Effect of New-Onset Diabetes Mellitus on Renal Outcomes and Mortality in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort and Design
2.3. Outcome Measures and Relevant Variables
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
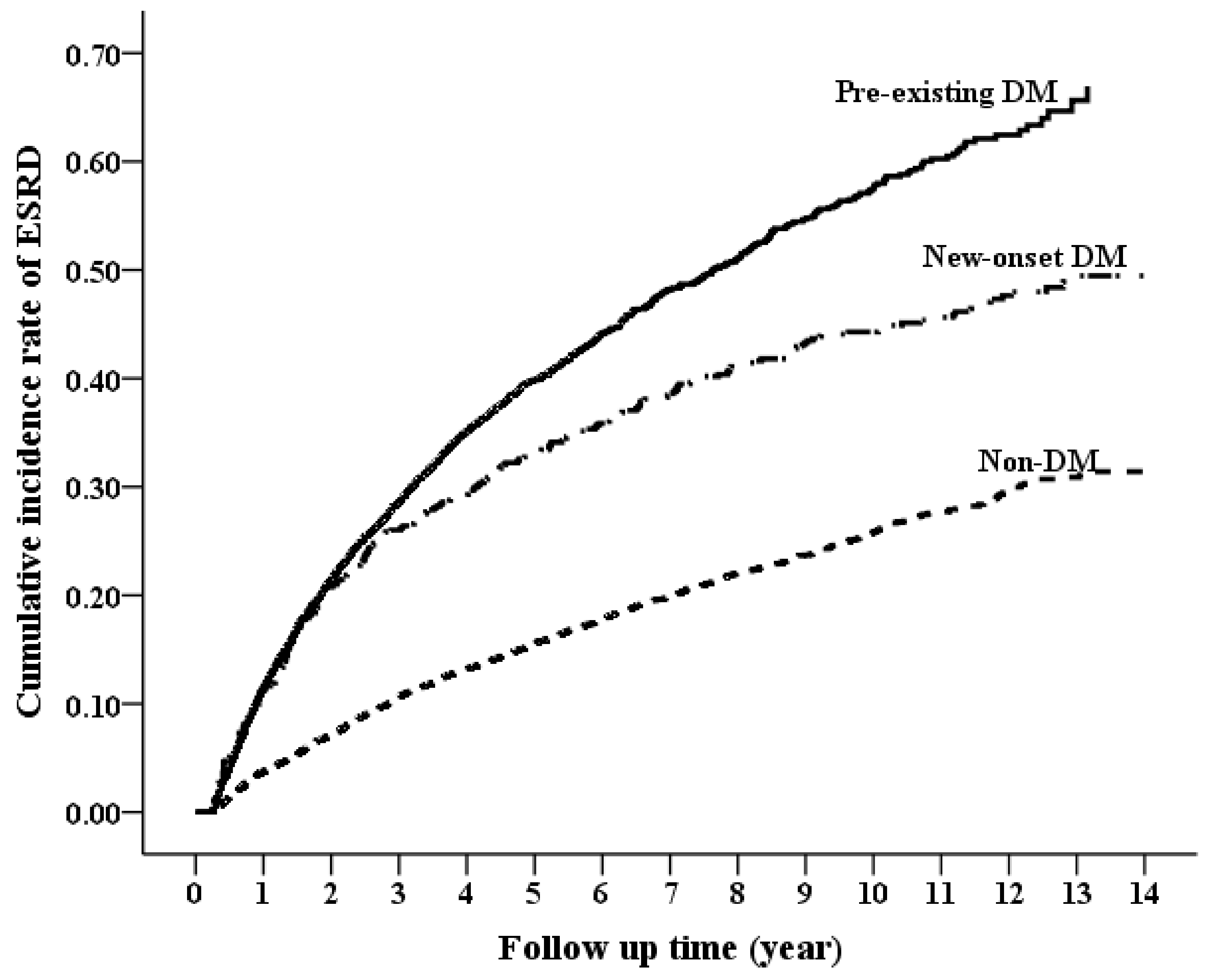
3.2. Long-Term Risk of ESRD by DM Status
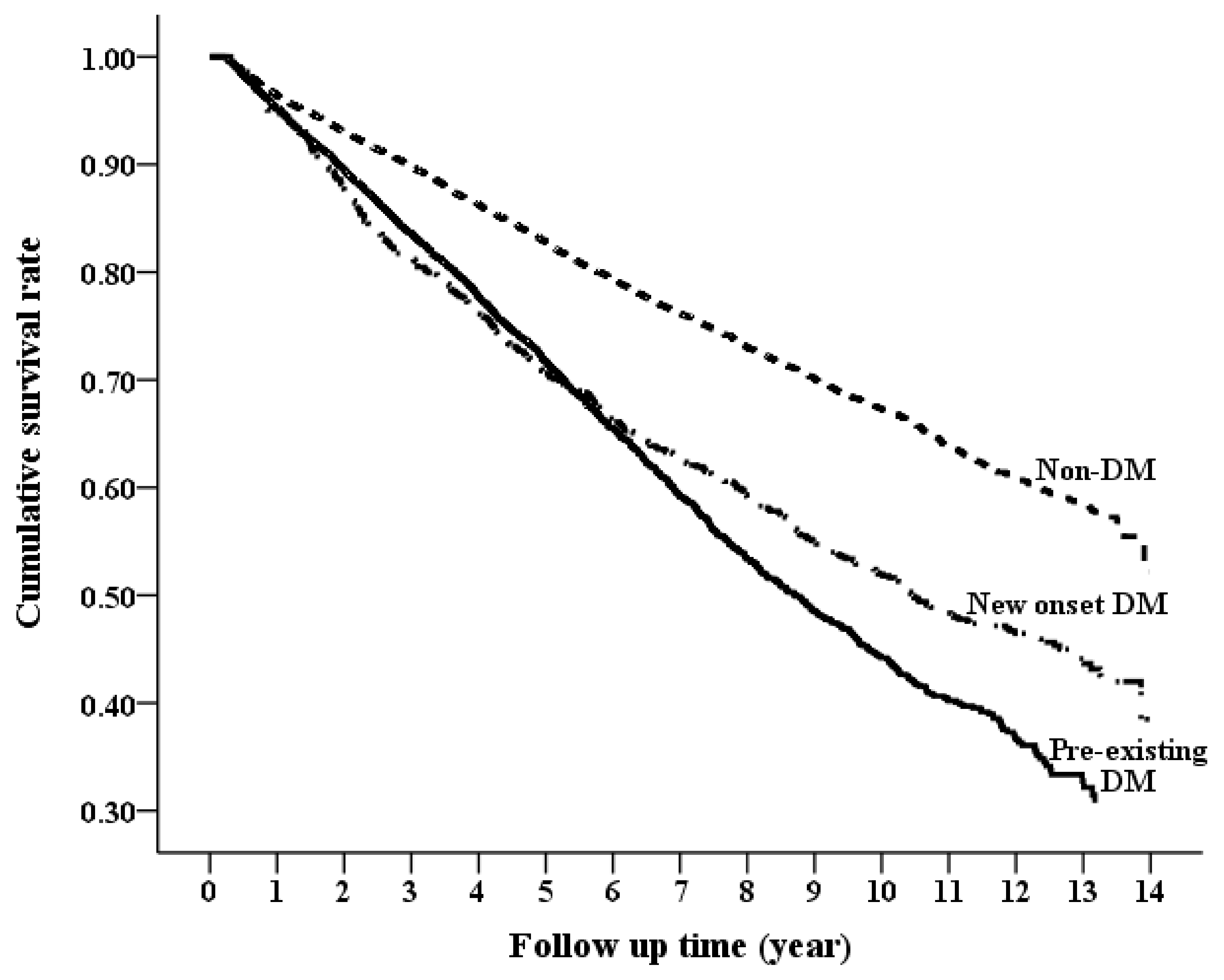
3.3. Long-Term Risk of Mortality by DM Status
3.4. Long-Term Risk of Composite Outcome (ESRD or Death) by DM Status
3.5. Significant Risk Factors for Incident DM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arogundade, F.A.; Barsoum, R.S. CKD prevention in Sub-Saharan Africa: A call for governmental, nongovernmental, and community support. Am. J. Kidney Dis. 2008, 51, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Hallan, S.I.; Miller, E.R.; Yeung, E.; Coresh, J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am. J. Epidemiol. 2008, 167, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Hemmelgarn, B.; Lloyd, A.; James, M.T.; Manns, B.J.; Klarenbach, S.; Tonelli, M.; Alberta Kidney Disease Network. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin. J. Am. Soc Nephrol. 2011, 6, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; Manns, B.J.; Lloyd, A.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Tonelli, M.; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. J. Am. Med. Assoc. 2010, 303, 423–429. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G.; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Seshasai, S.R.K.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Mukamal, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. J. Am. Med. Assoc. 2004, 291, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Kastarinen, M.; Juutilainen, A.; Kastarinen, H.; Salomaa, V.; Karhapää, P.; Tuomilehto, J.; Grönhagen-Riska, C.; Jousilahti, P.; Finne, P. Risk factors for end-stage renal disease in a community-based population: 26-year follow-up of 25,821 men and women in eastern Finland. J. Intern. Med. 2010, 267, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Bilous, R.; Kelly, W.; Jones, S.; Roper, N.; Connolly, V. All-cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabet. Med. 2007, 24, 10–17. