Association between Abortion History and Perinatal and Neonatal Outcomes of Singleton Pregnancies after Assisted Reproductive Technology
Abstract
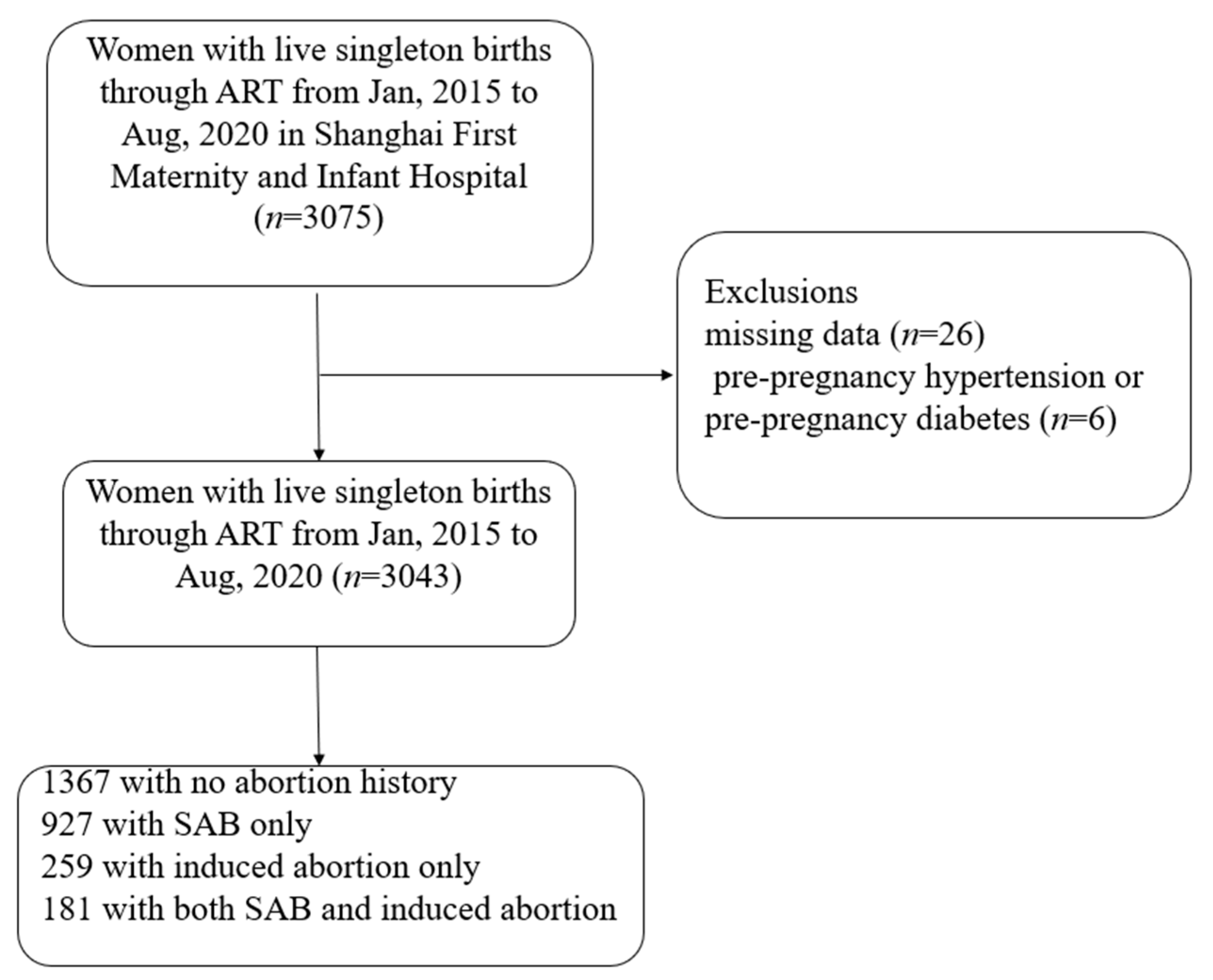
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil Steril. 2016, 105, 73–85.e856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinborg, A.; Wennerholm, U.B.; Romundstad, L.B.; Loft, A.; Aittomaki, K.; Söderström-Anttila, V.; Bergh, C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlev, A.; Walfisch, A.; Oran, E.; Har-Vardi, I.; Friger, M.; Lunenfeld, E.; Levitas, E. The effect of fertility treatment on adverse perinatal outcomes in women aged at least 40 years. Int. J. Gynaecol. Obstet. 2018, 140, 98–104. [Google Scholar] [CrossRef]
- Cavoretto, P.; Candiani, M.; Giorgione, V.; Inversetti, A.; Abu-Saba, M.M.; Tiberio, F.; Farina, A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: Meta-analysis of cohort studies. Ultrasound Obs. Gynecol. 2018, 51, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Sun, X.; Wang, C.; Sui, Y. Analysis of the risk of complications during pregnancy in pregnant women with assisted reproductive technology: A retrospective study using registry linkage from 2013 to 2018 in Shanghai, China. BMC Pregnancy Childbirth 2022, 22, 526. [Google Scholar] [CrossRef]
- Tai, W.; Hu, L.; Wen, J. Maternal and Neonatal Outcomes After Assisted Reproductive Technology: A Retrospective Cohort Study in China. Front. Med. 2022, 9, 837762. [Google Scholar] [CrossRef]
- Kouhkan, A.; Khamseh, M.E.; Pirjani, R.; Moini, A.; Arabipoor, A.; Maroufizadeh, S.; Baradaran, H.R. Obstetric and perinatal outcomes of singleton pregnancies conceived via assisted reproductive technology complicated by gestational diabetes mellitus: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 495. [Google Scholar] [CrossRef] [Green Version]
- Romundstad, L.B.; Romundstad, P.R.; Sunde, A.; von Düring, V.; Skjaerven, R.; Vatten, L.J. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum. Reprod. 2006, 21, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Y.; Li, C.; Zhang, W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014, 101, 385–391. [Google Scholar] [CrossRef]
- Wennerholm, U.B.; Henningsen AK, A.; Romundstad, L.B.; Bergh, C.; Pinborg, A.; Skjaerven, R.; Tiitinen, A. Perinatal outcomes of children born after frozen-thawed embryo transfer: A Nordic cohort study from the CoNARTaS group. Hum. Reprod. 2013, 28, 2545–2553. [Google Scholar] [CrossRef]
