Acute Responses of Novel Cardiac Biomarkers to a 24-h Ultra-Marathon
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Echocardiography
2.3. Biochemical Analyses
2.4. Statistics
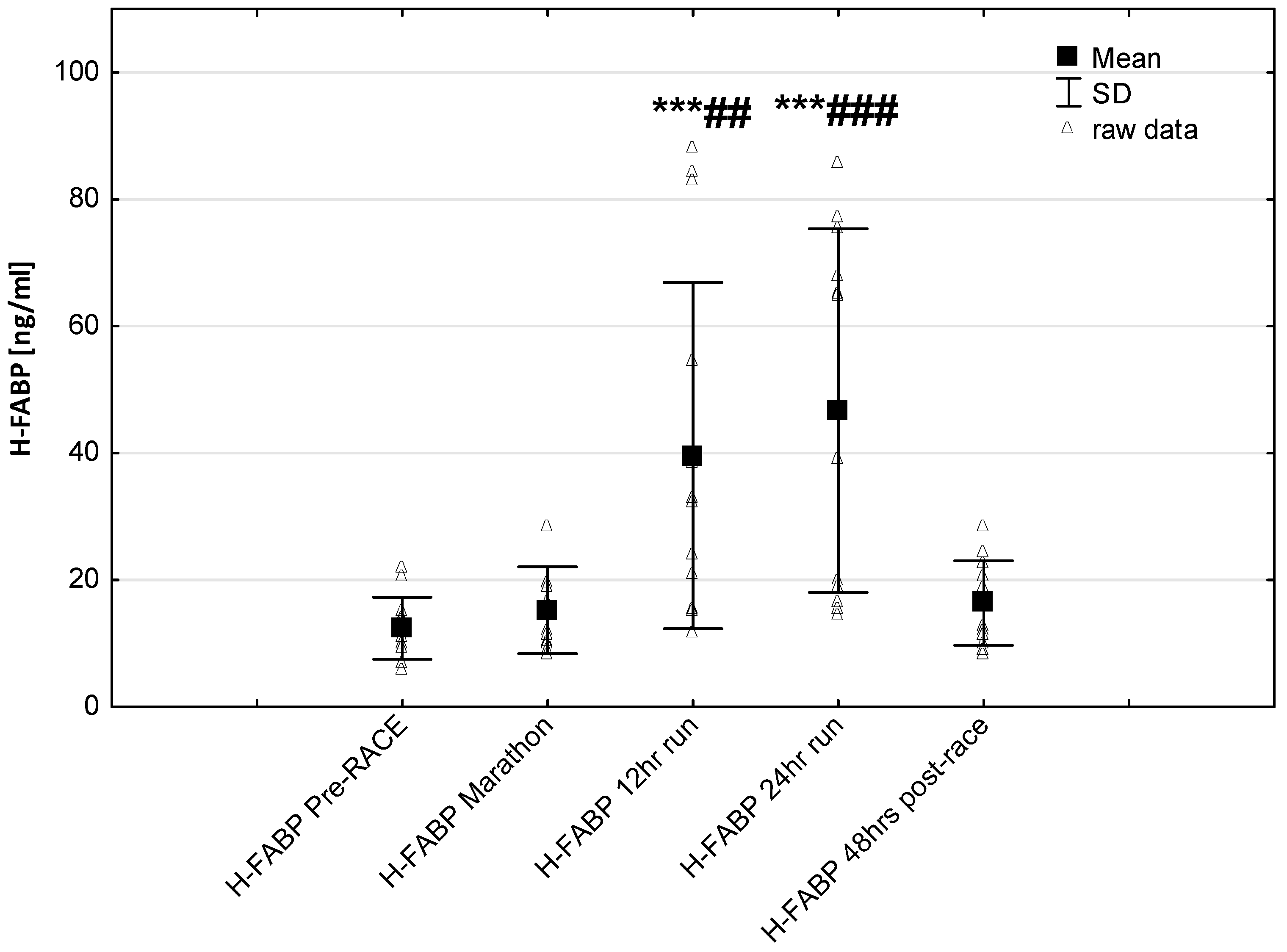
3. Results
4. Discussion
4.1. Echocardiography Examinations
4.2. Cardiac Biomarkers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, D.C.; Brellenthin, A.G.; Thompson, P.D.; Sui, X.; Lee, I.M.; Lavie, C.J. Running as a key lifestyle medicine for longevity. Prog. Cardiovasc. Dis. 2017, 60, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Caso, P.; Scarafile, R.; Salerno, G.; De Corato, G.; Mita, C.; Di Salvo, G.; Allocca, F.; Colonna, D.; Caprile, M.; et al. Biventricular myocardial adaptation to different training protocols in competitive master athletes. Int. J. Cardiol. 2007, 115, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Spence, A.; Rowley, N.; Thijssen, D.H.J.; Naylor, L.H. Vascular adaptation in athletes: Is there an ‘athlete’s artery’? Exp. Physiol. 2012, 97, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Shin, K.A.; Kim, C.H.; Lee, Y.H.; Park, Y.; Ahn, J.; Kim, Y.J. Effects of long-distance running on cardiac markers and biomarkers in exercise-induced hypertension runners: An observational study. Ann. Rehabil. Med. 2018, 42, 575–583. [Google Scholar] [CrossRef]
- Ross, E.; Middleton, N.; Shave, R.; George, K.; McConnell, A. Changes in respiratory muscle and lung function following marathon running in man. J. Sports Sci. 2008, 26, 1295–1301. [Google Scholar] [CrossRef]
- Predel, H.G. Marathon run: Cardiovascular adaptation and cardiovascular risk. Eur. Heart J. 2014, 35, 3091–3096. [Google Scholar] [CrossRef]
- Kłapcińska, B.; Waåkiewicz, Z.; Chrapusta, S.J.; Sadowska-Krȩpa, E.; Czuba, M.; Langfort, J. Metabolic responses to a 48-h ultra-marathon run in middle-aged male amateur runners. Eur. J. Appl. Physiol. 2013, 113, 2781–2793. [Google Scholar] [CrossRef]
- Shin, K.A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.J. Comparison of changes in biochemical markers for skeletal muscles, hepatic metabolism, and renal function after three types of long-distance running. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Schattke, S.; Xing, Y.; Lock, J.; Brechtel, L.; Schroeckh, S.; Spethmann, S.; Baumann, G.; Borges, A.C.; Knebel, F. Increased longitudinal contractility and diastolic function at rest in well-trained amateur marathon runners: A speckle tracking echocardiography study. Cardiovasc. Ultrasound 2014, 12, 11. [Google Scholar] [CrossRef]
