Early Post-Rewarming Fever Is Associated with Favorable 6-Month Neurologic Outcomes in Patients with Out-Of-Hospital Cardiac Arrest: A Multicenter Registry Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Population
2.2. Targeted Temperature Management
2.3. Data Collection
2.4. Statistical Analysis
3. Results
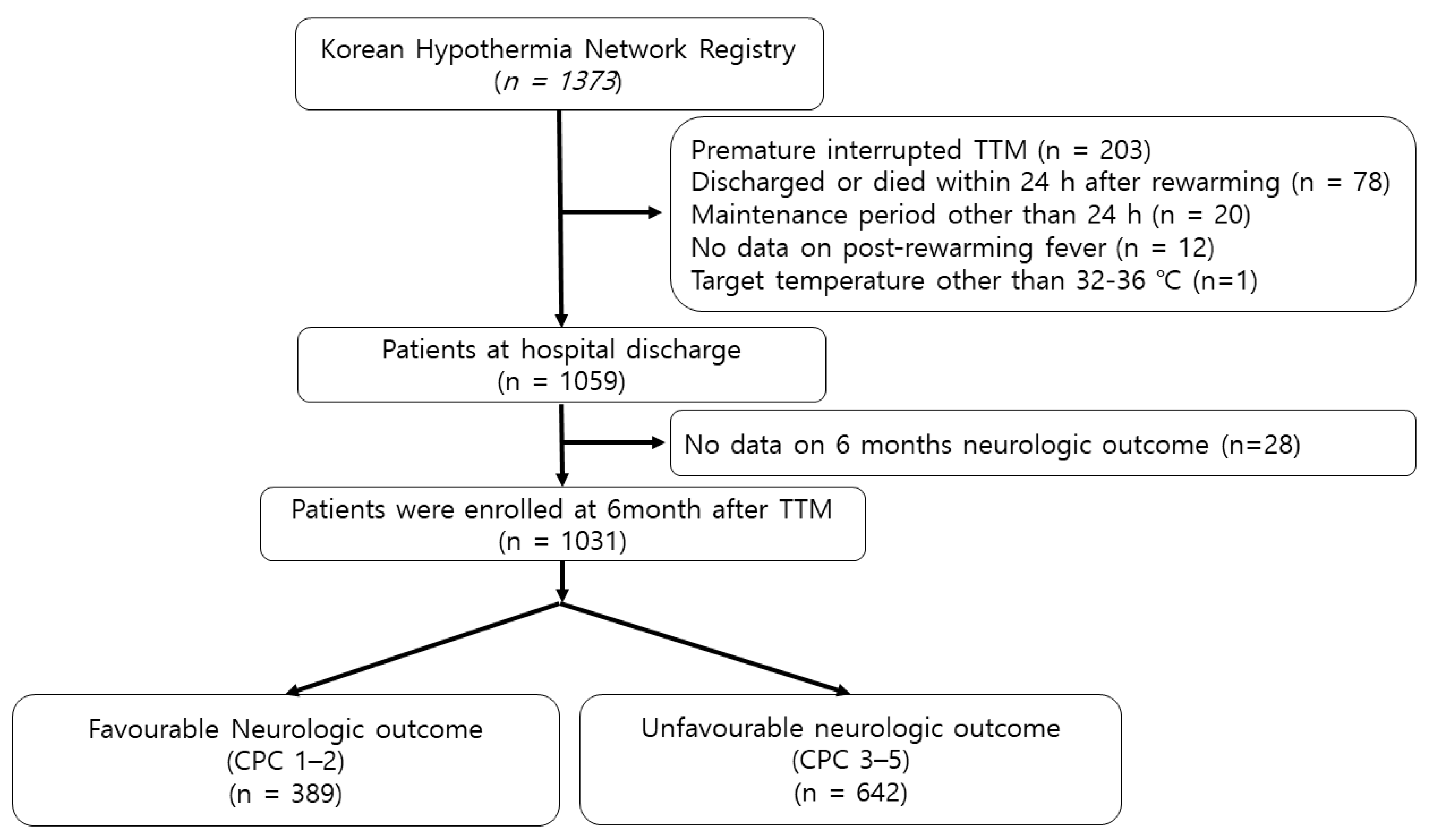
3.1. Study Population
3.2. Factors Associated with Development of Post-Rewarming Fever
3.3. Association between Post-Rewarming Fever and Mortality at Hospital Discharge
3.4. Association between Post-Rewarming Fever, and Timing of Post-rewarming Fever and Neurologic Outcomes at Hospital Discharge
3.5. Association of Post-Rewarming Fever, and Timing of Post-Rewarming Fever and Neurologic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, J.E. Thermogenic Mechanisms and Their Hormonal Regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Langhelle, A.; Tyvold, S.; Lexow, K.; Hapnes, S.; Sunde, K.; Steen, P. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest. A comparison between four regions in Norway. Resuscitation 2003, 56, 247–263. [Google Scholar] [CrossRef]
- Suffoletto, B.; Peberdy, M.A.; Van Der Hoek, T.; Callaway, C.W. Body temperature changes are associated with outcomes following in-hospital cardiac arrest and return of spontaneous circulation. Resuscitation 2009, 80, 1365–1370. [Google Scholar] [CrossRef]
- Zeiner, A.; Holzer, M.; Sterz, F.; Schörkhuber, W.; Eisenburger, P.; Havel, C.; Kliegel, A.; Laggner, A.N. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch. Intern. Med. 2001, 161, 2007–2012. [Google Scholar] [CrossRef]
- Takino, M.; Okada, Y. Hyperthermia following cardiopulmonary resuscitation. Intensiv. Care Med. 1991, 17, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Bernard, S.; Gray, T.W.; Buist, M.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjærgaard, J.; Kuiper, M.; et al. Targeted Temperature Management at 33 °C versus 36 °C after Cardiac Arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post–Cardiac Arrest Care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef] [Green Version]
