Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology
Abstract
:1. Introduction
2. Methods
2.1. General Information Concerning the Systematic Review
2.1.1. Inclusion Criteria
- Studies reporting either prevalence or incidence of ME/CFS, irrespective of age group, utilizing any of the following clinical diagnostic criteria: Centers for Disease Control & Prevention (CDC)−1994 [1], Canadian Consensus Criteria [21], London Criteria [22], International Consensus Criteria [2], or Institute of Medicine criteria [3].
- Studies from European countries; namely (by alphabetical order), Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Italy, Kazakhstan, Kosovo, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Moldova, Monaco, Montenegro, the Netherlands, North Macedonia (formerly Macedonia), Norway, Poland, Portugal, Romania, Russia, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, the United Kingdom and Vatican City.
- Studies conducted in community or primary care settings.
2.1.2. Exclusion Criteria
- Studies without primary data (e.g., reviews).
- Studies conducted in selected samples (e.g., post-infection, following vaccination or in high-risk population sub-groups such as war veterans).
- Studies based on self-reported diagnosis of ME/CFS.
- Studies with definitions inappropriate for the purposes of the present review (e.g., CFS-like illness or other clinical criteria, such as the Oxford criteria, due to lack of specificity [23]).
- Duplicate reports. When populations are overlapping, the study with the largest sample size was included.
- Studies published before 1994, when the first case definition of ME/CFS of those included in the present work was launched; namely, CDC−1994 [1].
2.2. Search Strategy for Identifying Relevant Studies
- Scopus: ({epidemiology} OR {prevalence} OR {incidence}) AND ({chronic fatigue syndrome} OR {myalgic encephalomyelitis} OR {CFS/ME})
- PubMed: (“Fatigue Syndrome, Chronic”(Mesh) AND ((“Incidence”(Mesh) OR “Epidemiology”(Mesh) OR “epidemiology” (Subheading)) OR “Prevalence”(Mesh) OR “Cross-Sectional Studies”(Mesh)))
- Web of Science: (“epidemiology” OR “prevalence” OR “incidence”) AND (“chronic fatigue syndrome” OR “myalgic encephalomyelitis” OR “CFS/ME” OR “ME/CFS”)
2.3. Selection of Studies for Inclusion to the Review
2.4. Assessment of Methodological Quality and Reporting of Data
2.5. Data Extraction and Management
2.6. Data Synthesis and Analysis
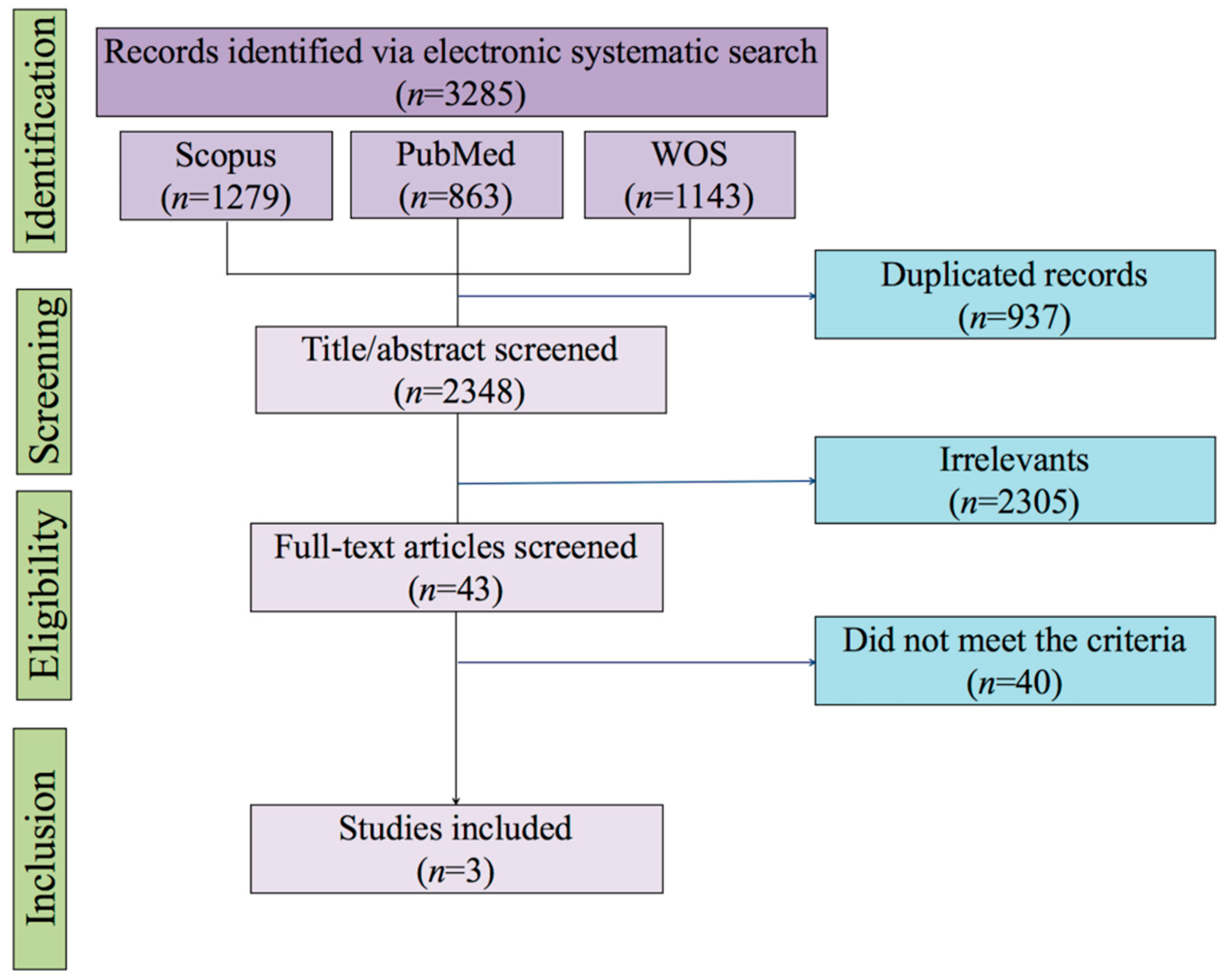
3. Results
4. Discussion
4.1. Implications
4.2. EUROMENE Research Recommendations for Epidemiology
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Original Protocol | Amendments (A) and Rationale (R) |
|---|---|
| Section: The primary systematic literature search on electronic databases. Text: Two independent reviewers (FE-L and JC-M) will perform a primary electronic search in PubMed, Scopus and Web of Science on 9 January 2018 | A: The search will be updated to include works published up to 15 June 2019. R: Due to budget restrictions to cover the publication fee, it was decided to postpone the preparation of this work. |
| Section: Exclusion criteria Text: Studies published more than 10 years ago (i.e., before 2008). | A: Studies published before 1994. R: The decision lacked of a strong rationale and it was too restrictive. Given that studies that used the CDC−1994, Canadian Consensus Criteria, London Criteria, International Consensus Criteria or Institute of Medicine criteria will be considered, we will search for the literature that has been published from 1994, when the CDC−1994 were launched. |