- Waśkiewicz, Z.; Kápcińska, B.; Sadowska-Krȩpa, E.; Czuba, M.; Kempa, K.; Kimsa, E.; Gerasimuk, D. Acute metabolic responses to a 24-h ultra-marathon race in male amateur runners. Eur. J. Appl. Physiol. 2012, 112, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Karlstedt, E.; Chelvanathan, A.; Da Silva, M.; Cleverley, K.; Kumar, K.; Bhullar, N.; Lytwyn, M.; Bohonis, S.; Oomah, S.; Nepomuceno, R.; et al. The impact of repeated marathon running on cardiovascular function in the aging population. J. Cardiovasc. Magn. Reson. 2012, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, K.K.; Laaksonen, M.S.; Luotolahti, M.; Laine, H.; Takala, T.O.; Nuutila, P.; Knuuti, J. Myocardial perfusion after marathon running. Scand. J. Med. Sci. Sports 2004, 14, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, H.; Keithahn, A.; Hertel, G.; Drexel, V.; Stern, H.; Schuster, T.; Lorang, D.; Beer, A.J.; Schmidt-Trucks, A.; Nickel, T.; et al. Magnetic resonance imaging of myocardial injury and ventricular torsion after marathon running. Clin. Sci. 2011, 120, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Malhotra, R.; Chiampas, G.; D’Hemecourt, P.; Troyanos, C.; Cianca, J.; Smith, R.N.; Wang, T.J.; Roberts, W.O.; Thompson, P.D.; et al. Cardiac arrest during long-distance running races. N. Engl. J. Med. 2012, 366, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Januzzi, J.L.; Lee-Lewandrowski, E.; Ton-Nu, T.T.; Yoerger, D.M.; Jassal, D.S.; Lewandrowski, K.B.; Siegel, A.J.; Marshall, J.E.; Douglas, P.S.; et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the boston marathon. Circulation 2006, 114, 2325–2333. [Google Scholar] [CrossRef]
- Leers, M.P.G.; Schepers, R.; Baumgarten, R. Effects of a long-distance run on cardiac markers in healthy athletes. Clin. Chem. Lab. Med. 2006, 44, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Knebel, F.; Schimke, I.; Schroeckh, S.; Peters, H.; Eddicks, S.; Schattke, S.; Brechtel, L.; Lock, J.; Wernecke, K.D.; Dreger, H.; et al. Myocardial function in older male amateur marathon runners: Assessment by tissue doppler echocardiography, speckle tracking, and cardiac biomarkers. J. Am. Soc. Echocardiogr. 2009, 22, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Khodaee, M.; Spittler, J.; VanBaak, K.; Changstrom, B.G.; Hill, J.C. Effects of running an ultramarathon on cardiac, hematologic, and metabolic biomarkers. Int. J. Sports Med. 2015, 36, 867–871. [Google Scholar] [CrossRef]
- Fortescue, E.B.; Shin, A.Y.; Greenes, D.S.; Mannix, R.C.; Agarwal, S.; Feldman, B.J.; Shah, M.I.; Rifai, N.; Landzberg, M.J.; Newburger, J.W.; et al. Cardiac troponin increases among runners in the boston marathon. Ann. Emerg. Med. 2007, 49, 137–143.e1. [Google Scholar] [CrossRef] [PubMed]
- Jassal, D.S.; Moffat, D.; Krahn, J.; Ahmadie, R.; Fang, T.; Eschun, G.; Sharma, S. Cardiac injury markers in non-elite marathon runners. Int. J. Sports Med. 2009, 30, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Yoerger, D.M.; Douglas, P.S.; Marshall, J.E.; Halpern, E.F.; Lawlor, D.; Picard, M.H.; Wood, M.J. Persistent and reversible cardiac dysfunction among amateur marathon runners. Eur. Heart J. 2006, 27, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Carranza-García, L.E.; George, K.; Serrano-Ostáriz, E.; Casado-Arroyo, R.; Caballero-Navarro, A.L.; Legaz-Arrese, A. Cardiac biomarker response to intermittent exercise bouts. Int. J. Sports Med. 2011, 32, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hetland, Ø.; Dickstein, K. Cardiac markers in the early hours of acute myocardial infarction: Clinical performance of creatine kinase, creatine kinase mb isoenzyme (activity and mass concentration), creatine kinase MM and MB subform ratios, myoglobin and cardiac troponin T. Scand. J. Clin. Lab. Investig. 1996, 56, 701–713. [Google Scholar] [CrossRef]
- Herrmann, M.; Scharhag, J.; Miclea, M.; Urhausen, A.; Herrmann, W.; Kindermann, W. Post-race kinetics of cardiac troponin T and I and N-terminal pro-brain natriuretic peptide in marathon runners. Clin. Chem. 2003, 49, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Legaz-Arrese, A.; George, K.; Carranza-García, L.E.; Munguía-Izquierdo, D.; Moros-García, T.; Serrano-Ostáriz, E. The impact of exercise intensity on the release of cardiac biomarkers in marathon runners. Eur. J. Appl. Physiol. 2011, 111, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Quist, H.E.; Otto, A.P.; Mathews, W.E.; Murakami, M.M. Release characteristics of cardiac biomarkers and ischemia-modified albumin as measured by the albumin cobalt-binding test after a marathon race. Clin. Chem. 2002, 48, 1097–1100. [Google Scholar] [PubMed]
- Bakula, M.; Milicevic, G.; Bakula, M.; Kozic, I.; Rumenjak, V.; Dominkovic, A. Kinetics of ischemia-modified albumin following exercise-induced myocardial ischemia. Clin. Lab. 2016, 62, 563–571. [Google Scholar] [CrossRef]
- Middleton, N.; Shave, R.; George, K.; Whyte, G.; Forster, J.; Oxborough, D.; Gaze, D.; Collinson, P. Novel application of flow propagation velocity and ischaemia-modified albumin in analysis of postexercise cardiac function in man. Exp. Physiol. 2006, 91, 511–519. [Google Scholar] [CrossRef]
- Okamoto, F.; Sohmiya, K.; Ohkaru, Y.; Kawamura, K.; Asayama, K.; Kimura, H.; Nishimura, S.; Ishii, H.; Sunahara, N.; Tanaka, T. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin. Chem. Lab. Med. 2000, 38, 231–238. [Google Scholar] [CrossRef]
- Cunningham, T.C.; Maghrabi, K.; Sanatani, S. Morbidities in the ultra-athlete and marathoner. Cardiol. Young 2017, 27, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Jastrzȩbski, Z.; Zychowska, M.; Jastrzȩbska, M.; Prusik, K.; Prusik, K.; Kortas, J.; Ratkowski, W.; Konieczna, A.; Radzimiński, Ł. Changes in blood morphology and chosen biochemical parameters in ultra-marathon runners during a 100-km run in relation to the age and speed of runners. Int. J. Occup. Med. Environ. Health 2015, 29, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.L.; Espino, D.; Infante-Ramírez, R.; Cervantes-Borunda, M.S.; Hernández-Torres, R.P.; Rivera-Cisneros, A.E.; Castillo, D.; Westgate, K.; Terzic, D.; Brage, S.; et al. Transient cardiac dysfunction but elevated cardiac and kidney biomarkers 24 h following an ultra-distance running event in mexican tarahumara. Extrem. Physiol. Med. 