 (10.9) | 31 (24) | 2 (1.6) | 9 (7) | NR | 6 (4.7) |
| PLAC_TOP | 130 | 0 | 1 (0.8) | 1 (0.8) | 2 (1.5) | 0 | 0 | NR | 1 (0.8) | |||
| Athenex, Inc 2019a NCT03285477 [53] | ITT | Up to 57 days after start of treatment | TIRBA | 175 | 5 (3) | 11 (6) | 0 | 2 (1) | 1 (0.6) | 1 (0.6) | 1 (0.6) | NR |
| PLAC_TOP | 176 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | |||
| Athenex, Inc 2019b NCT03285490 [54] | ITT | Up to 57 days after start of treatment | TIRBA | 178 | 17 (10) | 20 (11) | 0 | 5 (3) | 1 (0.6) | 1 (0.6) | 0 | NR |
| PLAC_TOP | 173 | 0 | 1 (0.6) * | 0 | 0 | 0 | 0 | 0 | NR | |||
| Pariser 2016 [74] | ITT | Week 24, i.e., 16 weeks after second available session | ALA_PDT | 44 | 0 | 0 | NR | NR | NR | 0 | NR | NR |
| PLAC_PDT | 46 | 0 | 0 | NR | NR | NR | 0 | NR | NR | |||
| Swanson 2010 [59] | ITT | Up to 8 weeks after end of treatment | IMQ3.75% | 160 | 40 (25.2) | 13 (8.2) | 17 (10.7) | 22 (13.8) | NR | 9 (5.7) | NR | 9 (5.7) |
| PLAC_TOP | 159 | 0 | 2 (1.3) | 0 | 0 | NR | 0 | NR | 0 | |||
| Study Identifier | Population | Assesses an Area of ≤25 cm2? | Intervention Code for Networks | Treatment Regimen | Retreatment Offered by Study? | Timepoint of Assessment | N Experiencing Event/N Analyzed (%) |
|---|---|---|---|---|---|---|---|
| Actavis Inc, 2016: NCT03200912 [69] | PP | Yes | IM0.015% | Once daily for 3 days | No | Day 57 | 44/144 (30.6%) |
| PLAC_TOP | Once daily for 3 days | No | Day 57 | 7/139 (5%) | |||
| Almirall Hermal GmbH, 2007: EUCTR-2007-003889 [79] | FAS | Yes | 5FU0.5% + SA | Once daily for 12 weeks ** | No | 8 weeks after end of treatment | 98 */177 (55.4%) |
| DICLO3% | Twice daily for 12 weeks ** | No | 8 weeks after end of treatment/week 20 | 59 */183 (32%) | |||
| PLAC_TOP | Once daily for 12 weeks ** | No | 8 weeks after end of treatment/week 20 | 14 */96 (15.1%) | |||
| Almirall S.A., 2010: EUCTR-2010-022244-20 [71] | FAS | No: up to 75 cm2 total | DICLO3% | Twice daily for 90 days ** | No | 150 days | 89/380 (23.4%) |
| PLAC_TOP | Twice daily for 90 days ** | No | 150 days | 16/127 (12.6%) | |||
| Alomar 2007 [61] | ITT | Yes | IMQ5%_EU | Once daily on three alternate days per week for 4 weeks of treatment | A second 4 week course of treatment was permitted at week 8 if complete clearance was not achieved | 4 weeks after end of last treatment cycle | 71/129 (55%) |
| PLAC_TOP | Once daily on three alternate days per week for 4 weeks of treatment | 4 weeks after end of last treatment cycle | 3/130 (2.3%) | ||||
| Arisi 2020 [84] | PP | Yes | MAL_PDT | One session | A second session delivered only “if needed” after 3 months | 90 days after final PDT | 6/26 (23.07%) |
| IM0.015% | Once daily for 3 days | No | 90 days after end of treatment | 9/30 (30%) | |||
| DICLO3% | Twice daily for 90 days | No | 90 days after end of treatment | 4/28 (14.28%) | |||
| Athenex, Inc 2019a NCT03285477 [53] | ITT | Yes | TIRBA1% | Once daily for 5 days | No | Day 57 | 77/175 (44%) |
| PLAC_TOP | Once daily for 5 days | No | Day 57 | 8/176 (5%) | |||
| Athenex, Inc 2019b NCT03285490 [54] | ITT | Yes | TIRBA1% | Once daily for 5 days | No | Day 57 | 97/178 (54%) |
| PLAC_TOP | Once daily for 5 days | No | Day 57 | 22/173 (13%) | |||
| Biofrontera Bioscience GmbH, 2006: EUCTR-2006-000314-20 [81] | ITT | No: up to 200 cm2 total | ALA_PDT | One session | No | 12 weeks after first (only) PDT | 7 */28 (25.9%) |
| PLAC_PDT | One session | No | 12 weeks after first (only) PDT | 1 */27 (3.7%) | |||
| Dirschka 2012 [73] | ITT | Study assessed lesion-directed treatment: area did not have to be contiguous | ALA_PDT | One session | A second session was permitted at week 12 if AKs remained | 12 weeks after last PDT | 194 */248 (78.2%) |