| Nacul et al., [26] | Lindal et al., [13] | Rimes et al., [27] | |
|---|---|---|---|
| Title and abstract | |||
| 1a. Indicate the study design | Yes | Yes | Yes |
| 1b. Informative and balanced abstract | Yes | Yes | Yes |
| Introduction | |||
| 2. Background/rationale | Yes | Yes | Yes |
| 3. Objectives | Yes | Yes | Yes |
| Methods | |||
| 4. Study design | Yes | No/Unclear | No/Unclear |
| 5. Setting | Yes | Yes | No/Unclear |
| 6a. Participants | Yes | Yes | Yes |
| 7. Variables | Yes | Yes | Yes |
| 8. Data sources/measurement | Yes | Yes | Yes |
| 9. Bias | No/Unclear | No/Unclear | No/Unclear |
| 10. Study size | Yes | No/Unclear | No/Unclear |
| 12a. Statistics: description of all methods | Yes | Yes | Yes |
| 12b. Statistics: subgroups and interactions | Yes | Yes | Yes |
| 12c. Statistics: missing data | Yes | Yes | Yes |
| 12d. Statistics: loss to follow-up | Yes | Not applicable | Yes |
| 12e. Statistics: sensitivity analyses | Yes | No/Unclear | No/Unclear |
| Results | |||
| 13a. Participants: individual at each stage | Yes | Yes | Yes |
| 13b. Participants: reasons for non-participation | Yes | No/Unclear | Yes |
| 13c. Participants: flow diagram | Yes | No/Unclear | No/Unclear |
| 14a. Descriptive data: characteristics of participants | Yes | Yes | Yes |
| 14b. Descriptive data: missing data | Yes | Yes | Yes |
| 14c. Descriptive data: follow-up | Yes | Not applicable | Yes |
| 15. Outcome data | Yes | Yes | Yes |
| 16a. Main results | Yes | Yes | Yes |
| Discussion | |||
| 18. Key results | Yes | Yes | Yes |
| 19. Limitations | Yes | Yes | Yes |
| 20. Interpretation | Yes | No/Unclear | No/Unclear |
| 21. Generalisability | Yes | No/Unclear | No/Unclear |
| Other information | |||
| 22. Funding | No/Unclear | No/Unclear | No/Unclear |
References
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine, I.O.M. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Natl. Acad. Press 2015. [Google Scholar]
- Prins, J.B.; van der Meer, J.W.M.; Bleijenberg, G. Chronic fatigue syndrome. Lancet 2006, 367, 346–355. [Google Scholar] [CrossRef]
- Lowry, T.J.; Pakenham, K.I. Health-related quality of life in chronic fatigue syndrome: Predictors of physical functioning and psychological distress. Psychol. Health Med. 2008, 13, 222–238. [Google Scholar] [CrossRef]
- de Carvalho Leite, J.C.; de L Drachler, M.; Killett, A.; Kale, S.; Nacul, L.; McArthur, M.; Hong, C.S.; O’Driscoll, L.; Pheby, D.; Campion, P.; et al. Social support needs for equity in health and social care: A thematic analysis of experiences of people with chronic fatigue syndrome/myalgic encephalomyelitis. Int. J. Equity Health 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Nacul, L.C.; Lacerda, E.M.; Campion, P.; Pheby, D.; Drachler, M.; de, L.; Leite, J.C.; Poland, F.; Howe, A.; Fayyaz, S.; et al. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health 2011, 11, 402. [Google Scholar] [CrossRef] [Green Version]