- Lei, L.L.; Lan, Y.L.; Wang, S.Y.; Feng, W.; Zhai, Z.J. Perinatal complications and live-birth outcomes following assisted reproductive technology: A retrospective cohort study. Chin. Med. J. 2019, 132, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Elbarazi, I.; Ghazal-Aswad, S.; Al-Maskari, F.; Al-Rifai, R.H.; Oulhaj, A.; Ahmed, L.A. Impact of Recurrent Miscarriage on Maternal Outcomes in Subsequent Pregnancy: The Mutaba’ah Study. Int. J. Womens Health 2020, 12, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Ausbeck, E.B.; Blanchard, C.; Tita, A.T.; Szychowski, J.M.; Harper, L. Perinatal Outcomes in Women with a History of Recurrent Pregnancy Loss. Am. J. Perinatol. 2021, 38, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Field, K.; Murphy, D.J. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: A retrospective cohort study. Hum. Reprod. 2015, 30, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jivraj, S.; Anstie, B.; Cheong, Y.C.; Fairlie, F.M.; Laird, S.M.; Li, T.C. Obstetric and neonatal outcome in women with a history of recurrent miscarriage: A cohort study. Hum. Reprod. 2001, 16, 102–106. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Di, W.; Kuang, Y.P.; Xu, B. Association between induced abortion history and later in vitro fertilization outcomes. Int. J. Gynaecol. Obstet. 2018, 141, 321–326. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Luo, H.N.; Zhang, Y.J.; Shi, R.; Ma, J.F.; Zhang, Y.S. Effect of the number of previous spontaneous abortions on the first in vitro fertilization cycle. Zhonghua Fu Chan Ke Za Zhi 2019, 54, 803–807. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Fan, K.; Jin, L. Association of History of Spontaneous or Induced Abortion With Subsequent Risk of Gestational Diabetes. JAMA Netw Open 2022, 5, e220944. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef]
- Shapira, E.; Ratzon, R.; Shoham-Vardi, I.; Serjienko, R.; Mazor, M.; Bashiri, A. Primary vs. secondary recurrent pregnancy loss--epidemiological characteristics, etiology, and next pregnancy outcome. J. Perinat Med. 2012, 40, 389–396. [Google Scholar] [CrossRef]
- Mohamedain, A.; Rayis, D.A.; AlHabardi, N.; Adam, I. Association between previous spontaneous abortion and preeclampsia: A case-control study. BMC Pregnancy Childbirth 2022, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Rasmark Roepke, E.; Christiansen, O.B.; Källén, K.; Hansson, S.R. Women with a History of Recurrent Pregnancy Loss Are a High-Risk Population for Adverse Obstetrical Outcome: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdottir, J.; Stephansson, O.; Cnattingius, S.; Akerud, H.; Wikström, A.K. Risk of placental dysfunction disorders after prior miscarriages: A population-based study. Am. J. Obs. Gynecol. 