| MAL_PDT | One session | 12 weeks after last PDT | 159 */247 (64.2%) | ||||
| PLAC_PDT | One session | 12 weeks after last PDT | 13 */76 (17.1%) | ||||
| Dohil 2016: Study 2 [63] | ITT | No: no set target area defined | 5FU4% | Once daily for 4 weeks | No | 4 weeks after end of treatment | 192 */353 (54.4%) |
| 5FU5% | Twice daily for 4 weeks | No | 4 weeks after end of treatment | 202 */349 (57.9%) | |||
| PLAC_TOP | Once daily for 4 weeks | No | 4 weeks after end of treatment | 3/70 (4.3%) | |||
| PLAC_TOP | Twice daily for 4 weeks | No | 4 weeks after end of treatment | NR/69 | |||
| Foley 2011 [86] | PP | Study assessed lesion-directed treatment: area did not have to be contiguous | IMQ5%_EU | Three times per week for 3–4 weeks | A second course was permitted 4 weeks after end of course 1 if AKs remained | 40 weeks after end of treatment | 17/25 (68%) |
| CRYO | One session | Additional sessions permitted at 3, 6 and 9 months post-treatment if AKs remained | Unclear. Ranges from 12 to 40 weeks post final treatment | 28/31 (90.3%) | |||
| Gage Development Company, 2016: NCT02952898 [68] | mITT | No: no set target area defined | DICLO3% | Twice daily for 60 days | No | 90 days | 56 */218 (25.7%) |
| PLAC_TOP | Twice daily for 60 days | No | 90 days | 21 */221 (9.5%) | |||
| Hauschild 2009 AK 03 [78] | FAS | No: set target area not defined. Up to eight 4 cm2 patches applied | ALA_PDT | One session | No | 12 weeks after first (only) PDT | 41/66 (62%) |
| PLAC_PDT | One session | No | 12 weeks after first (only) PDT | 2/33 (6%) | |||
| Hauschild 2009 AK 04 [78] | PP | No: set target area not defined. Up to eight 4 cm2 patches applied | ALA_PDT | One session | No | 12 weeks after first (only) PDT | 86/129 (67%) |
| PLAC_PDT | One session | No | 12 weeks after first (only) PDT | 5/43 (12%) | |||
| CRYO | One session | No | 12 weeks after first (only) session | 66/126 (52%) | |||
| Jorizzo 2007 [60] | ITT | Yes | IMQ5%_EU | 3 times per week for 4 weeks | A second course was offered 4 weeks after end of course 1 if AKs remained | 4 weeks after first treatment | 33 */123 (26.8%) |
| PLAC_TOP | 3 times per week for 4 weeks | 4 weeks after first treatment | 5 */123 (4.1%) | ||||
| Pariser 2003 [64] | PP | Study assessed lesion-directed treatment: area did not have to be contiguous | MAL_PDT | Two sessions one week apart | No, but all patients received the initial 2 sessions | 3 months after second PDT | 32/39 (82%) |
| PLAC_PDT | Two sessions one week apart | 3 months after second PDT | 8/38 (21%) | ||||
| Pariser 2008 [72] | ITT | Study assessed lesion-directed treatment: area did not have to be contiguous | MAL_PDT | Two sessions one week apart | No | 3 months after second PDT | 29/49 (59.2%) |
| PLAC_PDT | Two sessions one week apart | No | 3 months after second PDT | 7/47 (14.9%) | |||
| Pariser 2016 [74] | ITT | Study assessed lesion-directed treatment: area did not have to be contiguous | ALA_PDT | One session | A second session was offered at week 8 if AKs remained | 16 weeks after second (final) PDT | 12/47 (25.5%) |
| PLAC_PDT | One session | 16 weeks after second (final) PDT | 1/46 (2.2%) | ||||
| Peplin, 2008: NCT00700063 [65] | ITT | No: no set target area defined | IM0.015% | Once daily for three days | No | Day 57 | 16/32 (50.0 *%) |
| PLAC_TOP | Once daily for three days | No | Day 57 | 3/33 (9.1 *%) | |||
| Peplin, 2009a: NCT00915551 [66] | ITT | Yes | IM0.015% | Once daily for three days | No | Day 57 | 67/142 (47.2 *%) |
| PLAC_TOP | Once daily for three days | No | Day 57 | 7/136 (5.1 *%) | |||
| Peplin, 2009b: NCT00916006 [67] | ITT | Yes | IM0.015% | Once daily for three days | No | Day 57 | 50/135 (37.0 *%) |