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Zelnick, L.; Afkarian, M.; Ayers, E.; Curtin, L.; Himmelfarb, J.; Ikizler, T.A.; Kahn, S.E.; Kestenbaum, B.; Utzschneider, K. Impaired Glucose and Insulin Homeostasis in Moderate-Severe CKD. J. Am. Soc. Nephrol. 2016, 27, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-P.; Kor, C.-T.; Hsieh, M.-C.; Hsieh, Y.-P. Association between End-Stage Renal Disease and Incident Diabetes Mellitus—A Nationwide Population-Based Cohort Study. J. Clin. Med. 2018, 7, 343. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, H.Y.; Li, C.Y.; Lee, M.S.; Su, Y.C. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014, 85, 1200–1207. [Google Scholar] [CrossRef]
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef]
- Raghavan, S.; Liu, W.G.; Saxon, D.R.; Grunwald, G.K.; Maddox, T.M.; Reusch, J.E.B.; Berkowitz, S.A.; Caplan, L. Oral diabetes medication monotherapy and short-term mortality in individuals with type 2 diabetes and coronary artery disease. BMJ Open Diabetes Res. Care 2018, 6, e000516. [Google Scholar] [CrossRef]
- Fiorentino, M.; Bolignano, D.; Tesar, V.; Pisano, A.; Biesen, W.V.; Tripepi, G.; D’Arrigo, G.; Gesualdo, L.; ERA-EDTA Immunonephrology Working Group. Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol. Dial. Transplant. 2017, 32, 97–110. [Google Scholar]
- Hemmelgarn, B.R.; Zhang, J.; Manns, B.J.; Tonelli, M.; Larsen, E.; Ghali, W.A.; Southern, D.A.; McLaughlin, K.; Mortis, G.; Culleton, B.F. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006, 69, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Miyazaki, M.; Yamada, G.; Nakayama, M.; Yamamoto, T.; Satoh, M.; Sato, H.; Ito, S. Diabetes mellitus as a cause or comorbidity of chronic kidney disease and its outcomes: The Gonryo study. Clin. Exp. Nephrol. 2018, 22, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zwi, L.J.; Collins, J.F.; Marshall, M.R.; Cundy, T. Presentation, pathology and prognosis of renal disease in type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000412. [Google Scholar] [CrossRef] [PubMed]
- Tien, K.J.; Lin, Z.Z.; Chio, C.C.; Wang, J.J.; Chu, C.C.; Sun, Y.M.; Kan, W.C.; Chien, C.C. Epidemiology and mortality of new-onset diabetes after dialysis: Taiwan national cohort study. Diabetes Care 2013, 36, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Muzumdar, R.H. Pancreatic function, type 2 diabetes, and metabolism in aging. Int. J. Endocrinol. 2012, 2012, 320482. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Virtanen, M.; Kawachi, I.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Bjorner, J.B.; Borritz, M.; Brunner, E.J.; Burr, H.; et al. Long working hours, socioeconomic status, and the risk of incident type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 27–34. [Google Scholar] [CrossRef]
