Intraocular Pressure Measurement in Childhood Glaucoma under Standardized General Anaesthesia: The Prospective EyeBIS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
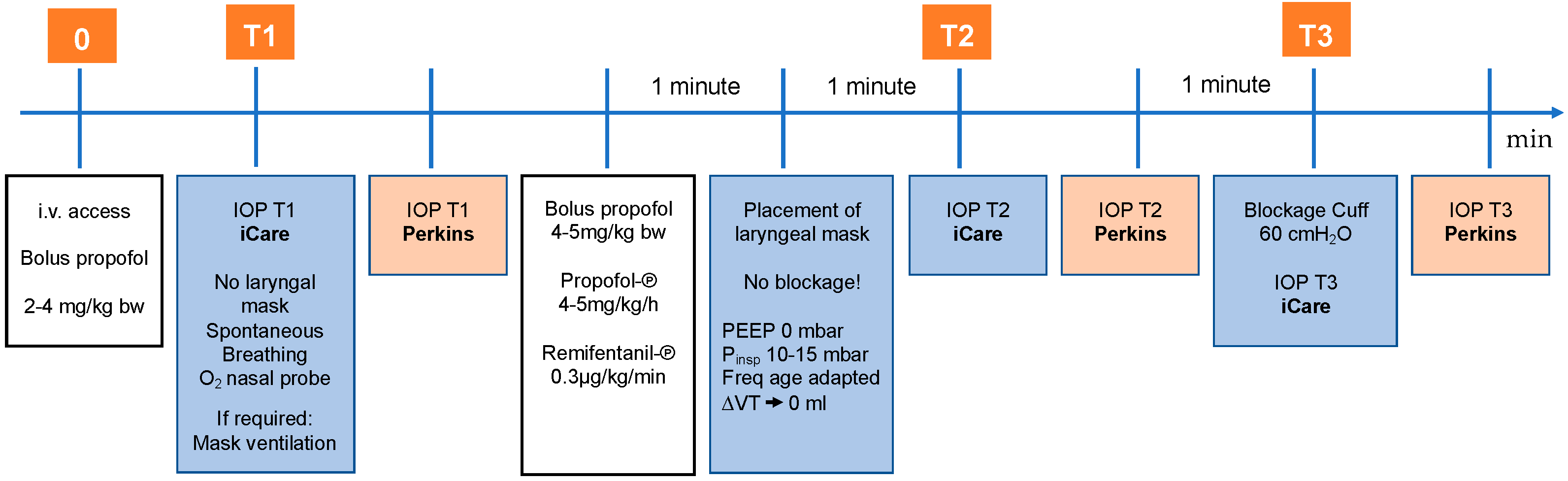
2.2. Intraocular Pressure Measurement
2.3. Sequence of Measurements
2.4. Corneal Thickness Measurement
2.5. Inclusion Criteria
2.6. Exclusion Criteria
2.7. Childhood Glaucoma Subjects
2.8. Healthy Subjects
2.9. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Primary Endpoint—Correlation between iCare and Perkins
3.3. Secondary Endpoints
3.3.1. Median IOP with iCare and Perkins
3.3.2. Correlation of CCT and IOP
3.3.3. Correlation of Age and IOP
4. Discussion
Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genĉík, A. Epidemiology and Genetics of Primary Congenital Glaucoma in Slovakia. Description of a Form of Primary Congenital Glaucoma in Gypsies with Autosomal-Recessive Inheritance and Complete Penetrance. Dev. Ophthalmol. 1989, 16, 76–115. [Google Scholar] [PubMed]
- Lundvall, A.; Svedberg, H.; Chen, E. Application of the ICare Rebound Tonometer in Healthy Infants. J. Glaucoma 2011, 20, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Durnian, J.M.; Cheeseman, R.; Kumar, A.; Raja, V.; Newman, W.; Chandna, A. Childhood Sight Impairment: A 10-Year Picture. Eye 2010, 24, 112–117. [Google Scholar] [CrossRef]
- Dorairaj, S.K.; Bandrakalli, P.; Shetty, C.; Vathsala, R.; Misquith, D.; Ritch, R. Childhood Blindness in a Rural Population of Southern India: Prevalence and Etiology. Ophthalmic Epidemiol. 2008, 15, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.H.; Al-Muhaylib, A.A.; Al Owaifeer, A.M.; Al-Essa, R.S.; Al-Shahwan, S.A. Primary Congenital Glaucoma: An Updated Review. Saudi J. Ophthalmol. 2019, 33, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Thau, A.; Lloyd, M.; Freedman, S.; Beck, A.; Grajewski, A.; Levin, A.V. New Classification System for Pediatric Glaucoma: Implications for Clinical Care and a Research Registry. Curr. Opin. Ophthalmol. 2018, 29, 385–394. [Google Scholar] [CrossRef]
- Fung, D.S.; Roensch, M.A.; Kooner, K.S.; Cavanagh, H.D.; Whitson, J.T. Epidemiology and Characteristics of Childhood Glaucoma: Results from the Dallas Glaucoma Registry. Clin. Ophthalmol. 2013, 7, 1739–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangiacomo, A.; Beck, A. Pediatric Glaucoma: Review of Recent Literature. Curr. Opin. Ophthalmol. 2017, 28, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihota, R.; Tuli, D.; Dada, T.; Gupta, V.; Sachdeva, M.M. Distribution and Determinants of Intraocular Pressure in a Normal Pediatric Population. J. Pediatr. Ophthalmol. Strabismus 2006, 43, 14–18, quiz 36–37. [Google Scholar] [PubMed]
