Thyroid Dysfunction under Amiodarone in Patients with and without Congenital Heart Disease: Results of a Nationwide Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Availability Statement
2.2. Statistical Analysis
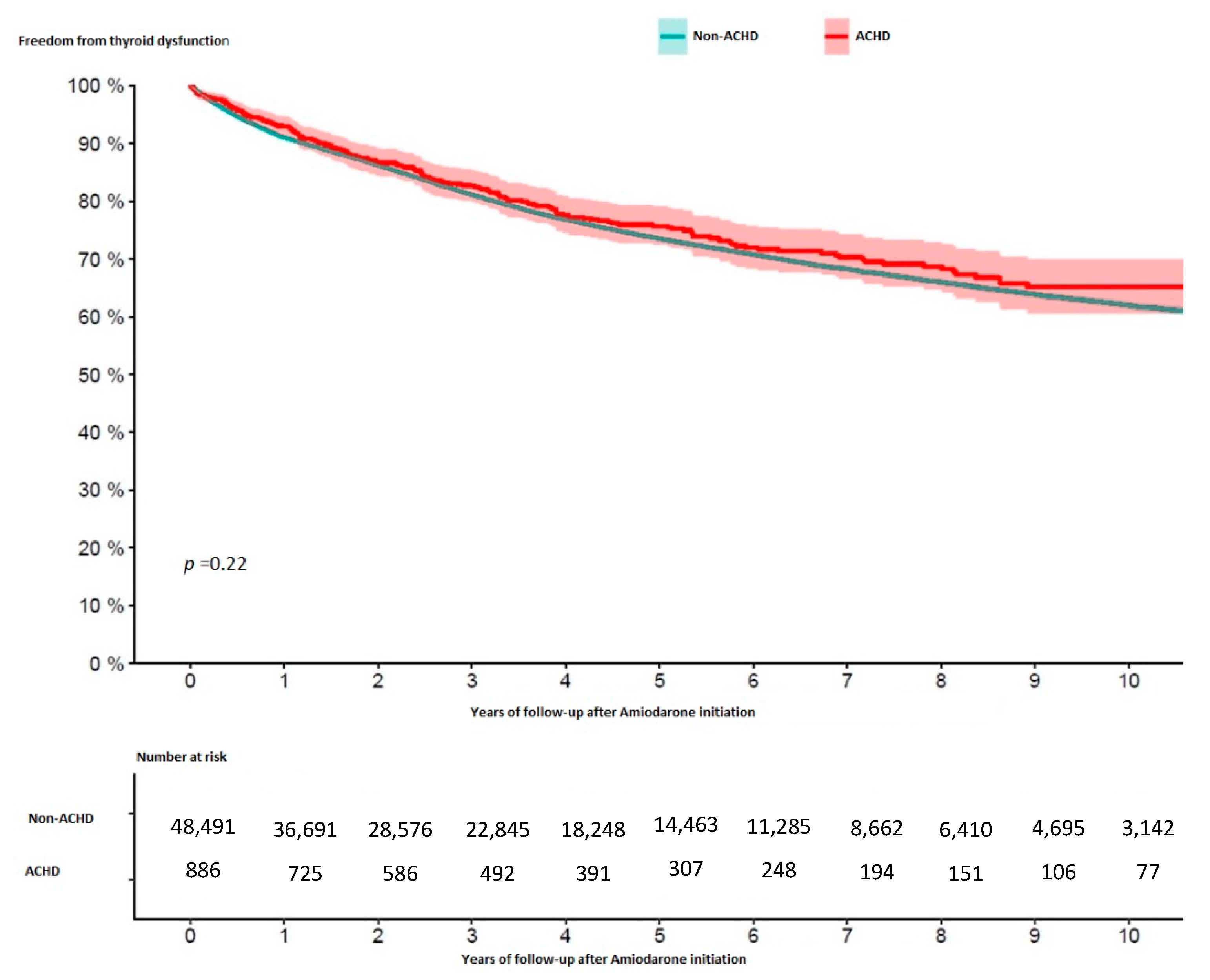
3. Results
4. Discussion
4.1. Incidence of Thyroid Dysfunction and Independent Risk Factors
4.2. Consequences of Diagnosis
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verheugt, C.L.; Uiterwaal, C.S.P.M.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.P.J.; Vliegen, H.W.; Grobbee, D.E.; Mulder, B.J.M. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, S.; Choi, S.H.; Weng, L.-C.; Wang, E.Y.; Trinquart, L.; Benjamin, E.J.; Ellinor, P.T.; Lubitz, S.A. Frequency of Cardiac Rhythm Abnormalities in a Half Million Adults. Circ. Arrhythmia Electrophysiol. 2018, 11, e006273. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, J.N. The cardiac arrhythmia suppression trial (CAST). N. Engl. J. Med. 1989, 321, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N.; Fletcher, R.D.; Fisher, S.G.; Singh, B.N.; Lewis, H.D.; Deedwania, P.C.; Massie, B.M.; Colling, C.; Lazzeri, D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Koyak, Z.; Kroon, B.; de Groot, J.R.; Wagenaar, L.J.; van Dijk, A.P.; Mulder, B.A.; Van Gelder, I.C.; Post, M.C.; Mulder, B.J.; Bouma, B.J. Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am. J. Cardiol. 2013, 112, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Cooper, D.S. Amiodarone and the thyroid. Am. J. Med. 2005, 118, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Scognamiglio, G.; Guarguagli, S.; Merola, A.; Palma, M.; Barracano, R.; Borrelli, N.; Correra, A.; Grimaldi, N.; Colonna, D.; et al. Prognostic Relevance of Thyroid Disorders in Adults With Congenital Heart Disease. Am. J. Cardiol. 2022, 166, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Eskes, S.A.; Wiersinga, W.M. Amiodarone and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 735–751. [Google Scholar] [CrossRef]
- Rabkin, S.W. Effect of amiodarone on phospholipid content and composition in heart, lung, kidney and skeletal muscle: Relationship to alteration of thyroid function. Pharmacology 2006, 76, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pekary, A.E.; Hershman, J.M.; Reed, A.W.; Kannon, R.; Wang, Y.S. Amiodarone inhibits T4 to T3 conversion and alpha-glycerophosphate dehydrogenase and malic enzyme levels in rat liver. Horm. Metab. Res. 1986, 18, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.A.; Barnes, I.; Cullinan, P.; Somerville, J. Amiodarone-associated thyroid dysfunction: Risk factors in adults with congenital heart disease. Circulation 1999, 100, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, B.; Cordina, R.; McGuire, M.; Celermajer, D. Adverse Effects of Amiodarone Therapy in Adults with Congenital Heart Disease. Heart Lung Circ. 2018, 27, S391. [Google Scholar] [CrossRef]