- Pant, S.; Deshmukh, A.; Gurumurthy, G.S.; Pothineni, N.V.; Watts, T.E.; Romeo, F.; Mehta, J.L. Inflammation and atherosclerosis—Revisited. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Marfisi, R.; Levantesi, G.; Silletta, M.G.; Tavazzi, L.; Tognoni, G.; Valagussa, F.; Marchioli, R. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007, 370, 667–675. [Google Scholar] [CrossRef]
- Emdin, C.A.; Anderson, S.G.; Woodward, M.; Rahimi, K. Usual Blood Pressure and Risk of New-Onset Diabetes: Evidence From 4.1 Million Adults and a Meta-Analysis of Prospective Studies. J. Am. Coll. Cardiol. 2015, 66, 1552–1562. [Google Scholar] [CrossRef] [PubMed]

| CKD Cohort | Maximum Standardization Difference between Groups | |||||
|---|---|---|---|---|---|---|
| Non-DM | Pre-Existing DM | Incident DM | p-Value | Before IPW a (%) | After IPW a (%) | |
| Sample size | 10356 | 6982 | 1103 | -- | -- | -- |
| Age, years | 65.45 ± 15.72 | 67.03 ± 12.02 | 65.21 ± 12.89 | <0.001 | 0.128 | 0.037 |
| Gender, Male | 6254 (60.39%) | 3834 (54.91%) | 641 (58.11%) | <0.001 | 0.111 | 0.039 |
| Monthly income, US dollars | 471.28 ± 471.09 | 401.91 ± 386.44 | 409.58 ± 433.35 | <0.001 | 0.158 | 0.090 |
| Geographic location | ||||||
| Northern | 4465 (43.12%) | 2973 (42.58%) | 481 (43.61%) | 0.705 | -- | -- |
| Middle | 2006 (19.37%) | 1251 (17.92%) | 214 (19.4%) | 0.049 | 0.038 | 0.043 |
| Southern | 3599 (34.75%) | 2540 (36.38%) | 386 (35%) | 0.086 | 0.034 | 0.051 |
| Eastern | 286 (2.76%) | 218 (3.12%) | 22 (1.99%) | 0.079 | 0.068 | 0.044 |
| Comorbidities within 1 year before the index date | ||||||
| Hypertension | 6362 (61.43%) | 5509 (78.9%) | 751 (68.09%) | <0.001 | 0.376 | 0.058 |
| Hyperlipidemia | 2465 (23.8%) | 3137 (44.93%) | 334 (30.28%) | <0.001 | 0.452 | 0.050 |
| Ischemic heart disease | 1973 (19.05%) | 1799 (25.77%) | 279 (25.29%) | <0.001 | 0.162 | 0.016 |
| Congestive heart failure | 1148 (11.09%) | 1159 (16.6%) | 137 (12.42%) | <0.001 | 0.163 | 0.019 |