- Castro-Marrero, J.; Faro, M.; Zaragozá, M.C.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: A community-based cross-sectional study from Spain. BMC Public Health 2019, 19, 840. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Olczyk, N.A.; Jason, L.; Alegre, J.; Fuentes-Llanos, J.; Castro-Marrero, J. A Cross-National Comparison of Myalgic Encephalomyelitis and Chronic Fatigue Syndrome at Tertiary Care Settings from the US and Spain. Am. J. Soc. Sci. Humanit. 2020, 5, 104–115. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N.M.; Johnson, C. Myalgic Encephalomyelitis (ME), Chronic Fatigue Syndrome (CFS), and Chronic Fatigue (CF) are distinguished accurately: Results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry Res. 2012, 200, 754–760. [Google Scholar] [CrossRef]
- Raine, R.; Carter, S.; Sensky, T.; Black, N. General practitioners’ perceptions of chronic fatigue syndrome and beliefs about its management, compared with irritable bowel syndrome: Qualitative study. BMJ 2004, 328, 1354–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacul, L.; Lacerda, E.M.; Kingdon, C.C.; Curran, H.; Bowman, E.W. How have selection bias and disease misclassification undermined the validity of myalgic encephalomyelitis/chronic fatigue syndrome studies? J. Health Psychol. 2017, 1359105317695803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Líndal, E.; Stefánsson, J.G.; Bergmann, S.; Lindal, E.; Stefansson, J.G.; Bergmann, S. The prevalence of chronic fatigue syndrome in Iceland - A national comparison by gender drawing on four different criteria. Nord. J. Psychiatry 2002, 56, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, G. Epidemiology of chronic fatigue syndrome. Occup. Med. 2005, 55, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinos, S.; Khoshaba, B.; Ashby, D.; White, P.D.; Nazroo, J.; Wessely, S.; Bhui, K.S. A systematic review of chronic fatigue, its syndromes and ethnicity: Prevalence, severity, co-morbidity and coping. Int. J. Epidemiol. 2009, 38, 1554–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, S.; Brenu, E.W.; Staines, D.R.; Marshall-Gradisnik, S. The adoption of chronic fatigue syndrome/myalgic encephalomyelitis case definitions to assess prevalence: A systematic review. Ann. Epidemiol. 2013, 23, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Brurberg, K.G.; Fønhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 2014, 4. [Google Scholar] [CrossRef]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Estévez-López, F.; Castro-Marrero, J.; Wang, X.; Bakken, I.J.; Ivanovs, A.; Nacul, L.; Sepúlveda, N.; Strand, E.B.; Pheby, D.; Alegre, J.; et al. Prevalence and incidence of myalgic encephalomyelitis/chronic fatigue syndrome in Europe - the Euro-epiME study from the European network EUROMENE: A protocol for a systematic review. BMJ Open 2018, 8. [Google Scholar] [CrossRef]
- Carruthers, B.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; et al. Myalgic encephalomelitis/chronic fatigue syndromw: Clinical working case definiton, diagnostic and treatment protocols. J. Chronic Fatigue Syndome 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Dowsett, E.G.G.E.; Goudsmit, E.; Macintyre, A.; Shepherd, C.B. The National Task Force on Chronic Fatigue Syndrome (CFS), Post Viral Fatigue Syndrome (PVFS), Myalgic Encephalomyelitis (ME). Westcare 1994. [Google Scholar]
- Sharpe, M.C.; Archard, L.C.; Banatvala, J.E.; Borysiewicz, L.K.; Clare, A.W.; David, A.; Edwards, R.H.; Hawton, K.E.; Lambert, H.P.; Lane, R.J. A report--chronic fatigue syndrome: Guidelines for research. J. R. Soc. Med. 1991, 84, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based. Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 4, e296. [Google Scholar] [CrossRef] [Green Version]