- Oberacher-Velten, I.; Prasser, C.; Rochon, J.; Ittner, K.-P.; Helbig, H.; Lorenz, B. The Effects of Midazolam on Intraocular Pressure in Children during Examination under Sedation. Br. J. Ophthalmol. 2011, 95, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Dear, G.D.; Hammerton, M.; Hatch, D.J.; Taylor, D. Anaesthesia and Intra-Ocular Pressure in Young Children. A Study of Three Different Techniques of Anaesthesia. Anaesthesia 1987, 42, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, E.; Murai, Y. The Effect of Ketamine on Intraocular Pressure in Children. Surv. Anesthesiol. 1972, 16, 252. [Google Scholar] [CrossRef]
- Adams, A.K. Ketamine in Paediatric Ophthalmic Practice. Anaesthesia 1973, 28, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.; Hill, R.; Lipham, W.J.; Weatherwax, K.J.; El-Moalem, H.E. Remifentanil Prevents an Increase in Intraocular Pressure after Succinylcholine and Tracheal Intubation. Br. J. Anaesth. 1998, 81, 606–607. [Google Scholar] [CrossRef] [Green Version]
- Hanna, S.F.; Ahmad, F.; Pappas, A.L.S.; Mikat-Stevens, M.; Jellish, W.S.; Kleinman, B.; Avramov, M.N. The Effect of Propofol/remifentanil Rapid-Induction Technique without Muscle Relaxants on Intraocular Pressure. J. Clin. Anesth. 2010, 22, 437–442. [Google Scholar] [CrossRef]
- Termühlen, J.; Gottschalk, A.; Eter, N.; Hoffmann, E.M.; Van Aken, H.; Grenzebach, U.; Prokosch, V. Does General Anesthesia Have a Clinical Impact on Intraocular Pressure in Children? Paediatr. Anaesth. 2016, 26, 936–941. [Google Scholar] [CrossRef]
- Darlong, V.; Kalaiyarasan, R.; Baidya, D.K.; Pandey, R.; Sinha, R.; Punj, J.; Dada, T. Effect of Airway Device and Depth of Anesthesia on Intra-Ocular Pressure Measurement during General Anesthesia in Children: A Randomized Controlled Trial. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 226–230. [Google Scholar]
- García-Resúa, C.; González-Meijome, J.M.; Gilino, J.; Yebra-Pimentel, E. Accuracy of the New ICare Rebound Tonometer vs. Other Portable Tonometers in Healthy Eyes. Optom. Vis. Sci. 2006, 83, 102. [Google Scholar] [CrossRef] [Green Version]
- Nakakura, S.; Mori, E.; Yamamoto, M.; Tsushima, Y.; Tabuchi, H.; Kiuchi, Y. Intraocular Pressure of Supine Patients Using Four Portable Tonometers. Optom. Vis. Sci. 2013, 90, 700–706. [Google Scholar] [CrossRef]
- Martinez-de-la-Casa, J.M.; Garcia-Feijoo, J.; Saenz-Frances, F.; Vizzeri, G.; Fernandez-Vidal, A.; Mendez-Hernandez, C.; Garcia-Sanchez, J. Comparison of Rebound Tonometer and Goldmann Handheld Applanation Tonometer in Congenital Glaucoma. J. Glaucoma 2009, 18, 49–52. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kanamori, A.; Fujihara, M.; Yamada, Y.; Nakamura, M.; Negi, A. Assessment of IcareONE Rebound Tonometer for Self-Measuring Intraocular Pressure. Acta Ophthalmol. 2014, 92, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Daxer, A.; Misof, K.; Grabner, B.; Ettl, A.; Fratzl, P. Collagen Fibrils in the Human Corneal Stroma: Structure and Aging. Investig. Ophthalmol. Vis. Sci. 1998, 39, 644–648. [Google Scholar] [PubMed]
- Vaughan, D.; Asbury, T. General Ophthalmology. In General Ophthalmology; Lange Medical Publications: Los Altos, CA, USA, 1977; p. 379. [Google Scholar]
