The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population and Study Design of the Retrospective Cohort
2.2. Laboratory Analyses in the Retrospective Cohort
2.3. Meta-Analysis
2.4. Statistical Analysis
3. Results
3.1. The Prevalence of Incidental Endometriosis in Women with CC-Resistant PCOS: Results of the Retrospective Cohort
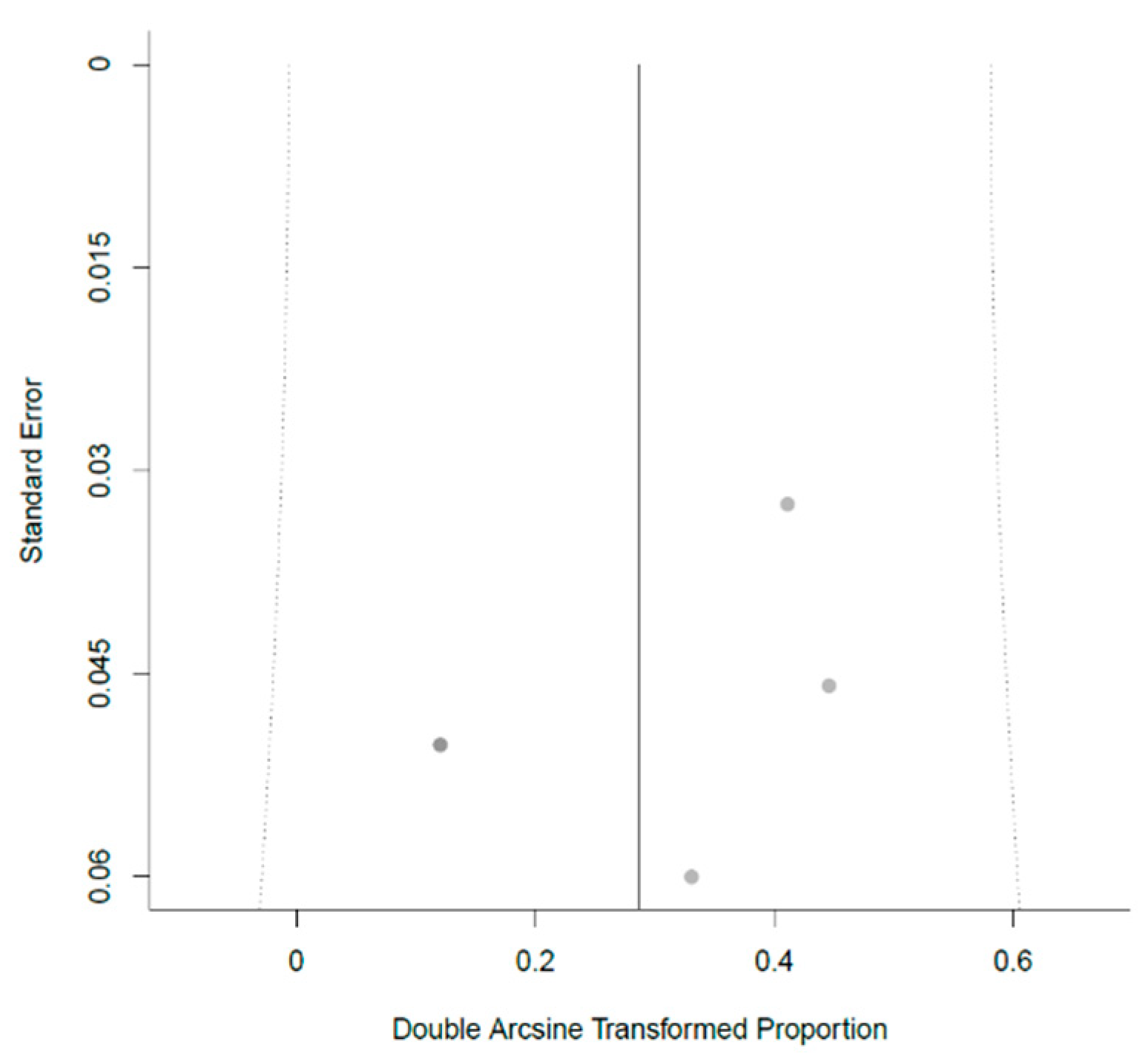
3.2. The Prevalence of Incidental Endometriosis in Women with CC-Resistant PCOS: Results of the Meta-Analysis
3.3. Additional Findings: Fallopian Tube Patency and Predictive Parameters for Endometriosis
4. Discussion
4.1. Incidental Endometriosis in Women with CC-Resistant PCOS and Its Possible Clinical Relevance
4.2. Endometriosis Prevalence in Women Undergoing LOD and Its Relevance for the Transvaginal Hydrolaparoscopic Approach
4.3. Fallopian Tube Patency and Predictive Parameters for Endometriosis
4.4. Study Limitations
4.5. Conclusions
Author Contributions
Conflicts of Interest
References
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. European Society of Human Reproduction and Embryology. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Nayak, P.K.; Agrawal, S. Laparoscopic ovarian drilling: An alternative but not the ultimate in the management of polycystic ovary syndrome. J. Nat. Sci. Biol. Med. 2015, 6, 40–48. [Google Scholar] [PubMed]
- Sanchez, A.M.; Vanni, V.S.; Bartiromo, L.; Papaleo, E.; Zilberberg, E.; Candiani, M.; Orvieto, R.; Viganò, P. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Mayerhofer, K.; Nouri, K.; Walch, K.; Seemann, R.; Kurz, C. Perioperative androstenedione kinetics in women undergoing laparoscopic ovarian drilling: A prospective study. Endocrine 2014, 47, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, A.; Manchanda, R.; Agarwal, N.; Nayar, B. Laparoscopic ovarian drilling in clomiphene citrate-resistant women with polycystic ovary syndrome. J. Am. Assoc. Gynecol. Laparosc. 2001, 8, 511–518. [Google Scholar] [CrossRef]
- Salah, I.M. Office microlaparoscopic ovarian drilling (OMLOD) versus conventional laparoscopic ovarian drilling (LOD) for women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2013, 287, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ezedinma, N.A.; Phelps, J.Y. Transvaginal hydrolaparoscopy. JSLS 2012, 16, 461–465. [Google Scholar] [CrossRef]