- Bro-Jeppesen, J.; Hassager, C.; Wanscher, M.; Søholm, H.; Thomsen, J.H.; Lippert, F.; Moller, J.E.; Køber, L.; Kjaergaard, J. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resusc. 2013, 84, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.; Wolf, K.; Kettinger, S.; Seif, E.; Jones, J.; Bacon-Baguley, T. Assessment of risk factors for post-rewarming “rebound hyperthermia” in cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation 2013, 84, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.; Grossestreuer, A.V.; Iannacone, S.; Gonzalez, M.; Shofer, F.S.; Povey, C.; Wendell, G.; Archer, S.E.; Gaieski, D.; Abella, B.S. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation 2013, 84, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Bouwes, A.; Robillard, L.B.; Binnekade, J.M.; De Pont, A.-C.J.; Wieske, L.; Hartog, A.W.D.; Schultz, M.J.; Horn, J. The influence of rewarming after therapeutic hypothermia on outcome after cardiac arrest. Resuscitation 2012, 83, 996–1000. [Google Scholar] [CrossRef]
- Aldhoon, B.; Melenovsky, V.; Kettner, J.; Kautzner, J. Clinical predictors of outcome in survivors of out-of-hospital cardiac arrest treated with hypothermia. Cor et Vasa 2012, 54, e68–e75. [Google Scholar] [CrossRef] [Green Version]
- Benz-Woerner, J.; Delodder, F.; Benz, R.; Cueni-Villoz, N.; Feihl, F.; Rossetti, A.O.; Liaudet, L.; Oddo, M. Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation 2012, 83, 338–342. [Google Scholar] [CrossRef]
- Gebhardt, K.; Guyette, F.X.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation 2013, 84, 1062–1067. [Google Scholar] [CrossRef]
- Cocchi, M.N.; Boone, M.D.; Giberson, B.; Giberson, T.; Farrell, E.; Salciccioli, J.D.; Talmor, D.; Williams, N.; Donnino, M.W. Fever After Rewarming. J. Intensiv. Care Med. 2013, 29, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.K.; Song, K.H.; Jung, Y.H.; Lee, D.H.; Youn, C.S.; Lee, S.M.; Cho, Y.S.; Jeung, K.W. The influence of post-rewarming temperature management on post-rewarming fever development after cardiac arrest. Resuscitation 2015, 97, 20–26. [Google Scholar] [CrossRef]
- World Medical Association General Assembly World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Int. J. Bioeth. 2004, 15, 124–129. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed on 1 October 2015).