- Koyak, Z.; Harris, L.; de Groot Joris, R.; Candice, K.S.; Erwin, N.O.; Berto, J.B.; Budts, W.; Aeilko, H.Z.; Van Gelder Isabelle, C.; Barbara, J.M.M. Sudden Cardiac Death in Adult Congenital Heart Disease. Circulation 2012, 126, 1944–1954. [Google Scholar] [CrossRef] [Green Version]
- Gatzoulis, M.A.; Balaji, S.; Webber, S.A.; Siu, S.C.; Hokanson, J.S.; Poile, C.; Rosenthal, M.; Nakazawa, M.; Moller, J.H.; Gillette, P.C.; et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet 2000, 356, 975–981. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Jonckheer, M.; Blockx, P.; Kaivers, R.; Wyffels, G. Hyperthyroidism as a possible complication of the treatment of ischemic heart disease with amiodarone. Acta Cardiol. 1973, 28, 192–200. [Google Scholar]
- Van Schepdael, J.; Solvay, H. Clinical study of amiodarone in cardiac rhythmic disorders. Presse Med. 1970, 78, 1849–1850. [Google Scholar]
- Ahmed, S.; Van Gelder, I.C.; Wiesfeld, A.C.; Van Veldhuisen, D.J.; Links, T.P. Determinants and outcome of amiodarone-associated thyroid dysfunction. Clin. Endocrinol. 2011, 75, 388–394. [Google Scholar] [CrossRef]
- Trip, M.D.; Wiersinga, W.; Plomp, T.A. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am. J. Med. 1991, 91, 507–511. [Google Scholar] [CrossRef]
- Zhong, B.; Wang, Y.; Zhang, G.; Wang, Z. Environmental Iodine Content, Female Sex and Age Are Associated with New-Onset Amiodarone-Induced Hypothyroidism: A Systematic Review and Meta-Analysis of Adverse Reactions of Amiodarone on the Thyroid. Cardiology 2016, 134, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Vorperian, V.R.; Havighurst, T.C.; Miller, S.; January, C.T. Adverse effects of low dose amiodarone: A meta-analysis. J. Am. Coll. Cardiol. 1997, 30, 791–798. [Google Scholar] [CrossRef]
- Lee, K.F.; Lee, K.M.; Fung, T.T. Amiodarone-induced thyroid dysfunction in the Hong Kong Chinese population. Hong Kong Med. J. 2010, 16, 434–439. [Google Scholar] [PubMed]
- Uchida, T.; Kasai, T.; Takagi, A.; Sekita, G.; Komiya, K.; Takeno, K.; Shigihara, N.; Shimada, K.; Miyauchi, K.; Fujitani, Y.; et al. Prevalence of amiodarone-induced thyrotoxicosis and associated risk factors in Japanese patients. Int. J. Endocrinol. 2014, 2014, 534904. [Google Scholar] [CrossRef]
- Takeuchi, D.; Honda, K.; Shinohara, T.; Inai, K.; Toyohara, K.; Nakanishi, T. Incidence, Clinical Course, and Risk Factors of Amiodarone-Induced Thyroid Dysfunction in Japanese Adults with Congenital Heart Disease. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 79, 1828–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Aghini-Lombardi, F.; Mariotti, S.; Bartalena, L.; Braverman, L.; Pinchera, A. Amiodarone: A common source of iodine-induced thyrotoxicosis. Horm. Res. 1987, 26, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.K.; Woods, D.R.; Boos, C.J. Management of amiodarone-related thyroid problems. Adv. Endocrinol. Metab. 