- Fernandez, H.; Alby, J.D.; Gervaise, A.; de Tayrac, R.; Frydman, R. Operative transvaginal hydrolaparoscopy for treatment of polycystic ovary syndrome: A new minimally invasive surgery. Fertil. Steril. 2001, 75, 607–611. [Google Scholar] [CrossRef]
- Fernandez, H.; Watrelot, A.; Alby, J.D.; Kadoch, J.; Gervaise, A.; de Tayrac, R.; Frydman, D. Fertility after ovarian drilling by transvaginal fertiloscopy for treatment of polycystic ovary syndrome. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 374–378. [Google Scholar] [CrossRef]
- Giampaolino, P.; Morra, I.; Della Corte, L.; Sparice, S.; Di Carlo, C.; Nappi, C.; Bifulco, G. Serum anti-Mullerian hormone levels after ovarian drilling for the second-line treatment of polycystic ovary syndrome: A pilot-randomized study comparing laparoscopy and transvaginal hydrolaparoscopy. Gynecol. Endocrinol. 2017, 33, 26–29. [Google Scholar] [CrossRef]
- Jacobson, T.Z.; Duffy, J.M.; Barlow, D.; Farquhar, C.; Koninckx, P.R.; Olive, D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst. Rev. 2010, 12, CD001398. [Google Scholar]
- Watrelot, A. Place of transvaginal fertiloscopy in the management of tubal factor disease. Reprod. Biomed. Online 2007, 15, 389–395. [Google Scholar] [CrossRef]
- Signorile, P.G.; Petraglia, F.; Baldi, A. Anti-mullerian hormone is expressed by endometriosis tissues and induces cell cycle arrest and apoptosis in endometriosis cells. J. Exp. Clin. Cancer Res. 2014, 33, 46. [Google Scholar] [CrossRef]
- Wang, J.; Dicken, C.; Lustbader, J.W.; Tortoriello, D.V. Evidence for a Müllerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil. Steril. 2009, 91, 1195–1203. [Google Scholar] [CrossRef]
- Karakas, S.E. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 248–253. [Google Scholar] [CrossRef]
- Fiala, L.; Bob, P.; Raboch, J. Oncological markers CA-125, CA 19-9 and endometriosis. Medicine 2018, 97, e13759. [Google Scholar] [CrossRef]
- Evsen, M.S.; Sak, M.E.; Soydinc, H.E.; Guven, S.; Basaranoglu, S.; Hatipoglu, N.K.; Evliyaoglu, O.; Gul, T. Serum levels of androgens and prostate-specific antigen in endometriosis. Clin. Exp. Obstet. Gynecol. 2014, 41, 432–435. [Google Scholar]
- Liang, F.; Ren, N.; Zhang, H.; Zhang, J.; Wu, Q.; Song, R.; Shi, Z.; Zhang, Z.; Wang, K. A meta-analysis of the relationship between vitamin D receptor gene Apa1 polymorphisms and polycystic ovary syndrome. Adv. Clin. Exp. Med. 2019, 28, 255–262. [Google Scholar] [CrossRef]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and Endometrium: A Systematic Review of a Neglected Area of