Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. MDCT Imaging
2.3. Severity Score in CT
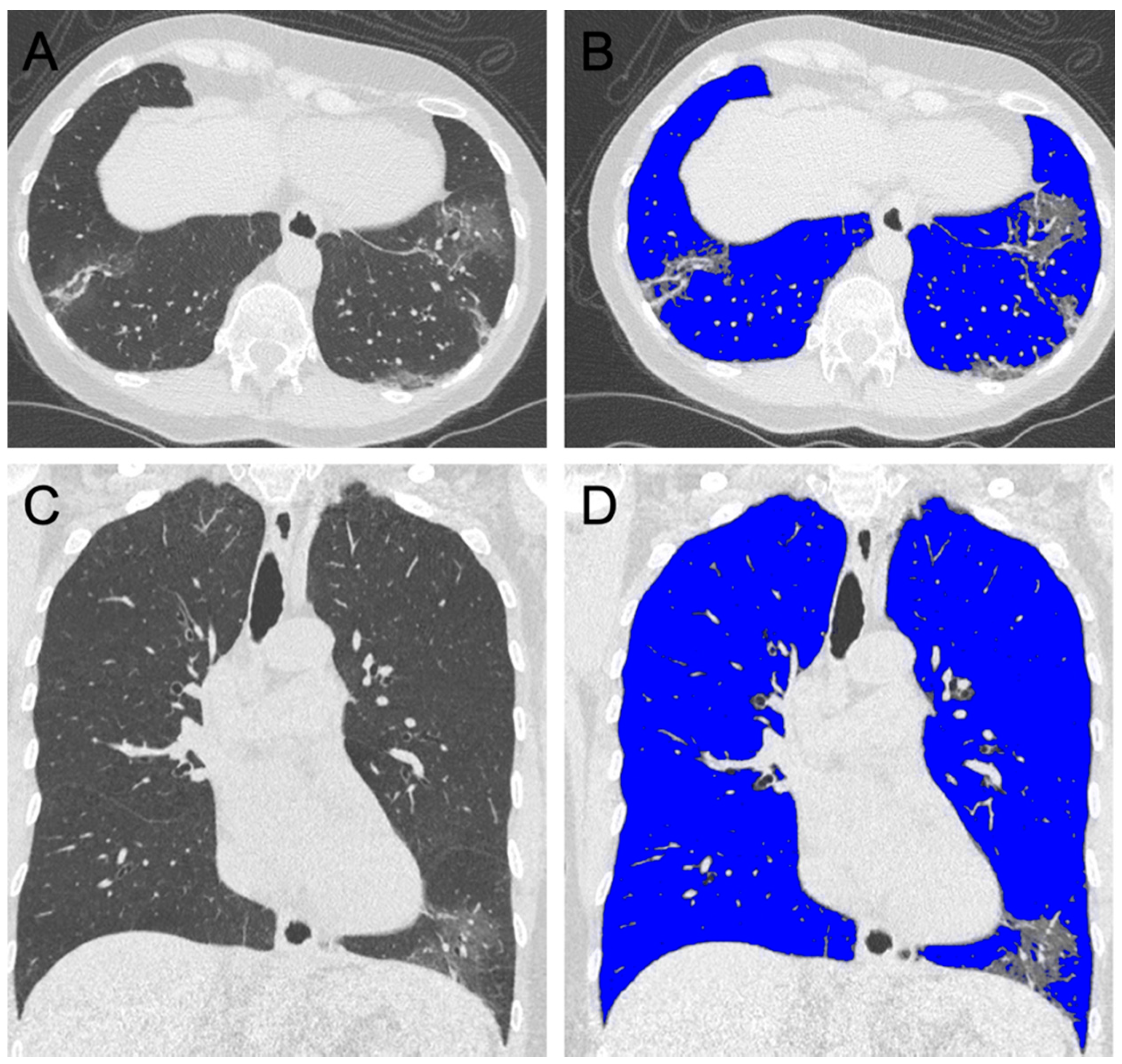
2.4. Semi-Automated Lung Parenchyma Volume Quantification
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Coronavirus Disease 2019 (COVID-19)—Situation Report-44. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200304-sitrep-44-covid-19.pdf?sfvrsn=93937f92_6 (accessed on 4 March 2020).