- Ajam, K.; Gold, L.; Beck, S.S.; Damon, S.; Phelps, R.; Rea, T.D. Reliability of the Cerebral Performance Category to classify neurological status among survivors of ventricular fibrillation arrest: A cohort study. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.E.; Trzeciak, S.; Kline, J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 2009, 37, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Grossestreuer, A.V.; Gaieski, D.F.; Donnino, M.W.; Wiebe, D.J.; Abella, B.S. Magnitude of temperature elevation is associated with neurologic and survival outcomes in resuscitated cardiac arrest patients with postrewarming pyrexia. J. Crit. Care 2017, 38, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hori, T. An Update on Thermosensitive Neurons in the Brain: From Cellular Biology to Thermal and Non-thermal Homeostatic Functions. Jpn. J. Physiol. 1991, 41, 1–22. [Google Scholar] [CrossRef]
- Payabvash, S.; Souza, L.C.; Wang, Y.; Schaefer, P.W.; Furie, K.L.; Halpern, E.F.; Gonzalez, R.G.; Lev, M.H. Regional ischemic vulnerability of the brain to hypoperfusion: The need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke 2011, 42, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Hickey, R.W.; Kochanek, P.; Ferimer, H.; Alexander, H.; Garman, R.H.; Graham, S.H. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit. Care Med. 2003, 31, 531–535. [Google Scholar] [CrossRef]
- Murnin, M.R.; Sonder, P.; Janssens, G.N.; Henry, C.L.; Polderman, K.H.; Rittenberger, J.C.; Dezfulian, C.; Service, A.T.P.C.A. Determinants of Heat Generation in Patients Treated With Therapeutic Hypothermia Following Cardiac Arrest. J. Am. Hear. Assoc. 2014, 3, e000580. [Google Scholar] [CrossRef] [Green Version]
- Perman, S.M.; Ellenberg, J.H.; Grossestreuer, A.V.; Gaieski, D.F.; Leary, M.; Abella, B.S.; Carr, B.G. Shorter time to target temperature is associated with poor neurologic outcome in post-arrest patients treated with targeted temperature management. Resuscitation 2014, 88, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Lefrant, J.-Y.; Müller, L.; De La Coussaye, J.E.; Benbabaali, M.; LeBris, C.; Zeitoun, N.; Mari, C.; Saïssi, G.; Ripart, J.; Eledjam, J.-J. Temperature measurement in intensive care patients: Comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensiv. Care Med. 2003, 29, 414–418. [Google Scholar] [CrossRef]

| Variables | Total (n = 1031) | Favorable (n = 389) | Unfavorable (n = 642) | p-Value |
|---|---|---|---|---|
| Age, years, IQR | 57 (46–68) | 54 (44–62) | 60 (48–71) | <0.001 |
| Male sex, n (%) | 737 (71.5) | 303 (77.9) | 434 (67.6) | <0.001 |
| Body mass index, kg m–2, IQR | 23.2 (20.9–25.7), 1023 * | 23.2 (21.3–25.6), 387 * | 23.2 (20.7–25.8), 636 * | 0.721 |
| Pre-existing illness | ||||
| Hypertension, n (%) | 358 (34.7) | 115 (29.6) | 243 (37.9) | 0.007 |
| Diabetes mellitus, n (%) | 232 (22.5) | 61 (15.7) | 171 (26.6) | <0.001 |
| AMI, n (%) | 65 (6.3) | 27 (6.9) | 38 (5.9) | 0.597 |
| Angina, n (%) | 64 (6.2) | 34 (8.7) | 30 (4.7) | 0.011 |
| Congestive heart failure, n (%) | 36 (3.5) | 12 (3.1) | 24 (3.7) | 0.606 |