- Schäfer, R.; Klett, J.; Auffarth, G.; Polarz, H.; Völcker, H.E.; Martin, E.; Böttiger, B.W. Intraocular Pressure More Reduced during Anesthesia with Propofol than with Sevoflurane: Both Combined with Remifentanil. Acta Anaesthesiol. Scand. 2002, 46, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Pirlich, N.; Grehn, F.; Mohnke, K.; Maucher, K.; Schuster, A.; Wittenmeier, E.; Schmidtmann, I.; Hoffmann, E.M. Anaesthetic Protocol for Paediatric Glaucoma Examinations: The Prospective EyeBIS Study Protocol. BMJ Open 2021, 11, e045906. [Google Scholar] [CrossRef]
- Kontiola, A.I.; Goldblum, D.; Mittag, T.; Danias, J. The Induction/impact Tonometer: A New Instrument to Measure Intraocular Pressure in the Rat. Exp. Eye Res. 2001, 73, 781–785. [Google Scholar] [CrossRef]
- Gloster, J.; Perkins, E.S. The Validity of the Imbert-Fick Law as Applied to Applanation Tonometry. Exp. Eye Res. 1963, 2, 274–283. [Google Scholar] [CrossRef]
- Dobson, V.; Brown, A.M.; Harvey, E.M.; Narter, D.B. Visual Field Extent in Children 3.5–30 Months of Age Tested with a Double-Arc LED Perimeter. Vision Res. 1998, 38, 2743–2760. [Google Scholar] [CrossRef] [Green Version]
- Mayer, D.L.; Dobson, V. Visual Acuity Development in Infants and Young Children, as Assessed by Operant Preferential Looking. Vision Res. 1982, 22, 1141–1151. [Google Scholar] [CrossRef]
- Getz, L.; Dobson, V.; Muna, B. Grating Acuity Development in 2-Week-Old to 3-Year-Old Children Born prior to Term. Clin. Version Sci. 1992, 7, 251–256. [Google Scholar]
- Salomao, S.R.; Ventura, D.F. Large Sample Population Age Norms for Visual Acuities Obtained with Vistech-Teller Acuity Cards. Investig. Ophthalmol. Vis. Sci. 1995, 36, 657–670. [Google Scholar]
- Borrego Sanz, L.; Morales-Fernandez, L.; Martínez de-la-Casa, J.M.; Sáenz-Francés, F.; Fuentes, M.; García-Feijóo, J. The Icare-Pro Rebound Tonometer Versus the Hand-Held Applanation Tonometer in Congenital Glaucoma. J. Glaucoma 2016, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann-Noor, A.H.; Puertas, R.; Tabasa-Lim, S.; El-Karmouty, A.; Kadhim, M.; Wride, N.K.; Lewis, A.; Grosvenor, D.; Rai, P.; Papadopoulos, M.; et al. Comparison of Handheld Rebound Tonometry with Goldmann Applanation Tonometry in Children with Glaucoma: A Cohort Study. BMJ Open 2013, 3, e001788. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, M.; Hirooka, K.; Baba, T.; Shiraga, F. Comparison of ICare Rebound Tonometer with Noncontact Tonometer in Healthy Children. J. Glaucoma 2011, 20, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Flemmons, M.S.; Hsiao, Y.-C.; Dzau, J.; Asrani, S.; Jones, S.; Freedman, S.F. Icare Rebound Tonometry in Children with Known and Suspected Glaucoma. J. AAPOS 2011, 15, 153–157. [Google Scholar] [CrossRef]
- Gandhi, N.G.; Prakalapakorn, S.G.; El-Dairi, M.A.; Jones, S.K.; Freedman, S.F. Icare ONE Rebound versus Goldmann Applanation Tonometry in Children with Known or Suspected Glaucoma. Am. J. Ophthalmol. 2012, 154, 843–849.e1. [Google Scholar] [CrossRef] [PubMed]