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Knechtle, B. Performance in 100-km ultra-marathoners—At which age it reaches its peak? J. Strength Cond. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Knechtle, B. Age of peak performance in 50-km ultramarathoners—Is it older than in marathoners? Open Access J. Sports Med. 2018, 9, 37–45. [Google Scholar] [CrossRef]
- Le Goff, C.; Laurent, T.; Kaux, J.F.; Chapelle, J.P. Intense physical exercise related to the emergent generation of cardio-vascular risk markers: A review. Biol. Sport 2012, 29, 11–16. [Google Scholar] [CrossRef]
- Zilinski, J.L.; Contursi, M.E.; Isaacs, S.K.; Deluca, J.R.; Lewis, G.D.; Weiner, R.B.; Hutter, A.M., Jr.; d’Hemecourt, P.A.; Troyanos, C.; Dyer, K.S.; et al. Myocardial adaptations to recreational marathon training among middle-aged men. Circ. Cardiovasc. Imaging 2015, 8, e002487. [Google Scholar] [CrossRef]
- Mingels, A.; Jacobs, L.; Michielsen, E.; Swaanenburg, J.; Wodzig, W.; Van Dieijen-Visser, M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin. Chem. 2009, 55, 101–108. [Google Scholar] [CrossRef]
- Mingels, A.M.A.; Jacobs, L.H.J.; Kleijnen, V.W.; Laufer, E.M.; Winkens, B.; Hofstra, L.; Wodzig, W.K.W.H.; Van Dieijen-Visser, M.P. Cardiac troponin t elevations, using highly sensitive assay, in recreational running depend on running distance. Clin. Res. Cardiol. 2010, 99, 385–391. [Google Scholar] [CrossRef]
- Mair, J. Tissue release of cardiac markers: From physiology to clinical applications. Clin. Chem. Lab. Med. 1999, 37, 1077–1084. [Google Scholar] [CrossRef]
- Sbarouni, E.; Georgiadou, P.; Theodorakis, G.N.; Kremastinos, D.T. Ischemia-modified albumin in relation to exercise stress testing. J. Am. Coll. Cardiol. 2006, 48, 2482–2484. [Google Scholar] [CrossRef] [PubMed]
- Pyati, A.K.; Devaranavadagi, B.B.; Sajjannar, S.L.; Nikam, S.V.; Shannawaz, M.; Sudharani. Heart-type fatty acid binding protein: A better cardiac biomarker than ck-mb and myoglobin in the early diagnosis of acute myocardial infarction. J. Clin. Diagn. Res. 2015, 9, BC08–BC11. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.; De Lemos, J.A.; Morrow, D.A.; Murphy, S.A.; Buros, J.L.; Cannon, C.P.; Sabatine, M.S. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation 2006, 114, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the american society of echocardiography. J. Am. Soc. Echocardiogr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, M.S.; Kalliokoski, K.K.; Luotolahti, M.; Kemppainen, J.; Teräs, M.; Kyröläinen, H.; Nuutila, P.; Knuuti, J. Myocardial perfusion during exercise in endurance-trained and untrained humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R837–R843. [Google Scholar] [CrossRef] [PubMed]