2014, 211, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Endler, M.; Saltvedt, S.; Cnattingius, S.; Stephansson, O.; Wikström, A.K. Retained placenta is associated with pre-eclampsia, stillbirth, giving birth to a small-for-gestational-age infant, and spontaneous preterm birth: A national register-based study. BJOG 2014, 121, 1462–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, Y.; Wang, X.Y.; Zhao, Y.Y.; Wang, J.; Zhao, Y.Y. Adverse Pregnancy Outcomes of Patients with History of First-Trimester Recurrent Spontaneous Abortion. Biomed. Res. Int. 2017, 2017, 4359424. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.G.; Mueller, B.A.; Daling, J.R. The relationship of placenta previa and history of induced abortion. Int. J. Gynaecol. Obstet. 2003, 81, 191–198. [Google Scholar] [CrossRef]
- Zhou, W.; Nielsen, G.L.; Larsen, H.; Olsen, J. Induced abortion and placenta complications in the subsequent pregnancy. Acta Obs. Gynecol. Scand 2001, 80, 1115–1120. [Google Scholar] [CrossRef]
- Lowit, A.; Bhattacharya, S.; Bhattacharya, S. Obstetric performance following an induced abortion. Best Pr. Res. Clin. Obs. Gynaecol. 2010, 24, 667–682. [Google Scholar] [CrossRef]
- Ganer Herman, H.; Volodarsky-Perel, A.; Nu TN, T.; Machado-Gedeon, A.; Cui, Y.; Shaul, J.; Dahan, M.H. Does a history of recurrent pregnancy loss affect subsequent obstetric outcomes and placental findings in in vitro fertilization? J. Assist. Reprod. Genet, 2022; in press. [Google Scholar] [CrossRef]
- Bucci, I.; Giuliani, C.; Di Dalmazi, G.; Formoso, G.; Napolitano, G. Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar] [CrossRef]
- Mazzilli, R.; Medenica, S.; Di Tommaso, A.M.; Fabozzi, G.; Zamponi, V.; Cimadomo, D.; Defeudis, G. The role of thyroid function in female and male infertility: A narrative review. J. Endocrinol. Invest. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Pan, X.; Teng, W.; Shan, Z. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.; Zeng, Z.; Zhou, F.; Wang, H.; Liu, J.; Wang, R.; Tang, L. Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 344–361. [Google Scholar] [CrossRef]
- Bernardi, L.A.; Cohen, R.N.; Stephenson, M.D. Impact of subclinical hypothyroidism in women with recurrent early pregnancy loss. Fertil Steril. 2013, 100, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
| History of Abortion (n = 1367) | History of Abortion | No Abortion History (n = 1676) | |||
|---|---|---|---|---|---|
| SAB Only (n = 927) | Induced Abortion Only (n = 259) | Both SAB and Induced Abortion (n = 181) | |||
| Age, y, n (%) | |||||
| ≤24 | 4 (0.29) | 4 (0.43) | 0 (0) | 0 (0) | 15 (0.89) |
| 25–29 | 160 (11.70) | 114 (12.30) | 28 (10.81) | 18 (9.94) | 273 (16.29) |