Research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Mirabi, P.; Basirat, Z.; Zeinalzadeh., M.; Khafri, S. Assocation between endometriosis and hyperprolactinemia in infertile women. Iran. J. Reprod. Med. 2015, 13, 155–160. [Google Scholar]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 2008, 23, 462–477. [Google Scholar] [CrossRef]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil. Steril. 2008, 89, 505–522. [Google Scholar] [CrossRef]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic review of endometriosis pain assessment: How to choose a scale? Hum. Reprod. Updat. 2015, 21, 136–152. [Google Scholar] [CrossRef]
- Buttram, V.C., Jr. Evolution of the revised American Fertility Society classification of endometriosis. Fertil. Steril. 1985, 43, 347–350. [Google Scholar] [CrossRef]
- Watrelot, A.; Nisolle, M.; Chelli, H.; Hocke, C.; Rongières, C.; Racinet, C. International Group for Fertiloscopy Evaluation. Is laparoscopy still the gold standard in infertility assessment? A comparison of fertiloscopy versus laparoscopy in infertility. Results of an international multicentre prospective trial: The ‘FLY’ (Fertiloscopy-LaparoscopY) study. Hum. Reprod. 2003, 18, 834–839. [Google Scholar]
- Seyam, E.; Hefzy, E. Laparoscopic ovarian drilling versus GnRH antagonist combined with cabergoline as a prophylaxis against the re-development of ovarian hyperstimulation syndrome. Gynecol. Endocrinol. 2018, 34, 616–622. [Google Scholar] [CrossRef]
- Zahiri, S.Z.; Sharami, S.H.; Tahersima, Z.; Salamat, F. Comparison between Unilateral and Bilateral Ovarian Drilling in Clomiphene Citrate Resistance Polycystic Ovary Syndrome Patients: A Randomized Clinical Trial of Efficacy. Int. J. Fertil. Steril. 2015, 9, 9–16. [Google Scholar]
- El-Sayed, M.L.M.; Ahmed, M.A.; Mansour, M.A.A.; Mansour, S.A.A. Unilateral Versus Bilateral Laparoscopic Ovarian Drilling Using Thermal Dose Adjusted According to Ovarian Volume in CC-Resistant PCOS, A Randomized Study. J. Obstet. Gynaecol. India 2017, 67, 356–362. [Google Scholar] [CrossRef]
- Trimbos, J.B.; Trimbos-Kemper, G.C.; Peters, A.A.; van der Does, C.D.; van Hall, E.V. Findings in 200 consecutive asymptomatic women, having a laparoscopic sterilization. Arch. Gynecol. Obstet. 1990, 247, 121–124. [Google Scholar] [CrossRef]
- Rawson, J.M. Prevalence of endometriosis in asymptomatic women. J. Reprod. Med. 1991, 36, 513–515. [Google Scholar]