- Nacul, L.C.; Lacerda, E.M.; Pheby, D.; Campion, P.; Molokhia, M.; Fayyaz, S.; Leite, J.C.D.C.; Poland, F.; Howe, A.; Drachler, M.L. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: A repeated cross-sectional study in primary care. BMC Med. 2011, 9, 91. [Google Scholar] [CrossRef]
- Rimes, K.A.; Goodman, R.; Hotopf, M.; Wessely, S.; Meltzer, H.; Chalder, T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: A prospective community study. Pediatrics 2007, 119, e603-9. [Google Scholar] [CrossRef] [Green Version]
- Lindal, E.; Stefansson, J.G.; Bergmann, S. The Prevalence of Chronic Fatigue Syndrome in Iceland--a National Comparison by Gender Drawing on Four Different Criteria. Nord. J. Psychiatry 2006, 60, 183. [Google Scholar] [CrossRef]
- Reyes, M.; Nisenbaum, R.; Hoaglin, D.C.; Unger, E.R.; Emmons, C.; Randall, B.; Stewart, J.A.; Abbey, S.; Jones, J.F.; Gantz, N.; et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch. Intern. Med. 2003, 163, 1530–1536. [Google Scholar] [CrossRef]
- Reeves, W.C.; Jones, J.F.; Maloney, E.; Heim, C.; Hoaglin, D.C.; Boneva, R.S.; Morrissey, M.; Devlin, R. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Popul. Health Metr. 2007, 5. [Google Scholar] [CrossRef] [Green Version]
- de Lourdes Drachler, M.; de Carvalho Leite, J.C.; Hooper, L.; Hong, C.S.; Pheby, D.; Nacul, L.; Lacerda, E.; Campion, P.; Killett, A.; McArthur, M.; et al. The expressed needs of people with chronic fatigue syndrome/myalgic encephalomyelitis: A systematic review. BMC Public Health 2009, 9, 458. [Google Scholar]
- Martín-Martínez, E.; Martín-Martínez, M. Varied Presentation of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Needs for Classification and Clinician Education: A Case Series. Clin. Ther. 2019, 41, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pheby, D.F.H.; Araja, D.; Berkis, U.; Brenna, E.; Cullinan, J.; de Korwin, J.-D.; Gitto, L.; Hughes, D.A.; Hunter, R.M.; Trepel, D.; et al. The Development of a Consistent Europe-Wide Approach to Investigating the Economic Impact of Myalgic Encephalomyelitis (ME/CFS): A Report from the European Network on ME/CFS (EUROMENE). Healthcare 2020, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Fluge, Ø.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Kristoffersen, E.K.; Sørland, K.; Bruland, O.; Dahl, O.; et al. B-Lymphocyte Depletion in Myalgic Encephalopathy/Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef] [PubMed]
- White, P.D.; Goldsmith, K.A.; Johnson, A.L.; Potts, L.; Walwyn, R.; DeCesare, J.C.; Baber, H.L.; Burgess, M.; Clark, L.V.; Cox, D.L.; et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef] [Green Version]
- Wearden, A.J.; Dowrick, C.; Chew-Graham, C.; Bentall, R.P.; Morriss, R.K.; Peters, S.; Riste, L.; Richardson, G.; Lovell, K.; Dunn, G.; et al. Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: Randomised controlled trial. BMJ 2010, 340, c1777. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.; Lin, J.C.; Sheriff, S.; Maudsley, A.A.; Younger, J.W. Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: Assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020, 14, 562–572. [Google Scholar] [CrossRef]