| 30–34 | 636 (46.53) | 426 (45.95) | 119 (45.95) | 91 (50.28) | 849 (50.66) |
| ≥35 | 567 (41.48) | 383 (41.32) | 112 (43.24) | 72 (39.78) | 539 (32.16) |
| Pre-pregnancy BMI, kg/m2, n (%) | |||||
| <18.5 | 116 (8.49) | 81 (8.74) | 23 (8.88) | 12 (6.63) | 151 (9.01) |
| 18.5–24.9 | 1013 (74.10) | 679 (73.25) | 200 (77.22) | 134 (74.03) | 1268 (75.66) |
| ≥25 | 238 (17.41) | 167 (18.02) | 36 (13.90) | 35 (19.34) | 257 (15.33) |
| Parity, n (%) | |||||
| Nulliparous | 1230 (89.98) | 869 (93.74) | 217 (83.78) | 144 (79.56) | 1608 (95.94) |
| Multiparous | 137 (10.02) | 58 (6.26) | 42 (16.22) | 37 (20.44) | 68 (4.06) |
| Mode of delivery, n (%) | |||||
| Vaginal delivery | 589 (43.09) | 390 (42.07) | 125 (48.26) | 74 (40.88) | 793 (47.32) |
| Cesarean section | 778 (56.91) | 537 (57.93) | 134 (51.74) | 107 (59.12) | 883 (52.68) |
| Year of delivery, n (%) | |||||
| 2015 | 130 (9.51) | 86 (9.28) | 26 (10.04) | 18 (9.94) | 158 (9.43) |
| 2016 | 228 (16.68) | 155 (16.72) | 47 (18.15) | 26 (14.36) | 260 (15.51) |
| 2017 | 235 (17.19) | 170 (18.34) | 46 (17.76) | 19 (10.50) | 289 (17.24) |
| 2018 | 235 (17.19) | 149 (16.07) | 48 (18.53) | 38 (20.99) | 277 (16.53) |
| 2019 | 308 (22.53) | 208 (22.44) | 58 (22.39) | 42 (23.20) | 386 (23.03) |
| 2020 | 231 (16.90) | 159 (17.15) | 34 (13.13) | 38 (20.99) | 306 (18.26) |
| History of Abortion (n = 1367) | History of Abortion | No Abortion History (n = 1676) | |||
|---|---|---|---|---|---|
| SAB Only (n = 927) | Induced Abortion Only (n = 259) | Both SAB and Induced Abortion (n = 181) | |||
| Gestational diabetes mellitus | 306 (22.38) * | 206 (22.22) * | 62 (23.94) * | 38 (20.99) | 297 (17.72) |
| Gestational hypertension and preeclampsia | 142 (10.39) | 101 (10.90) | 20 (7.72) | 21 (11.60) | 157 (9.37) |
| Premature birth | 73 (5.34) | 53 (5.72) | 14 (5.41) | 6 (3.31) | 79 (4.71) |
| Premature rupture of membranes | 184 (13.46) | 122 (13.16) | 37 (14.29) | 25 (13.81) | 245 (14.62) |
| Polyhydramnios | 17 (1.24) | 11 (1.19) | 3 (1.16) | 3 (1.66) | 20 (1.19) |
| Oligohydramnios | 33 (2.41) | 20 (2.16) | 3 (1.16) | 10 (5.52) * | 39 (2.33) |
| Thyroid disease during pregnancy | 188 (13.75) * | 137 (14.78) * | 25 (9.65) | 26 (14.36) * | 150 (8.95) |
| Placental related diseases | 224 (16.39) * | 151 (16.29) * | 40 (15.44) | 33 (18.23) * | 191 (11.40) |
| Intrahepatic cholestasis of pregnancy | 15 (1.10) | 9 (0.97) | 3 (1.16) | 3 (1.66) | 20 (1.19) |
| Group B Streptococcus infection | 16 (1.17) | 13 (1.40) | 2 (0.77) | 1 (0.55) | 17 (1.01) |
| Postpartum hemorrhage | 7 (0.51) | 6 (0.65) | 1 (0.39) | 0 (0) | 7 (0.42) |
| Umbilical cord-related abnormality | 25 (1.83) | 23 (2.37) | 2 (0.77) | 1 (0.55) | 33 (1.97) |