- Hubble, K.M.; Fatovich, D.M.; Grasko, J.M.; Vasikaran, S.D. Cardiac troponin increases among marathon runners in the perth marathon: The troponin in marathons (trim) study. Med. J. Aust. 2009, 190, 91–93. [Google Scholar] [PubMed]
- Fagard, R. Athlete’s heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Frank, A.G.; Ben-Ari, E.; Kessler, G.; Pines, A.; Drory, Y.; Kellermann, J.J. Altered left ventricular volume and ejection fraction responses to supine dynamic exercise in athletes. J.Am. Coll. Cardiol. 1990, 15, 582–588. [Google Scholar] [CrossRef]
- Cowie, M.R.; Jourdain, P.; Maisel, A.; Dahlstrom, U.; Follath, F.; Isnard, R.; Luchner, A.; McDonagh, T.; Mair, J.; Nieminen, M.; et al. Clinical applications of B-type natriuretic peptide (BNP) testing. Eur. Heart J. 2003, 24, 1710–1718. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, Y.O.; Lee, J.B.; Lee, Y.H.; Shin, K.A.; Kim, A.C.; Goh, C.W.; Kim, C.; Oh, J.K.; Min, Y.K.; et al. The effects of running a 308 km ultra-marathon on cardiac markers. Eur. J. Sport Sci. 2014, 14, S92–S97. [Google Scholar] [CrossRef]
- Trivax, J.E.; Franklin, B.A.; Goldstein, J.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Dejong, A.T.; Colar, J.M.; Haines, D.E.; McCullough, P.A. Acute cardiac effects of marathon running. J. Appl. Physiol. 2010, 108, 1148–1153. [Google Scholar] [CrossRef]
- Sinha, M.K.; Gaze, D.C.; Tippins, J.R.; Collinson, P.O.; Kaski, J.C. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation 2003, 107, 2403–2405. [Google Scholar] [CrossRef]
- Delacour, H.; Nervale, A.; Servonnet, A.; Pagliano, B.; Dehan, C.; Gardet, V. Variations of plasma concentrations of h-fabp during a muscular exercise. Ann. Biol. Clin. 2007, 65, 27–32. [Google Scholar]
- Shave, R.; Baggish, A.; George, K.; Wood, M.; Scharhag, J.; Whyte, G.; Gaze, D.; Thompson, P.D. Exercise-induced cardiac troponin elevation: Evidence, mechanisms, and implications. J. Am. Coll. Cardiol. 2010, 56, 169–176. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, H.; He, Y.; Cao, W.; Liu, Y.; Kong, Z.; George, K. The impact of high-intensity interval training on the ctnt response to acute exercise in sedentary obese young women. Scand. J. Med. Sci. Sports 2018. [Google Scholar] [CrossRef]
- Van der Linden, N.; Klinkenberg, L.J.; Leenders, M.; Tieland, M.; Verdijk, L.B.; Niens, M.; van Suijlen, J.D.; de Groot, L.C.; Bekers, O.; van Loon, L.J.; et al. The effect of exercise training on the course of cardiac troponin t and i levels: Three independent training studies. Sci. Rep. 2015, 5, 18320. [Google Scholar] [CrossRef]
- Hindieh, W.; Adler, A.; Weissler-Snir, A.; Fourey, D.; Harris, S.; Rakowski, H. Exercise in patients with hypertrophic cardiomyopathy: A review of current evidence, national guideline recommendations and a proposal for a new direction to fitness. J. Sci. Med. Sport 2017, 20, 333–338. [Google Scholar] [CrossRef]