- Maksoud, R.; du Preez, S.; Eaton-Fitch, N.; Thapaliya, K.; Barnden, L.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of neurological impairments in myalgic encephalomyelitis/chronic fatigue syndrome using neuroimaging techniques. PLoS ONE 2020, 15, e0232475. [Google Scholar] [CrossRef]
- Cockshell, S.J.; Mathias, J.L. Cognitive functioning in chronic fatigue syndrome: A meta-analysis. Psychol. Med. 2010, 40, 1253–1267. [Google Scholar] [CrossRef]
- Chapenko, S.; Krumina, A.; Logina, I.; Rasa, S.; Chistjakovs, M.; Sultanova, A.; Viksna, L.; Murovska, M. Association of active human herpesvirus-6, -7 and parvovirus b19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv. Virol. 2012, 2012, 205085. [Google Scholar] [CrossRef] [Green Version]
- Rasa-Dzelzkalēja, S.; Čapenko, S.; Krūmiņa, A.; Lin, Y.-C.; Murovska, M. Association of Human Parvovirus B19 Infection with Development and Clinical Course of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proc. Latv. Acad. Sci. Sect. B Nat. Exact. Appl. Sci. 2019, 73, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Shikova, E.; Reshkova, V.; Kumanova, A.; Raleva, S.; Alexandrova, D.; Capo, N.; Murovska, M.; European Network on ME/CFS, (EUROMENE). Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacul, L.C.; Mudie, K.; Kingdon, C.C.; Clark, T.G.; Lacerda, E.M. Hand Grip Strength as a Clinical Biomarker for ME/CFS and Disease Severity. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Sunnquist, M.; Brown, A.; Furst, J.; Cid, M.; Farietta, J.; Kot, B.; Bloomer, C.; Nicholson, L.; Williams, Y.; et al. Factor Analysis of the DePaul Symptom Questionnaire: Identifying Core Domains. J. Neurol. Neurobiol. 2015, 1. [Google Scholar]
- Lacerda, E.M.; Bowman, E.W.; Cliff, J.M.; Kingdon, C.C.; King, E.C.; Lee, J.-S.S.; Clark, T.G.; Dockrell, H.M.; Riley, E.M.; Curran, H.; et al. The UK ME/CFS Biobank for biomedical research on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Multiple Sclerosis. Open J. Bioresour. 2017, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Castro-Marrero, J.; Faro, M.; Aliste, L.; Saez-Francas, N.; Calvo, N.; Martinez-Martinez, A.; de Sevilla, T.F.; Alegre, J. Comorbidity in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Nationwide Population-Based Cohort Study. Psychosomatics 2017, 58, 533–543. [Google Scholar] [CrossRef]
- Shepherd, C.; Chaudhuri, A. ME/CFS/PVFS: An Exploration of the Key Clinical Issues. Gawcott ME Assoc. 2013. [Google Scholar]
- Słomko, J.; Newton, J.L.; Kujawski, S.; Tafil-Klawe, M.; Klawe, J.; Staines, D.; Marshall-Gradisnik, S.; Zalewski, P. Prevalence and characteristics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in Poland: A cross-sectional study. BMJ Open 2019, 9, e023955. [Google Scholar] [CrossRef] [Green Version]
| Reference | Procedure | Settings, Country | Sample, Total (% Women) | Age Range | Case Definition | Prevalence Estimate % (95% CI) | Prevalence Estimate by Gender |
|---|---|---|---|---|---|---|---|
| Nacul et al., [26] | Electronic search (GPs databases), queries to GPs, clinical review of cases | Primary care, The United Kingdom | 143,000 (51%) | Adults, 18 to 64 years old | CDC−1994 CCC−2003 | 0.20 (0.18 to 0.23) 0.10 (0.09 to 0.12) | Women = 0.31 (0.27 to 0.35) Men = 0.08 (0.06 to 0.10) Women = 0.18 (0.15 to 0.21) Men = 0.04 (0.03 to 0.06) |