| PLAC_TOP | Once daily for three days | No | Day 57 | 3/134 (2.2 *%) | |||
| Piacquadio 2004 [85] | PP | No: no set target area defined | ALA_PDT | One session | A second session was offered at week 8 if AKs remained | 4 weeks after second (final) PDT | 109/149 (73%) |
| PLAC_PDT | One session | 4 weeks after second (final) PDT | 4/52 (8%) | ||||
| Reinhold 2016 [52] | ITT | Yes | ALA_PDT | One session | A second session was offered at week 12 if AKs remained | 12 weeks after last PDT | 50 */55 (91%) |
| PLAC_PDT | One session | 12 weeks after last PDT | 7 */32 (22%) | ||||
| Serra-Guillen 2012 [92] | Completers | Yes | MAL_PDT | One session | No | 1 month after first (only) PDT | 4/40 (10%) |
| IMQ5%_EU | Three times a week on alternate nights, for 4 weeks | No | 1 month after end of first (only) course | 9/33 (27%) | |||
| Simon 2015 [89] | Completers | Yes | 5FU0.5% + SA | Once daily for 6 weeks | No | 8 weeks after end of treatment | 11/33 (33.3%) |
| CRYO | One session | Most patients (87.9%) received a second session 3 weeks following the first session | 11 weeks after second (final) session | 8/32 (25%) | |||
| Stockfleth 2017 [62] | ITT | Yes | 5FU0.5% + SA | Once daily for 12 weeks | No | 8 weeks after end of treatment | 53 */108 (49.5%) |
| PLAC_TOP | Once daily for 12 weeks | No | 8 weeks after end of treatment | 10 */55 (18.2%) | |||
| Stockfleth 2018 [75] | The FAS included all randomized patients | Yes | IM0.015% | Once daily for 3 days | A second course was offered 8 weeks after the first course if AKs were present | Week 8 or week 17, i.e., 56 days after start of last treatment course | 136 */255 (53.3%) |
| DICLO3% | Twice daily for 90 days | No | End of last treatment course, defined as week 17. So, 29 days after end of treatment | 58/247 (23.5%) | |||
| Swanson 2010 [59] | ITT | No: area defined as greater than 25 cm2 | IMQ3.75% | Daily for 2 weeks | All patients received a second course 2 weeks after the end of the first course | 8 weeks after end of treatment | 57 */160 (35.6%) |
| PLAC_TOP | Daily for 2 weeks | 8 weeks after end of treatment | 10 */159 (6.3%) | ||||
| Szeimies 2010 [82] | FAS | Study assessed lesion-directed treatment: area did not have to be contiguous | ALA_PDT | One session | A second session was given at week 12 if AKs remained | 12 weeks after last PDT | 53 */80 (66.3%) |
| PLAC_PDT | One session | 12 weeks after last PDT | 5 */40 (12.5%) | ||||
| Zane 2014 [91] | PP | Study assessed lesion-directed treatment: area did not have to be contiguous | DICLO3% | Twice daily for 90 days | No | 90 days after end of treatment | 27/100 (27%) |
| MAL_PDT | One session | A second session was given at month 3 if AKs remained | 90 days (approximately 13 weeks) after final PDT | 67/98 (68%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heppt, M.V.; Dykukha, I.; Graziadio, S.; Salido-Vallejo, R.; Chapman-Rounds, M.; Edwards, M. Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 1654. https://doi.org/10.3390/jcm11061654
Heppt MV, Dykukha I, Graziadio S, Salido-Vallejo R, Chapman-Rounds M, Edwards M. Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2022; 11(6):1654. https://doi.org/10.3390/jcm11061654
Chicago/Turabian StyleHeppt, Markus V., Igor Dykukha, Sara Graziadio, Rafael Salido-Vallejo, Matt Chapman-Rounds, and Mary Edwards. 2022. "Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 11, no. 6: 1654. https://doi.org/10.3390/jcm11061654
APA StyleHeppt, M. V., Dykukha, I., Graziadio, S., Salido-Vallejo, R., Chapman-Rounds, M., & Edwards, M. (2022). Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 11(6), 1654. https://doi.org/10.3390/jcm11061654