- Dias, K.A.; Link, M.S.; Levine, B.D. Exercise training for patients with hypertrophic cardiomyopathy: Jacc review topic of the week. J. Am. Coll. Cardiol. 2018, 72, 1157–1165. [Google Scholar] [CrossRef]
- Barakat, B.; Pezzilli, R.; Prestinenza, P. Elevated serum high-sensitive cardiac troponin t in adolescent runner: Exercise or something else? Emerg. Care J. 2014, 10, 5–7. [Google Scholar] [CrossRef]
- Galderisi, M.; Cardim, N.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; Edvardsen, T.; Freitas, A.; Habib, G.; et al. The multi-modality cardiac imaging approach to the athlete’s heart: An expert consensus of the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Rowley, N.J.; Green, D.J.; George, K.; Thijssen, D.H.; Oxborough, D.; Sharma, S.; Somauroo, J.D.; Jones, J.; Sheikh, N.; Whyte, G. Peripheral vascular structure and function in hypertrophic cardiomyopathy. Br. J. Sports Med. 2012, 46 (Suppl. 1), i98–i103. [Google Scholar] [CrossRef] [PubMed]
- Gebka, A.; Rajtar-Salwa, R.; Dziewierz, A.; Petkow-Dimitrow, P. Painful and painless myocardial ischemia detected by elevated level of high-sensitive troponin in patients with hypertrophic cardiomyopathy. Postepy w Kardiologii Interwencyjnej (Adv. Interv. Cardiol.) 2018, 14, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; Ball, C.A.; Hebl, V.B.; Ong, K.C.; Siontis, K.C.; Olson, T.P.; Ackerman, M.J.; Ommen, S.R.; Allison, T.G.; Geske, J.B. Effect of body mass index on exercise capacity in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2018, 121, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Dov, F.; Arad, M. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: Results of a structured exercise training program in a cardiac rehabilitation center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Lee, Y.H.; Chae, J.H.; Kim, C.K. Creatine kinase isoenzyme activity during and after an ultra-distance (200 km) run. Biol. Sport 2015, 32, 357–361. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Nakata, T.; Hashimoto, A.; Hase, M.; Tsuchihashi, K.; Shimamoto, K. Human heart-type fatty acid-binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology 2003, 99, 96–104. [Google Scholar] [CrossRef]
- Young, J.M.; Pickering, J.W.; George, P.M.; Aldous, S.J.; Wallace, J.; Frampton, C.M.; Troughton, R.W.; Richards, M.A.; Greenslade, J.H.; Cullen, L.; et al. Heart fatty acid binding protein and cardiac troponin: Development of an optimal rule-out strategy for acute myocardial infarction. BMC Emerg. Med. 2016, 16, 34. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Yang, F. Role of heart-type fatty acid binding protein in early detection of acute myocardial infarction in comparison with cTni, CK-MB and myoglobin. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 449–451. [Google Scholar]