- Rosenbaum, L. Facing Covid-19 in Italy-ethics, logistics, and therapeutics on the epidemic’s front line. N. Engl. J. Med. 2020, 382, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, A.Z.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K. Chest CT findings in Coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology 2020, 200463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guo, D.; Li, C.; Fang, Z.; Chen, L.; Yang, R.; Li, X.; Zeng, W. Coronavirus disease 2019: Initial chest CT findings. Eur. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur. Radiol. 2020. [Google Scholar] [CrossRef]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.-T.; Leung, S.T.; Chin, T.W.-Y.; Lo, C.S.Y.; Lui, M.M.-S.; Lee, J.C.Y.; Chiu, K.W.-H.; Chung, T. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology 2019, 201160. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, D.; Liu, Y.; Fan, Y.; Zhao, L.; Li, X.; Zhu, W. Clinical and high.-resolution CT features of the COVID-19 infection: Comparison of the initial and follow-up changes. Invest. Radiol. 2020. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Tanne, J.H. Covid-19: New York city deaths pass 1000 as Trump tells Americans to distance for 30 days. BMJ 2020, 369, m1333. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- National Health Commission of the People’s republic of China. Diagnosis and Treatment Protocols of Pneumonia Caused by a Novel Coronavirus (6th Version). Available online: http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml (accessed on 15 April 2020).
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with Coronavirus disease 2019 in Wuhan. China Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing in Coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 200642. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair allocation of scarce medical resources in the time of Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Koonin, L.M.; Pillai, S.; Kahn, E.B.; Moulia, D.; Patel, A. Strategies to inform allocation of stockpiled ventilators to healthcare facilities during a pandemic. Health Secur. 2020, 18, 69–74. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichol, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L. Covid-19 in critically Ill patients in the seattle region-case series. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, H.; Hu, J.; Lian, J.; Gu, J.; Zhang, S.; Ye, C.; Lu, Y.; Jin, C.; Yu, G. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020, 94, 81–87. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C. Virological assessment of hospitalized patients with COVID-2019. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Grieser, C.; Goldmann, A.; Steffen, I.G.; Kastrup, M.; Fernández, C.M.; Engert, U.; Deja, M.; Lojewski, C.; Denecke, T. Computed tomography findings from patients with ARDS due to Influenza A (H1N1) virus-associated pneumonia. Eur. J. Radiol. 2012, 81, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Sheshadri, A.; Shah, D.P.; Godoy, M.; Erasmus, J.J.; Song, J.; Li, L.; Evans, S.E.; Chemaly, R.F.; Dickey, B.F.; Ost, D.E. Progression of the radiologic severity index predicts mortality in patients with parainfluenza virus-associated lower respiratory infections. PLoS ONE 2018, 13, e0197418. [Google Scholar] [CrossRef] [PubMed]