- Barbosa, C.P.; Souza, A.M.; Bianco, B.; Christofolini, D.; Bach, F.A.; Lima, G.R. Frequency of endometriotic lesions in peritoneum samples from asymptomatic fertile women and correlation with CA125 values. Sao Paulo Med. J. 2009, 127, 342–345. [Google Scholar] [CrossRef]
- Gylfason, J.T.; Kristjansson, K.A.; Sverrisdottir, G.; Jonsdottir, K.; Rafnsson, V.; Geirsson, R.T. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am. J. Epidemiol. 2010, 172, 237–243. [Google Scholar] [CrossRef]
- Fuentes, A.; Escalona, J.; Céspedes, P.; Espinoza, A.; Johnson, M.C. Prevalencia de la endometriosis en mujeres sometidas a esterilización quirúrgica laparoscópica en un hospital de Santiago de Chile. Rev. Med. Chil. 2014, 142, 16–19. [Google Scholar] [CrossRef]
- Tissot, M.; Lecointre, L.; Faller, E.; Afors, K.; Akladios, C.; Audebert, A. Clinical presentation of endometriosis identified at interval laparoscopic tubal sterilization: Prospective series of 465 cases. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 647–650. [Google Scholar] [CrossRef]
- Pantou, A.; Simopoulou, M.; Sfakianoudis, K.; Giannelou, P.; Rapani, A.; Maziotis, E.; Grigoriadis, S.; Tsioulou, P.; Syrkos, S.; Souretis, K.; et al. The Role of Laparoscopic Investigation in Enabling Natural Conception and Avoiding in vitro Fertilization Overuse for Infertile Patients of Unidentified Aetiology and Recurrent Implantation Failure Following in vitro Fertilization. J. Clin. Med. 2019, 8, 548. [Google Scholar] [CrossRef]
- Marcoux, S.; Maheux, R.; Bérubé, S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N. Engl. J. Med. 1997, 337, 217–222. [Google Scholar] [CrossRef]
- Luo, L.; Gu, F.; Jie, H.; Ding, C.; Zhao, Q.; Wang, Q.; Zhou, C. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer—A matched-pair study. Reprod. Biomed. Online 2017, 35, 576–582. [Google Scholar] [CrossRef]
- Santulli, P.; Marcellin, L.; Menard, S.; Thubert, T.; Khoshnood, B.; Gayet, V.; Goffinet, F.; Ancel, P.Y.; Chapron, C. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum. Reprod. 2016, 31, 1014–1023. [Google Scholar] [CrossRef]
- Parazzini, F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: A randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum. Reprod. 1999, 14, 1332–1334. [Google Scholar]
- Kandil, M.; Rezk, M.; Al-Halaby, A.; Emarh, M.; El-Nasr, I.S. Impact of Ultrasound-Guided Transvaginal Ovarian Needle Drilling Versus Laparoscopic Ovarian Drilling on Ovarian Reserve and Pregnancy Rate in Polycystic Ovary Syndrome: A Randomized Clinical Trial. J. Minim. Invasive Gynecol. 2018, 25, 1075–1079. [Google Scholar] [CrossRef]