| Stroke | 1273 (12.29%) | 1264 (18.1%) | 139 (12.6%) | <0.001 | 0.165 | 0.084 |
| Rheumatoid disease | 284 (2.74%) | 101 (1.45%) | 20 (1.81%) | <0.001 | 0.088 | 0.044 |
| Cancer | 831 (8.02%) | 479 (6.86%) | 59 (5.35%) | <0.001 | 0.102 | 0.041 |
| COPD | 1195 (11.54%) | 655 (9.38%) | 157 (14.23%) | <0.001 | 0.156 | 0.022 |
| Charlson comorbidity index | 2.16 ± 1.85 | 2.53 ± 1.89 | 2.07 ± 1.76 | <0.001 | 0.246 | 0.076 |
| Long-term medication use | ||||||
| Anti-hypertensive drugs | ||||||
| ACEI/ARB | 3460 (33.41%) | 4577 (65.55%) | 376 (34.09%) | <0.001 | 0.645 | 0.057 |
| beta-blocker | 3109 (30.02%) | 3127 (44.79%) | 363 (32.91%) | <0.001 | 0.308 | 0.041 |
| Diuretics | 2366 (22.85%) | 3095 (44.33%) | 264 (23.93%) | <0.001 | 0.464 | 0.044 |
| Statin | 1570 (15.16%) | 3343 (47.88%) | 181 (16.41%) | <0.001 | 0.732 | 0.060 |
| NSAIDs | 1565 (15.11%) | 1240 (17.76%) | 148 (13.42%) | <0.001 | 0.118 | 0.018 |
| Pentoxifylline | 525 (5.07%) | 756 (10.83%) | 37 (3.35%) | <0.001 | 0.290 | 0.090 |
| Outpatient visit within 1 year before the index date | 29.16 ± 20.32 | 33.22 ± 20.94 | 31.28 ± 21.56 | <0.001 | 0.196 | 0.055 |
| Outcome | Non-DM | Pre-Existing DM | Incident DM | Time-Dependent Cox Model † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | IR (95% CI) | Event | IR (95% CI) | Event | IR (95% CI) | Pre-Existing DM vs. Non-DM aHR (95% CI) | p-Value | Incident DM vs. Non-DM aHR (95% CI) | p-Value | |
| Composite Endpoint | 3294 | 63.34 (61.18−65.51) | 3075 | 143.66 (138.58−148.74) | 307 | 95.4 (84.72−106.07) | 2.29 (2.21−2.36) | <0.0001 | 1.77 (1.70−1.84) | <0.0001 |
| ESRD | 1735 | 33.36 (31.79−34.93) | 2168 | 101.28 (97.02−105.55) | 111 | 34.49 (28.07−40.91) | 2.54 (2.43−2.65) | <0.0001 | 1.12 (1.06−1.19) | 0.0002 |
| All-cause mortality | 2219 | 39.07 (37.45–40.7) | 1895 | 69.91 (66.76–73.06) | 303 | 65.45 (58.08–72.82) | 2.23 (2.14−2.33) | <0.0001 | 2.48 (2.37−2.60) | <0.0001 |
| Cardiovascular death | 267 | 4.70 (4.14−5.27) | 364 | 13.43 (12.05−14.81) | 44 | 9.50 (6.70−12.31) | 3.00 (2.68−3.35) | <0.0001 | 2.68 (2.37−3.04) | <0.0001 |
| Infection-related death | 714 | 12.57 (11.65–13.49) | 600 | 22.14 (20.36–23.91) | 113 | 24.41 (19.91–28.91) | 2.33 (2.16−2.52) | <0.0001 | 2.97 (2.74−3.21) | <0.0001 |
| Variables | Crude HR (95% CI) | p-Value | Adjusted HR † (95% CI) | p-Value |
|---|---|---|---|---|