| Lindal et al., [13] | Postal delivery to randomly selected people | Community, Iceland | 2471 (57%) | Adults, 19 to 75 years old | CDC−1994 | 2.2 (not reported) | Women = 3.0% (not reported) Men = 1.1% (not reported) |
| Rimes et al., [27] | Random selection from the Child Benefit Register | Community, The United Kingdom | 842 (not reported) | Adolescents, 11 to 15 years old | CDC−1994 | 0.1 (not reported) | Not reported |
| Reference | Follow-up, Procedure | Settings, Country | Sample, Total (Women, %) | Age Range | Case Definition | Incidence Estimate | Incidence Estimate by Gender |
|---|---|---|---|---|---|---|---|
| Nacul et al., [26] | 12 months, Electronic search (GPs databases), queries to GPs, clinical review of cases | Primary care, The United Kingdom | 143,153 (51%) | Adults, 18 to 64 years old | CDC−1994 CCC−2003 | 15 new cases per 100,000 adults per year 5 new cases per 100,000 adults per year | Women = 23 new cases per 100,000 adults per year Men = 7 new cases per 100,000 adults per year Women = 6 new cases per 100,000 adults per year Men = 3 new cases per 100,000 adults per year |
| Rimes et al., [27] | 4 to 6 months, random selection from the Child Benefit Register | Community, The United Kingdom | 842 (not reported) | Adolescents, 11 to 15 years old | CDC−1994 | 5 new cases per 1000 adolescents per 6 months | Not reported |
| Nacul et al., [26] | Lindal et al., [13] | Rimes et al., [27] | |
|---|---|---|---|
| 1. Appropriate sample frame | Yes | Yes | Yes |
| 2. Participants were sampled appropriately | Yes | Yes | Yes |
| 3. Adequate sample size | Yes | Yes | Yes |
| 4. Participants and settings were well described | Yes | Yes | Yes |
| 5. Data analysis with sufficient coverage | Yes | No/Unclear | Yes |
| 6. Valid methods for identifying the condition | Yes | No/Unclear | Yes |
| 7. Standard and reliable measure of the condition | Yes | Yes | Yes |
| 8. Appropriate statistical analyses | Yes | Yes | Yes |
| 9. Adequate response rate | Yes | Yes | No/Unclear |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estévez-López, F.; Mudie, K.; Wang-Steverding, X.; Bakken, I.J.; Ivanovs, A.; Castro-Marrero, J.; Nacul, L.; Alegre, J.; Zalewski, P.; Słomko, J.; et al. Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. J. Clin. Med. 2020, 9, 1557. https://doi.org/10.3390/jcm9051557
Estévez-López F, Mudie K, Wang-Steverding X, Bakken IJ, Ivanovs A, Castro-Marrero J, Nacul L, Alegre J, Zalewski P, Słomko J, et al. Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. Journal of Clinical Medicine. 2020; 9(5):1557. https://doi.org/10.3390/jcm9051557
Chicago/Turabian StyleEstévez-López, Fernando, Kathleen Mudie, Xia Wang-Steverding, Inger Johanne Bakken, Andrejs Ivanovs, Jesús Castro-Marrero, Luis Nacul, Jose Alegre, Paweł Zalewski, Joanna Słomko, and et al. 2020. "Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology" Journal of Clinical Medicine 9, no. 5: 1557. https://doi.org/10.3390/jcm9051557
APA StyleEstévez-López, F., Mudie, K., Wang-Steverding, X., Bakken, I. J., Ivanovs, A., Castro-Marrero, J., Nacul, L., Alegre, J., Zalewski, P., Słomko, J., Strand, E. B., Pheby, D., Shikova, E., Lorusso, L., Capelli, E., Sekulic, S., Scheibenbogen, C., Sepúlveda, N., Murovska, M., & Lacerda, E., on behalf of The European Network on ME/CFS (EUROMENE). (2020). Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. Journal of Clinical Medicine, 9(5), 1557. https://doi.org/10.3390/jcm9051557