| Arrhythmia, n (%) | 50 (4.8) | 21 (5.4) | 29 (4.5) | 0.551 |
| Renal disease, n (%) | 79 (7.7) | 17 (4.4) | 62 (9.7) | 0.002 |
| Pulmonary disease, n (%) | 60 (5.8) | 8 (2.1) | 52 (8.1) | <0.001 |
| TIA or ischemic stroke, n (%) | 59 (5.7) | 18 (4.6) | 41 (6.4) | 0.270 |
| Liver cirrhosis, n (%) | 12 (1.2) | 1 (0.3) | 11 (1.7) | 0.037 |
| Malignancy, n (%) | 55 (5.3) | 20 (5.1) | 35 (5.5) | 0.887 |
| Cardiac etiology, n (%) | 644 (62.5) | 340 (87.4) | 304 (47.4) | <0.001 |
| Downtime, min, IQR | 27 (16–40) | 18 (12–27) | 33 (22–46) | <0.001 |
| Witness, n (%) | 730 (70.8) | 325 (83.5) | 405 (63.1) | <0.001 |
| Bystander CPR, n (%) | 648 (62.9) | 262 (67.4) | 386 (60.1) | 0.020 |
| Shockable rhythm, n (%) | 379 (36.8) | 269 (71.0) | 110 (17.1) | <0.001 |
| SOFA score, IQR | 11 (8–12), 954 * | 9 (7–11), 369 * | 11 (9–13), 585 * | <0.001 |
| Serum lactate, mg dL–1, IQR | 9.4 (5.8–12.5), 997 * | 9.4 (5.5–12.4), 377 * | 9.4 (6.0–12.6), 620 * | 0.481 |
| pH, IQR | 7.11 (6.94–7.25), 997 * | 7.22 (7.09–7.30), 371 * | 7.04 (6.89–7.18), 626 * | <0.001 |
| PaCO2, mmHg, IQR | 47 (35.0–69.0), 997 * | 39.0 (32.0–49.0), 371 * | 56.0 (38.8–80.1), 626 * | <0.001 |
| HCO3, mEq dL–1, IQR | 15.3 (12.0–18.8), 995 * | 15.8 (12.9–19.1), 371 * | 15.0 (11.6–18.8), 624 * | 0.028 |
| Peak CRP, mg dL–1, IQR | 15.8 (9.4–24.3), 994 * | 12.9 (7.4–18.8), 371 * | 18.1 (11.4–27.0), 623 * | <0.001 |
| Time from ROSC to initiation of TTM, min | 210.0 (132.0–301.0) | 214.0 (146.0–299.0) | 208.0 (122.8–304.3) | 0.163 |
| Pre-TTM Temperature (°C), IQR | 36 (34.9–36.8), 1006 * | 36.4 (35.8–37.0), 379 * | 35.6 (34.5–36.4), 627 * | <0.001 |
| Peak temperature, (°C), within 72 h, IQR | 37.7 (37.2–38.2) | 38.0 (37.6–38.3) | 37.5 (37.0–38.0) | <0.001 |
| Pre-TTM Shock, n (%) | 474 (46.0) | 132 (33.9) | 342 (53.3) | <0.001 |
| PRTM, n (%) | 648 (62.9) | 248 (63.8) | 400 (62.3) | 0.642 |
| Target temperature, n (%) | 0.812 | |||
| 32.0−34.0 °C | 820 (79.5) | 311 (79.9) | 509 (79.3) | |
| 34.1−36.0 °C | 211 (20.5) | 78 (20.1) | 133 (20.7) | |
| Seizure, n (%) | 279 (27.1) | 51 (13.1) | 228 (35.5) | <0.001 |
| Infection, n (%) | 563 (54.6) | 190 (48.8) | 373 (58.1) | 0.004 |
| Variables | PRF (n = 389) | No PRF (n = 642) | p-Value |
|---|---|---|---|
| Age, years, IQR | 56 (45–66) | 58 (48–69) | 0.007 |
| Male sex, n (%) | 302 (77.6) | 435 (67.8) | 0.001 |
| Body mass index, kg m–2, IQR | 23.6 (21.4–25.9), 386 * | 23.1 (20.7–25.4), 637 * | 0.066 |
| Pre-existing illness | |||
| Hypertension, n (%) | 128 (32.9) | 230 (35.8) | 0.346 |
| Diabetes mellitus, n (%) | 77 (19.8) | 155 (24.1) | 0.107 |
| AMI, n (%) | 25 (6.4) | 40 (6.2) | 1.000 |
| Angina, n (%) | 32 (8.2) | 32 (5.0) | 0.045 |
| Congestive heart failure, n (%) | 12 (3.1) | 24 (3.7) | 0.606 |
| Arrhythmia, n (%) | 20 (5.1) | 30 (4.7) | 0.766 |
| Renal disease, n (%) | 20 (5.1) | 59 (9.2) | 0.021 |
| Pulmonary disease, n (%) | 14 (3.6) | 46 (7.2) | 0.019 |
| TIA or ischemic stroke, n (%) | 20 (5.1) | 39 (6.1) | 0.582 |
| Liver cirrhosis, n (%) | 2 (0.5) | 10 (1.6) | 0.229 |