| History of Sbortion (n = 1367) | History of Abortion | No Abortion History (n = 1676) | |||
|---|---|---|---|---|---|
| SAB Only (n = 927) | Induced Abortion Only (n = 259) | Both SAB and Induced Abortion (n = 181) | |||
| Fetal distress | 66 (4.83) | 49 (5.29) | 8 (3.09) | 9 (4.97) | 99 (5.91) |
| Low birth weight | 68 (4.97) | 49 (5.29) | 11 (4.25) | 8 (4.42) | 68 (4.06) |
| Very low birth weight | 11 (0.80) | 5 (0.54) | 3 (1.16) | 3 (1.66) | 10 (0.60) |
| Gender of newborn (female babies) | 651 (47.62) | 457 (49.30) | 114 (44.02) | 80 (44.20) | 775 (46.24) |
| Apgar score at one minute <7 | 7 (0.51) | 4 (0.43) | 1 (0.39) | 2 (1.10) | 11 (0.66) |
| History of Abortion (n = 1367) | History of Abortion | No Abortion History (n = 1676) | |||
|---|---|---|---|---|---|
| SAB Only (n = 927) | Induced Abortion Only (n = 259) | Both SAB and Induced Abortion (n = 181) | |||
| Gestational diabetes mellitus Crude OR (95% CI) | 1.339 (1.120–1.601) * | 1.327 (1.087–1.619) * | 1.461 (1.070–1.996) * | 1.234 (0.845–1.802) | 1 (Reference) |
| Adjusted OR (95% CI) | 1.239 (1.030–1.492) * | 1.240 (1.010–1.522) * | 1.316 (0.948–1.827) | 1.052 (0.705–1.569) | 1 (Reference) |
| Thyroid disease during pregnancy Crude OR (95% CI) | 1.622 (1.292–2.037) * | 1.764 (1.378–2.259) * | 1.087 (0.696–1.696) | 1.706 (1.090–2.671) * | 1 (Reference) |
| Adjusted OR (95% CI) | 1.589 (1.261–2.002) * | 1.724 (1.344–2.213) * | 1.075 (0.682–1.696) | 1.657 (1.042–2.634) * | 1 (Reference) |
| Placental-related diseases Crude OR (95% CI) | 1.524 (1.238–1.876) * | 1.513 (1.202–1.905) * | 1.420 (0.982–2.054) | 1.734 (1.155–2.602) * | 1 (Reference) |
| Adjusted OR (95% CI) | 1.465 (1.183–1.815) * | 1.433 (1.132–1.814) * | 1.526 (1.038–2.242) * | 1.747 (1.142–2.673) * | 1 (Reference) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Su, X.; Liu, Y.; Li, G.; Liu, X.; Du, Q. Association between Abortion History and Perinatal and Neonatal Outcomes of Singleton Pregnancies after Assisted Reproductive Technology. J. Clin. Med. 2023, 12, 1. https://doi.org/10.3390/jcm12010001
Sun H, Su X, Liu Y, Li G, Liu X, Du Q. Association between Abortion History and Perinatal and Neonatal Outcomes of Singleton Pregnancies after Assisted Reproductive Technology. Journal of Clinical Medicine. 2023; 12(1):1. https://doi.org/10.3390/jcm12010001
Chicago/Turabian StyleSun, Hanxiang, Xiujuan Su, Yang Liu, Guohua Li, Xiaosong Liu, and Qiaoling Du. 2023. "Association between Abortion History and Perinatal and Neonatal Outcomes of Singleton Pregnancies after Assisted Reproductive Technology" Journal of Clinical Medicine 12, no. 1: 1. https://doi.org/10.3390/jcm12010001