| Mitigated Group | ICU Group | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p | |
| Age (years) | 37 | 59.0 | 17.1 | 28 | 64.9 | 16.6 | 0.170 |
| Percutaneous oxygen saturation (%) | 32 | 95.9 | 2.6 | 20 | 91.5 | 8.3 | 0.007 |
| Temperature (°C) | 37 | 37.8 | 0.9 | 21 | 37.6 | 17.9 | 0.173 |
| Leucocytes (G/L) | 37 | 5.6 | 2.5 | 28 | 8.2 | 4.0 | 0.002 |
| Lymphocytes (%) | 37 | 29.1 | 49.6 | 25 | 26.2 | 64.6 | <0.001 |
| CRP (mg/dL) | 37 | 4.8 | 6.3 | 28 | 12.4 | 9.7 | <0.001 |
| Creatinine (mg/dL) | 37 | 1.3 | 1.9 | 28 | 1.4 | 0.7 | 0.855 |
| D-dimer (µg/mL) | 31 | 1631.9 | 2573.3 | 18 | 2407.2 | 2856.5 | 0.334 |
| LDH (U/L) | 35 | 394.1 | 529.3 | 24 | 487.6 | 277.7 | 0.431 |
| Creatine kinase (U/L) | 34 | 153.91 | 165.2 | 24 | 632.4 | 1622.1 | 0.092 |
| CK-MB (U/L) | 1 | 37.0 | 32.4 | 5 | 42.8 | 25.3 | |
| Troponin-T (ng/mL) | 7 | 0.3 | 0.7 | 8 | 0.1 | 0.1 | 0.499 |
| IL-6 (pg/mL) | 25 | 51.7 | 65.6 | 12 | 103.9 | 43.6 | 0.017 |
| Mitigated Group | ICU Group | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p | |
| CT severity score | 37 | 2.9 | 0.9 | 28 | 4.0 | 1.0 | <0.001 |
| Lung ventilated (%) | 37 | 80.7 | 11.2 | 27 | 58.4 | 15.9 | <0.001 |
| Right lung (%) | 37 | 80.7 | 10.7 | 27 | 57.0 | 17.6 | <0.001 |
| Right upper lobe (%) | 37 | 83.1 | 12.4 | 27 | 56.4 | 21.7 | <0.001 |
| Right middle lobe (%) | 37 | 88.0 | 7.4 | 27 | 71.0 | 17.4 | <0.001 |
| Right lower lobe (%) | 37 | 74.5 | 13.9 | 27 | 49.4 | 20.2 | <0.001 |
| Left lung (%) | 37 | 80.5 | 12.6 | 27 | 58.8 | 17.2 | <0.001 |
| Left upper lobe (%) | 37 | 85.5 | 10.3 | 27 | 62.9 | 17.0 | <0.001 |
| Left lower lobe (%) | 37 | 74.1 | 16.9 | 27 | 52.2 | 21.5 | <0.001 |
| Feature | Mean Importance | Standard Deviation |
|---|---|---|
| Ventilation upper lung | 0.184 | 0.025 |
| Ventilation lower lung | 0.123 | 0.030 |
| CRP | 0.074 | 0.018 |
| Radiological Severity Score | 0.067 | 0.011 |
| IL-6 | 0.058 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burian, E.; Jungmann, F.; Kaissis, G.A.; Lohöfer, F.K.; Spinner, C.D.; Lahmer, T.; Treiber, M.; Dommasch, M.; Schneider, G.; Geisler, F.; et al. Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort. J. Clin. Med. 2020, 9, 1514. https://doi.org/10.3390/jcm9051514
Burian E, Jungmann F, Kaissis GA, Lohöfer FK, Spinner CD, Lahmer T, Treiber M, Dommasch M, Schneider G, Geisler F, et al. Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort. Journal of Clinical Medicine. 2020; 9(5):1514. https://doi.org/10.3390/jcm9051514
Chicago/Turabian StyleBurian, Egon, Friederike Jungmann, Georgios A. Kaissis, Fabian K. Lohöfer, Christoph D. Spinner, Tobias Lahmer, Matthias Treiber, Michael Dommasch, Gerhard Schneider, Fabian Geisler, and et al. 2020. "Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort" Journal of Clinical Medicine 9, no. 5: 1514. https://doi.org/10.3390/jcm9051514
APA StyleBurian, E., Jungmann, F., Kaissis, G. A., Lohöfer, F. K., Spinner, C. D., Lahmer, T., Treiber, M., Dommasch, M., Schneider, G., Geisler, F., Huber, W., Protzer, U., Schmid, R. M., Schwaiger, M., Makowski, M. R., & Braren, R. F. (2020). Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort. Journal of Clinical Medicine, 9(5), 1514. https://doi.org/10.3390/jcm9051514