| Malignancy, n (%) | 14 (3.6) | 41 (6.4) | 0.063 |
| Cardiac etiology n (%) | 270 (69.4) | 374 (58.3) | <0.001 |
| Downtime, min, IQR | 27 (16–40) | 30 (18–43) | <0.001 |
| Witness n (%) | 279 (71.7) | 451 (70.2) | 0.622 |
| Bystander CPR n (%) | 239 (61.4) | 409 (63.7) | 0.506 |
| Shockable rhythm, n (%) | 176 (46.4) | 203 (31.6) | <0.001 |
| SOFA score, IQR | 10 (7–12), 364 * | 11 (9–13), 590 * | <0.001 |
| Serum lactate, mg dL–1, IQR | 9.2 (5.5–12.5), 383* | 9.5 (6.0–12.5), 614 * | 0.161 |
| pH, IQR | 7.16 (7.00–7.26), 376 * | 7.08 (6.92–7.24), 621 * | <0.001 |
| PaCO2, mmHg, IQR | 44.3 (34.3–63.0), 376 * | 49.2 (35.9–73.0), 621 * | 0.008 |
| HCO3, mEq dL–1, IQR | 15.3 (12.4–18.9), 375 * | 15.3 (11.8–18.8), 620 * | 0.580 |
| Peak CRP, mg dL–1, IQR | 15.8 (10.6–22.4), 369 * | 15.7 (8.6–25.6), 625 * | 0.933 |
| Time from ROSC to initiation of TTM, min | 224 (152.5–330.5) | 201.0 (123.0–289.3) | <0.001 |
| Pre-TTM Temperature, °C, IQR | 36.2 (35.4–37.0), 380 * | 35.8 (34.6–36.5), 626 * | <0.001 |
| Pre-TTM Shock, n (%) | 155 (39.8) | 319 (49.7) | 0.001 |
| PRTM, n (%) | 217 (55.8) | 431 (67.1) | <0.001 |
| Target temperature of TTM, n (%) | 0.691 | ||
| 32.0–34.0 °C | 312 (80.2) | 508 (79.1) | |
| 34.1–36.0 °C | 77 (19.8) | 134 (20.9) | |
| Seizure n (%) | 113 (29.0) | 166 (25.9) | 0.278 |
| Infection n (%) | 228 (58.6) | 335 (52.2) | 0.046 |
| Variables | AOR (95% CI) | p-Value |
|---|---|---|
| Age, years | 0.988 (0.978–0.998) | 0.015 |
| Male sex | 1.323 (0.950–1.844) | 0.098 |
| Body mass index, kg m–2 | 1.032 (0.996–1.071) | 0.084 |
| Angina | 2.336 (1.265–4.315) | 0.007 |
| Diabetes mellitus | 1.114 (0.755–1.643) | 0.588 |
| Renal disease | 0.723 (0.380–1.375) | 0.323 |
| Pulmonary disease | 0.726 (0.360–1.467) | 0.373 |
| Malignancy | 0.569 (0.291–1.114) | 0.100 |
| Cardiac etiology | 1.462 (1.067–2.004) | 0.018 |
| Downtime, min | 0.993 (0.984–1.002) | 0.119 |
| Shockable rhythm | 1.164 (0.792–1.712) | 0.440 |
| SOFA score | 0.878 (0.834–0.924) | <0.001 |
| Lactate, mg dL–1 | 0.993 (0.965–1.021) | 0.610 |
| pH | 0.631 (0.273–1.458) | 0.281 |
| PaCO2, mmHg | 0.999 (0.990–1.007) | 0.804 |
| Time from ROSC to initiation of TTM, min | 1.002 (1.001–1.002) | <0.001 |
| Pre-TTM shock | 0.833 (0.602–1.152) | 0.270 |
| Pre-TTM temperature, °C | 1.214 (1.093–1.348) | <0.001 |
| PRTM | 0.634 (0.470–0.856) | 0.003 |
| Infection | 1.340 (0.997–1.802) | 0.053 |
| Variables | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| Age, years | 1.032 (1.017–1.047) | 1.029 (1.014–1.044) | 1.030 (1.015–1.045) | 1.030 (1.015–1.045) |
| Male sex | 0.723 (0.459–1.138) | |||
| Hypertension | 1.374 (0.850–2.221) | |||
| Diabetes mellitus | 1.320 (0.765–2.277) | |||
| Angina | 0.613 (0.278–1.354) | |||
| Renal disease | 0.818 (0.346–1.932) | |||
| Pulmonary disease | 0.734 (0.268–2.005) | |||
| Liver cirrhosis | 10.142 (0.731–140.716) | |||
| Cardiac etiology | 0.246 (0.146–0.413) | 0.255 (0.152–0.430) | 0.251 (0.149–0.423) | 0.256 (0.152–0.434) |
| Downtime, min | 1.053 (1.038–1.069) | 1.051(1.035–1.066) | 1.051 (1.036–1.067) | 1.050 (1.035–1.065) |