2011, 2, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.B.; Chiale, P.A.; Haedo, A.; Lázzari, J.O.; Elizari, M.V. Ten years of experience with amiodarone. Am. Heart J. 1983, 106, 957–964. [Google Scholar] [CrossRef]
- Freisinger, E.; Gerß, J.; Makowski, L.; Marschall, U.; Reinecke, H.; Baumgartner, H.; Koeppe, J.; Diller, G.P. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: Results of a nationwide analysis including more than 44,000 patients. Eur. Heart J. 2020, 43, 4168–4177. [Google Scholar] [CrossRef] [PubMed]


| Non-ACHD, n = 48,891 | ACHD, n = 886 | p-Value | |
|---|---|---|---|
| Age (median years (IQR)) | 73.4 (66.1–79.4) | 65.9 (55.0–74.7) | <0.001 |
| Sex, female, n (%) | 18,066 (37.0) | 301 (34.0) | 0.07 |
| Complexity of congenital heart disease | |||
| Simple, n (%) | 577 (65.1) | ||
| Moderate, n (%) | 206 (23.3) | ||
| Severe, n (%) | 103 (11.6) | ||
| Left heart failure, n (%) | 26,661 (54.5) | 497 (56.1) | 0.36 |
| Right heart failure, n (%) | 10,192 (20.8) | 216 (24.4) | 0.01 |
| Pacemaker, n (%) | 4118 (8.4) | 81 (9.1) | 0.43 |
| Implantable cardioverter defibrillator, n (%) | 4332 (8.9) | 63 (7.1) | 0.07 |
| Obesity, n (%) | 18.478 (37.8) | 279 (31.5) | <0.001 |
| Smoking, n (%) | 6600 (13.5) | 120 (13.5) | 0.96 |
| Alcohol abuse, n (%) | 2671 (5.5) | 40 (4.5) | 0.23 |
| Chronic kidney disease, n (%) | 2136 (4.4) | 27 (3.0) | 0.06 |
| Liver dysfunction, n (%) | 53 (0.1) | 0 (0.0) | 1.00 |
| Arrhythmia | |||
| Atrial reentrant tachycardia, n (%) | 7023 (14.4) | 170 (19.2) | <0.001 |
| Atrial fibrillation, n (%) | 41,584 (85.1) | 757 (85.4) | 0.78 |
| Atrial flutter, n (%) | 3143 (6.4) | 73 (8.2) | 0.03 |
| Ventricular extrasystole, n (%) | 9438 (19.3) | 209 (23.6) | 0.002 |
| Ventricular flutter/fibrillation, n (%) | 2669 (5.5) | 42 (4.7) | 0.41 |
| Ventricular reentrant tachycardia, n (%) | 234 (0.5) | 6 (0.7) | 0.33 |
| Ventricular tachycardia, n (%) | 7250 (14.8) | 127 (14.3) | 0.74 |
| Cardiac arrest (not specified further), n (%) | 1742 (3.6) | 35 (4.0) | 0.52 |
| Heart failure drug therapy | |||
| Calcium channel blockers, n (%) | 20,958 (42.9%) | 331 (37.4) | <0.001 |
| ACE-Inhibitors/Angiotensin II receptor blockers, n (%) | 41,469 (84.8) | 706 (79.7) | <0.001 |
| Betablockers (excluding sotalol), n (%) | 43,386 (88.7) | 805 (90.9) | 0.05 |
| Cardiac glycosides, n (%) | 11,046 (22.6) | 198 (22.3) | 0.90 |
| Non-ACHD, n = 48,891 | ACHD, n = 886 | p-Value | |
|---|---|---|---|
| Combined thyroid dysfunction, n (%) | 10,677 (21.8) | 198 (22.3) | 0.71 |
| Hyperthyroidism, n (%) | 5094 (10.4) | 103 (11.6) | 0.24 |