- Ott, J.; Hager, M.; Nouri, K.; Marschalek, J.; Kurz, C. Assessment of Tubal Patency: A Prospective Comparison of Diagnostic Hysteroscopy and Laparoscopic Chromopertubation. J. Minim. Invasive Gynecol. 2019, S1553–S4650. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W. Association between body mass index and endometriosis risk: A meta-analysis. Oncotarget 2017, 8, 46928–46936. [Google Scholar] [CrossRef]
- Giampaolino, P.; Della Corte, L.; Foreste, V.; Bifulco, G. Is there a relationship between Vitamin D and Endometriosis? An overview of literature. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Gadalla, M.A.; Huang, S.; Wang, R.; Norman, R.J.; Abdullah, S.A.; El Saman, A.M.; Ismail, A.M.; van Wely, M.; Mol, B.W.J. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 64–76. [Google Scholar] [CrossRef]
- Wu, C.H.; Winkel, C.A. The effect of therapy initiation day on clomiphene citrate therapy. Fertil. Steril. 1989, 52, 564–568. [Google Scholar] [CrossRef]
- Dehbashi, S.; Vafaei, H.; Parsanezhad, M.D.; Alborzi, S. Time of initiation of clomiphene citrate and pregnancy rate in polycystic ovarian syndrome. Int. J. Gynaecol. Obstet. 2006, 93, 44–48. [Google Scholar] [CrossRef]
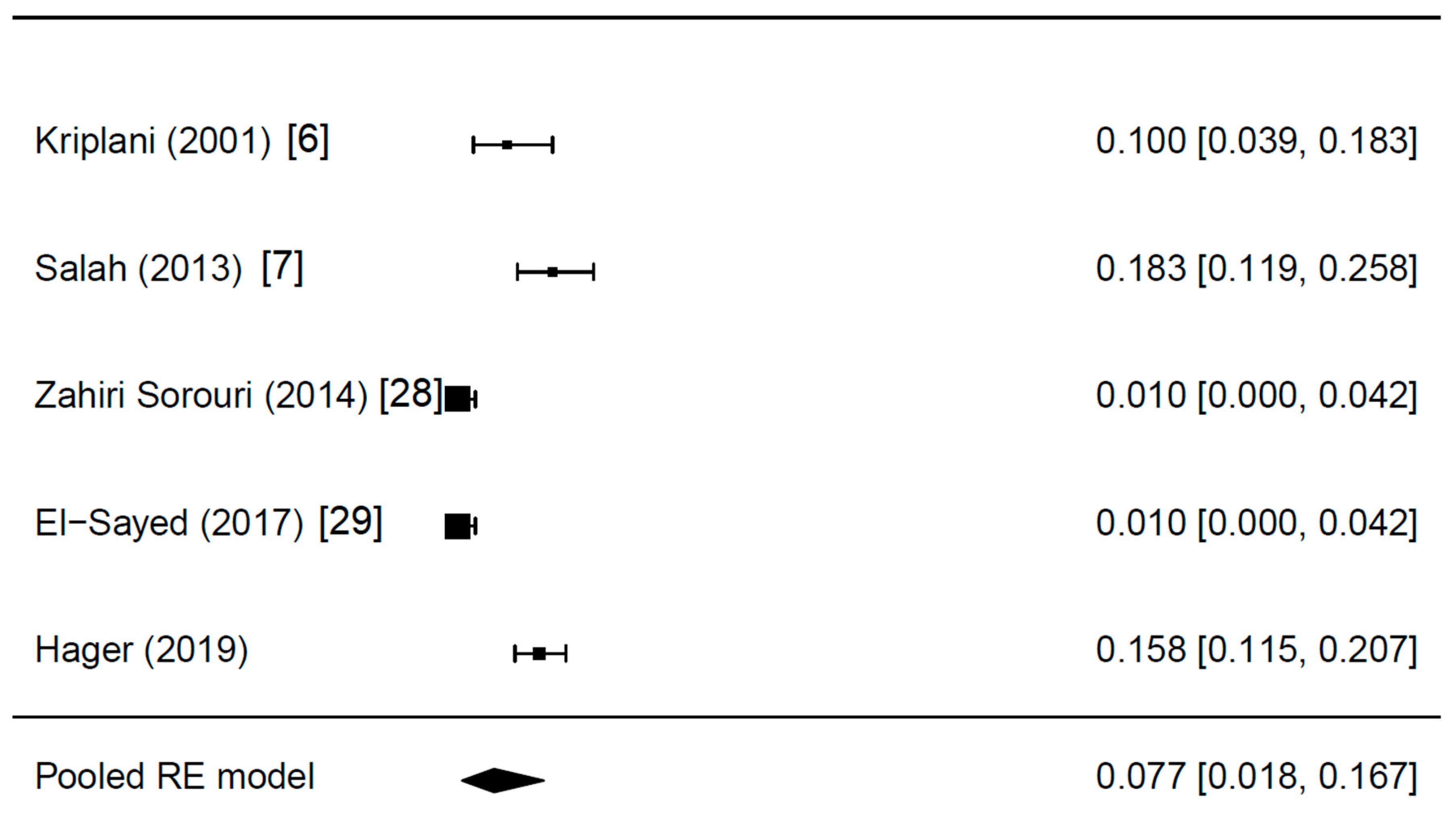
| First Author (year) | Women with PCOS (n) | Study Design | Years | Characteristics of PCOS Patients | Women with Endometriosis (n, %) | Additional Findings |
|---|---|---|---|---|---|---|
| Kriplani (2001) [6] | 70 | Retrospective | ? | Anovulatory, CC resistant | 7 (10.0) | Endometriotic cyst in n = 4 (5.7%) |
| Salah (2013) [7] | 120 | Retrospective | ? | Anovulatory, CC resistant | 22 (18.3) | – |
| Zahiri Sorouri (2014) [28] | 100 | Prospective | 2011–2012 | CC resistant | 1 (1.0) | – |
| El-Sayed (2017) [29] | 100 | Prospective | 2015–2017 | CC resistant, BMI < 30 kg/m2, LH >10 IU/mL or LH/FSH ratio >2, free androgen index >4, normal oGTT | 1 (1.0) | – |
| Hager (2019) | 240 | Retrospective | 2008–2018 | Anovulatory, CC resistant, women without signs of endometriosis | 38 (16.9) | – |
| Estimate Random Effect (95% CI) | Estimate Pooled (95% CI) | |
|---|---|---|
| All studies included | 0.077 (0.018; 0.167) | 0.110 (0.085; 0.134) |
| Without Kriplani (2001) [6] | 0.071 (0.007; 0.188) | 0.111 (0.085; 0.137) |
| Without Salah (2013) [7] | 0.056 (0.006; 0.145) | 0.092 (0.067; 0.117) |