| Age at diagnosis of CKD (years) | 1.01 (1.01–1.02) | <0.0001 | 1.01 (1–1.01) | 0.022 |
| Gender, Male | 0.949 (0.84–1.07) | 0.387 | ||
| Monthly income | 0.893 (0.85–0.94) | <0.001 | 0.93 (0.88–0.98) | 0.003 |
| Geographic location | ||||
| Northern | 1 | |||
| Central | 0.976 (0.83–1.15) | 0.764 | ||
| Southern | 1.042 (0.91–1.19) | 0.548 | ||
| Eastern | 1 (0.65–1.53) | 0.999 | ||
| Comorbidities within 1 year before the index date | ||||
| Hypertension | 1.532 (1.35–1.74) | <0.001 | 1.35 (1.18–1.55) | <0.0001 |
| Hyperlipidemia | 1.432 (1.26–1.63) | <0.001 | 1.36 (1.2–1.55) | <0.0001 |
| Ischemic heart disease | 1.425 (1.24–1.63) | <0.001 | 1.19 (1.03–1.37) | 0.016 |
| Congestive heart failure | 1.4 (1.17–1.67) | <0.001 | ||
| Stroke | 1.207 (1.01–1.44) | 0.038 | ||
| Rheumatoid disease | 0.684 (0.44–1.06) | 0.092 | ||
| Cancer | 0.904 (0.7–1.18) | 0.453 | ||
| COPD | 1.276 (1.08–1.51) | 0.005 | ||
| Charlson comorbidity index | 1.069 (1.03–1.11) | <0.001 | ||
| Long-term medication use | ||||
| ACEI/ARB | 1.285 (1.13–1.46) | <0.001 | ||
| beta-blocker | 1.328 (1.17–1.51) | <0.001 | ||
| Diuretics | 1.338 (1.16–1.54) | <0.001 | ||
| Statin | 1.337 (1.14–1.57) | <0.001 | ||
| NSAIDs | 1.099 (0.92–1.31) | 0.285 | ||
| Pentoxifylline | 0.815 (0.59–1.13) | 0.222 | 0.7 (0.51–0.98) | 0.037 |
| Outpatient visit within 1 year before the index date (per 1 visit) | 1.006 (1–1.01) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-K.; Kor, C.-T.; Hsieh, Y.-P. Effect of New-Onset Diabetes Mellitus on Renal Outcomes and Mortality in Patients with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 550. https://doi.org/10.3390/jcm7120550
Hsu P-K, Kor C-T, Hsieh Y-P. Effect of New-Onset Diabetes Mellitus on Renal Outcomes and Mortality in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2018; 7(12):550. https://doi.org/10.3390/jcm7120550
Chicago/Turabian StyleHsu, Po-Ke, Chew-Teng Kor, and Yao-Peng Hsieh. 2018. "Effect of New-Onset Diabetes Mellitus on Renal Outcomes and Mortality in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 7, no. 12: 550. https://doi.org/10.3390/jcm7120550
APA StyleHsu, P.-K., Kor, C.-T., & Hsieh, Y.-P. (2018). Effect of New-Onset Diabetes Mellitus on Renal Outcomes and Mortality in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 7(12), 550. https://doi.org/10.3390/jcm7120550