| Witness | 0.752 (0.454–1.246) | |||
| Bystander CPR | 1.305 (0.848–2.008) | |||
| Shockable rhythm | 0.218 (0.136–0.349) | 0.235 (0.147–0.377) | 0.228 (0.142–0.364) | 0.219 (0.136–0.353) |
| SOFA score | 1.109 (1.027–1.197) | 1.109 (1.027–1.197) | 1.107 (1.026–1.195) | 1.102 (1.020–1.190) |
| pH | 0.131 (0.042–0.410) | 0.123 (0.039–0.386) | 0.126 (0.040–0.396) | 0.125 (0.039–0.393) |
| PaCO2, mmHg | 1.006 (0.985–1.027) | |||
| HCO3, mEq dl–1 | 1.036 (0.991–1.084) | |||
| Peak CRP, mg dL–1, IQR | 1.025 (1.010–1.039) | 1.024 (1.010–1.039) | 1.024 (1.009–1.038) | 1.024 (1.009–1.038) |
| Time from ROSC to initiation of TTM, min | 1.000 (0.999–1.002) | |||
| Pre-TTM Temperature, °C | 0.727 (0.626–0.846) | 0.752 (0.646–0.876) | 0.745 (0.640–0.867) | 0.764 (0.656–0.890) |
| Pre-TTM Shock | 1.150 (0.732–1.806) | |||
| Seizure | 5.262 (3.243–8.538) | 5.308 (3.270–8.618) | 5.320 (3.278–8.634) | 5.443 (3.330–8.897) |
| Infection | 1.691 (1.122–2.550) | 1.763 (1.165–2.669) | 1.690 (1.121–2.547) | 1.614 (1.067–2.443) |
| Peak temperature, °C, within 72 h | NA | 0.660 (0.485–0.899) | NA | NA |
| PRF | NA | 0.633 (0.416–0.963) | NA | |
| No PRF | NA | NA | Reference | |
| PRF within 24 h | NA | NA | 0.355 (0.191–0.659) | |
| PRF 24–48 h | NA | NA | 0.797 (0.458–1.386) | |
| PRF 48–72 h | NA | NA | 0.980 (0.463–2.074) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.Y.; Lee, D.H.; Lee, B.K.; Jeung, K.W.; Jung, Y.H.; Choi, S.P.; Park, J.S.; Lee, J.H.; Han, K.S.; Min, Y.I., on behalf of the Korean Hypothermia Network Investigators. Early Post-Rewarming Fever Is Associated with Favorable 6-Month Neurologic Outcomes in Patients with Out-Of-Hospital Cardiac Arrest: A Multicenter Registry Study. J. Clin. Med. 2020, 9, 2927. https://doi.org/10.3390/jcm9092927
Lee HY, Lee DH, Lee BK, Jeung KW, Jung YH, Choi SP, Park JS, Lee JH, Han KS, Min YI on behalf of the Korean Hypothermia Network Investigators. Early Post-Rewarming Fever Is Associated with Favorable 6-Month Neurologic Outcomes in Patients with Out-Of-Hospital Cardiac Arrest: A Multicenter Registry Study. Journal of Clinical Medicine. 2020; 9(9):2927. https://doi.org/10.3390/jcm9092927
Chicago/Turabian StyleLee, Hyoung Youn, Dong Hun Lee, Byung Kook Lee, Kyung Woon Jeung, Yong Hun Jung, Seung Phil Choi, Jung Soo Park, Jae Hoon Lee, Kap Su Han, and Yong Il Min on behalf of the Korean Hypothermia Network Investigators. 2020. "Early Post-Rewarming Fever Is Associated with Favorable 6-Month Neurologic Outcomes in Patients with Out-Of-Hospital Cardiac Arrest: A Multicenter Registry Study" Journal of Clinical Medicine 9, no. 9: 2927. https://doi.org/10.3390/jcm9092927
APA StyleLee, H. Y., Lee, D. H., Lee, B. K., Jeung, K. W., Jung, Y. H., Choi, S. P., Park, J. S., Lee, J. H., Han, K. S., & Min, Y. I., on behalf of the Korean Hypothermia Network Investigators. (2020). Early Post-Rewarming Fever Is Associated with Favorable 6-Month Neurologic Outcomes in Patients with Out-Of-Hospital Cardiac Arrest: A Multicenter Registry Study. Journal of Clinical Medicine, 9(9), 2927. https://doi.org/10.3390/jcm9092927