| Without Zahiri Sorouri (2014) [28] | 0.101 (0.030; 0.204) | 0.128 (0.100; 0.157) |
| Without El-Sayed (2017) [29] | 0.101 (0.030; 0.204) | 0.128 (0.100; 0.157) |
| Without Hager (2019) | 0.059 (0.004; 0.159) | 0.079 (0.053; 0.106) |
| Women with Endometriosis (n = 38) | Women without Endometriosis (n = 187) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |||
| Age (years) * | 28.5 ± 4.8 | 28.3 ± 5.0 | 1.009 (0.941; 1.083) | 0.792 | – | – |
| Body mass index (kg/m2) * | 23.3 ± 5.2 | 25.3 ± 4.6 | 0.905 (0.830; 0.987) | 0.024 | 0.872 (0.792; 0.960) | 0.005 |
| Primary sterility # | 30 (78.9) | 122 (65.2) | 0.501 (0.217; 1.155) | 0.105 | – | – |
| Thyroid stimulating hormone (IU/mL) * | 1.6 ± 1.0 | 1.7 ± 1.0 | 0.888 (0.587; 1.343) | 0.573 | – | – |
| Prolactin (ng/mL) | 11.7 ± 5.1 | 13.5 ± 7.1 | 0.954 (0.896; 1.017) | 0.151 | ||
| 25 OH vitamin D (nmol/L) | 35.0 ± 24.8 | 43.8 ± 22.3 | 0.982 (0.965; 0.999) | 0.049 | 0.980 (0.962; 0.999) | 0.036 |
| LH (mIU/mL) * | 11.6 ± 7.5 | 12.6 ± 9.9 | 0.987 (0.945; 1.031) | 0.553 | – | – |
| FSH (mIU/mL) * | 5.7 ± 2.3 | 5.6 ± 2.0 | 1.019 (0.851; 1.219) | 0.838 | – | – |
| Testosterone (ng/mL) * | 0.43 ± 0.24 | 0.48 ± 0.25 | 0.432 (0.080; 2.344) | 0.331 | – | – |
| DHEAS (µg/mL) | 2.60 ± 1.20 | 2.57 ± 1.15 | 1.020 (0.732; 1.420) | 0.909 | – | – |
| AMH (ng/mL) * | 9.1 ± 6.2 | 9.5 ± 7.6 | 0.992 (0.935; 1.052) | 0.783 | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager, M.; Wenzl, R.; Riesenhuber, S.; Marschalek, J.; Kuessel, L.; Mayrhofer, D.; Ristl, R.; Kurz, C.; Ott, J. The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis. J. Clin. Med. 2019, 8, 1210. https://doi.org/10.3390/jcm8081210
Hager M, Wenzl R, Riesenhuber S, Marschalek J, Kuessel L, Mayrhofer D, Ristl R, Kurz C, Ott J. The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(8):1210. https://doi.org/10.3390/jcm8081210
Chicago/Turabian StyleHager, Marlene, René Wenzl, Sonja Riesenhuber, Julian Marschalek, Lorenz Kuessel, Daniel Mayrhofer, Robin Ristl, Christine Kurz, and Johannes Ott. 2019. "The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis" Journal of Clinical Medicine 8, no. 8: 1210. https://doi.org/10.3390/jcm8081210
APA StyleHager, M., Wenzl, R., Riesenhuber, S., Marschalek, J., Kuessel, L., Mayrhofer, D., Ristl, R., Kurz, C., & Ott, J. (2019). The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis. Journal of Clinical Medicine, 8(8), 1210. https://doi.org/10.3390/jcm8081210