| Variables | Participants, n = 14 |
|---|---|
| Age (years) | 40.0 ± 11.7 |
| Body mass (kg) | 78.4 ± 11.4 |
| Body height (cm) | 178.0 ± 5.0 |
| BSA (m2) | 2.0 ± 0.2 |
| BMI (kg·m−2) | 24.5 ± 2.4 |
| VO2max (mL·min−1·kg−1) | 55.3 ± 8.8 |
| Variables | Mean | Min–Max |
|---|---|---|
| Marathon time (h) | 4.8 ± 0.7 | 4.4–5.4 |
| Marathon running velocity (km/h) | 8.6 ± 0.8 | 7.8–9.5 |
| 12 h distance (km) | 83.4 ± 6.9 | 57.4–120.4 |
| 12-h running velocity (km/h) | 7.0 ± 0.6 | 4.8–9.9 |
| 24 h distance (km) | 149.4 ± 33.0 | 100.7–214.5 |
| 24-h running velocity (km/h) | 6.2 ± 1.4 | 4.2–8.9 |
| Variables | Pre Ultra-Marathon (Pre-Race) n = 14 | Post-Ultra-Marathon (48 h Post-Race) n = 14 |
|---|---|---|
| LVM (g) | 271.6 ± 37.7 | 271.8 ± 38.2 |
| LVMI (g/m2) | 138.0 ± 11.6 | 138.0 ± 11.2 |
| LVEDD (mm) | 51.5 ± 0.4 | 51.3 ± 0.3 |
| LVESD (mm) | 29.6 ± 0.4 | 29.4 ± 0.6 |
| IVSDD (mm) | 12.3 ± 0.7 | 12.3 ± 0.6 |
| LVPWTD (mm) | 10.5 ± 0.7 | 10.6 ± 0.7 |
| RWT | 0.44 ± 0.04 | 0.45 ± 0.05 |
| LA (mm) | 39.0 ± 8.5 | 38.0 ± 10.3 |
| RVDD (mm) | 29.0 ± 3.0 | 29.0 ± 3.2 |
| LVEF % | 61.4 ± 5.5 | 64.6 ± 8.5 |
| E/A | 1.9 ± 0.3 | 1.3 ± 0.1 ** |
| SBP (mm Hg) | 124.4 ± 14.5 | 120.0 ± 10.5 |
| DBP (mm Hg) | 77.0 ± 11.0 | 77.0 ± 9.0 |
| HR (beats/min) | 58.0 ± 10.0 | 62.0 ± 8.0 |
| Variables (24-h run) | Age | Running Distance |
|---|---|---|
| NT-proBNP (pg/mL) | r = 0.66 p < 0.01 | r = −0.69 p < 0.01 |
| CK-MB (U/L) | r = 0.47 p < 0.87 | r = 0.39 p < 0.17 |
| IMA (IU/mL) | r = 0.14 p < 0.62 | r = −0.42 p < 0.14 |
| cTnT (pg/mL) | r = −0.77 p < 0.79 | r = −0.72 p < 0.49 |
| CRP (ngmL) | r = −0.61 p < 0.02 | r = 0.52 p < 0.05 |
| H-FABP (ng/mL) | r = −0.19 p < 0.52 | r = 0.59 p < 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żebrowska, A.; Waśkiewicz, Z.; Nikolaidis, P.T.; Mikołajczyk, R.; Kawecki, D.; Rosemann, T.; Knechtle, B. Acute Responses of Novel Cardiac Biomarkers to a 24-h Ultra-Marathon. J. Clin. Med. 2019, 8, 57. https://doi.org/10.3390/jcm8010057
Żebrowska A, Waśkiewicz Z, Nikolaidis PT, Mikołajczyk R, Kawecki D, Rosemann T, Knechtle B. Acute Responses of Novel Cardiac Biomarkers to a 24-h Ultra-Marathon. Journal of Clinical Medicine. 2019; 8(1):57. https://doi.org/10.3390/jcm8010057
Chicago/Turabian StyleŻebrowska, Aleksandra, Zbigniew Waśkiewicz, Pantelis T. Nikolaidis, Rafał Mikołajczyk, Damian Kawecki, Thomas Rosemann, and Beat Knechtle. 2019. "Acute Responses of Novel Cardiac Biomarkers to a 24-h Ultra-Marathon" Journal of Clinical Medicine 8, no. 1: 57. https://doi.org/10.3390/jcm8010057
APA StyleŻebrowska, A., Waśkiewicz, Z., Nikolaidis, P. T., Mikołajczyk, R., Kawecki, D., Rosemann, T., & Knechtle, B. (2019). Acute Responses of Novel Cardiac Biomarkers to a 24-h Ultra-Marathon. Journal of Clinical Medicine, 8(1), 57. https://doi.org/10.3390/jcm8010057