| Hypothyroidism, n (%) | 7079 (14.5) | 138 (15.6) | 0.36 |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Complexity of congenital heart disease (moderate versus simple) | 1.07 (0.81–1.39) | 0.64 |
| Complexity of congenital heart disease (complex versus simple) | 1.48 (1.10–1.98) | 0.009 |
| Age/10 years | 0.94 (0.87–1.02) | 0.12 |
| Female gender | 1.78 (1.42–2.23) | <0.001 |
| Pacemaker therapy | 0.84 (0.49–1.45) | 0.53 |
| Implantable cardioverter defibrillator | 0.97 (0.46–2.05) | 0.94 |
| Alcohol abuse | 0.90 (0.54–1.49) | 0.68 |
| Nicotine abuse | 1.26 (0.95–1.66) | 0.10 |
| Obesity | 0.95 (0.75–1.22) | 0.71 |
| Intake of amiodarone at 1 year * | 3.68 (2.25–6.01) | <0.001 |
| Intake of amiodarone at 2 year * | 2.97 (1.70–5.18) | <0.001 |
| Intake of amiodarone at 3 year * | 4.89 (2.51–9.51) | <0.001 |
| Intake of amiodarone at 4 year * | 3.93 (2.52–6.12) | <0.001 |
| Non-ACHD, n = 10,677 | ACHD, n = 198 | p-Value | |
|---|---|---|---|
| Levothyroxine, n (%) | 4288 (40.2) | 72 (36.4) | 0.31 |
| Thiamazole, n (%) | 1129 (10.6) | 25 (12.6) | 0.35 |
| Propylthiouracil, n (%) | 36 (0.3) | 3 (1.5) | 0.03 |
| Sodiumperchlorate, n (%) | 501 (4.7) | 16 (8.1) | 0.04 |
| Radiotherapy (thyroid), n (%) | 76 (0.7) | 3 (1.5) | 0.17 |
| Thyroid surgery, n (%) | 170 (1.6) | 4 (2.0) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.J.; Enders, D.; Eckardt, L.; Köbe, J.; Wasmer, K.; Breithardt, G.; De Torres Alba, F.; Kaleschke, G.; Baumgartner, H.; Diller, G.-P. Thyroid Dysfunction under Amiodarone in Patients with and without Congenital Heart Disease: Results of a Nationwide Analysis. J. Clin. Med. 2022, 11, 2027. https://doi.org/10.3390/jcm11072027
Fischer AJ, Enders D, Eckardt L, Köbe J, Wasmer K, Breithardt G, De Torres Alba F, Kaleschke G, Baumgartner H, Diller G-P. Thyroid Dysfunction under Amiodarone in Patients with and without Congenital Heart Disease: Results of a Nationwide Analysis. Journal of Clinical Medicine. 2022; 11(7):2027. https://doi.org/10.3390/jcm11072027
Chicago/Turabian StyleFischer, Alicia Jeanette, Dominic Enders, Lars Eckardt, Julia Köbe, Kristina Wasmer, Günter Breithardt, Fernando De Torres Alba, Gerrit Kaleschke, Helmut Baumgartner, and Gerhard-Paul Diller. 2022. "Thyroid Dysfunction under Amiodarone in Patients with and without Congenital Heart Disease: Results of a Nationwide Analysis" Journal of Clinical Medicine 11, no. 7: 2027. https://doi.org/10.3390/jcm11072027
APA StyleFischer, A. J., Enders, D., Eckardt, L., Köbe, J., Wasmer, K., Breithardt, G., De Torres Alba, F., Kaleschke, G., Baumgartner, H., & Diller, G.-P. (2022). Thyroid Dysfunction under Amiodarone in Patients with and without Congenital Heart Disease: Results of a Nationwide Analysis. Journal of Clinical Medicine, 11(7), 2027. https://doi.org/10.3390/jcm11072027