- Molero-Senosiaín, M.; Morales-Fernández, L.; Saenz-Francés, F.; García-Feijoo, J.; Martínez-de-la-Casa, J.M. Analysis of Reproducibility, Evaluation, and Preference of the New iC100 Rebound Tonometer versus iCare PRO and Perkins Portable Applanation Tonometry. Eur. J. Ophthalmol. 2020, 30, 1349–1355. [Google Scholar] [CrossRef]
- Takagi, D.; Sawada, A.; Yamamoto, T. Evaluation of a New Rebound Self-Tonometer, Icare HOME: Comparison with Goldmann Applanation Tonometer. J. Glaucoma 2017, 26, 613–618. [Google Scholar] [CrossRef]
- Messenio, D.; Ferroni, M.; Boschetti, F. Goldmann Tonometry and Corneal Biomechanics. Appl. Sci. 2021, 11, 4025. [Google Scholar] [CrossRef]
- Ehlers, N.; Bramsen, T.; Sperling, S. Applanation Tonometry and Central Corneal Thickness. Acta Ophthalmol. 1975, 53, 34–43. [Google Scholar] [CrossRef]
- Barclay, K.; Wall, T.; Wareham, K.; Asai, T. Intra-Ocular Pressure Changes in Patients with Glaucoma. Comparison between the Laryngeal Mask Airway and Tracheal Tube. Anaesthesia 1994, 49, 159–162. [Google Scholar] [CrossRef]
- Muir, K.W.; Jin, J.; Freedman, S.F. Central Corneal Thickness and Its Relationship to Intraocular Pressure in Children. Ophthalmology 2004, 111, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Deol, M.; Taylor, D.A.; Radcliffe, N.M. Corneal Hysteresis and Its Relevance to Glaucoma. Curr. Opin. Ophthalmol. 2015, 26, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatzioufas, Z.; Labiris, G.; Stachs, O.; Hovakimyan, M.; Schnaidt, A.; Viestenz, A.; Käsmann-Kellner, B.; Seitz, B. Biomechanical Profile of the Cornea in Primary Congenital Glaucoma. Acta Ophthalmol. 2013, 91, e29–e34. [Google Scholar] [CrossRef]
- Touboul, D.; Roberts, C.; Kérautret, J.; Garra, C.; Maurice-Tison, S.; Saubusse, E.; Colin, J. Correlations between Corneal Hysteresis, Intraocular Pressure, and Corneal Central Pachymetry. J. Cataract Refract. Surg. 2008, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzalkowska, A.; Pirlich, N.; Stingl, J.V.; Schuster, A.K.; Rezapour, J.; Wagner, F.M.; Buse, J.; Hoffmann, E.M. Intraocular Pressure Measurement in Childhood Glaucoma under Standardized General Anaesthesia: The Prospective EyeBIS Study. J. Clin. Med. 2022, 11, 2846. https://doi.org/10.3390/jcm11102846
Strzalkowska A, Pirlich N, Stingl JV, Schuster AK, Rezapour J, Wagner FM, Buse J, Hoffmann EM. Intraocular Pressure Measurement in Childhood Glaucoma under Standardized General Anaesthesia: The Prospective EyeBIS Study. Journal of Clinical Medicine. 2022; 11(10):2846. https://doi.org/10.3390/jcm11102846
Chicago/Turabian StyleStrzalkowska, Alicja, Nina Pirlich, Julia V. Stingl, Alexander K. Schuster, Jasmin Rezapour, Felix M. Wagner, Justus Buse, and Esther M. Hoffmann. 2022. "Intraocular Pressure Measurement in Childhood Glaucoma under Standardized General Anaesthesia: The Prospective EyeBIS Study" Journal of Clinical Medicine 11, no. 10: 2846. https://doi.org/10.3390/jcm11102846






