Preparation and Photocatalytic Performance of MoS2/MoO2 Composite Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Photocatalysts
2.3. Characterization
2.4. Photocatalytic Degradation and Photoelectrochemical Test
3. Results and Discussion
3.1. Characterization and Properties of 1T-MoS2/MoO2 Composite Catalysts
3.1.1. XRD Characterization
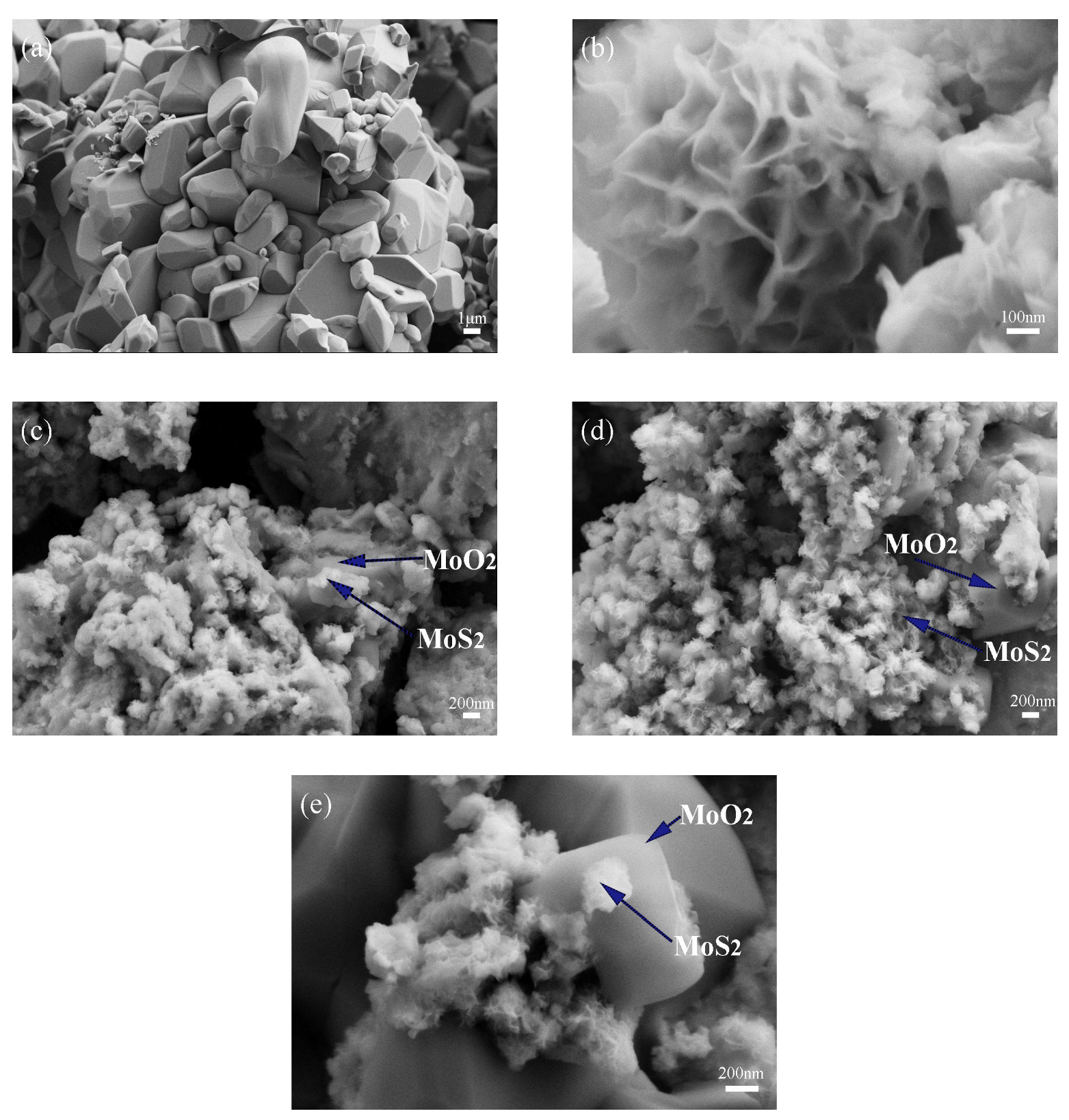
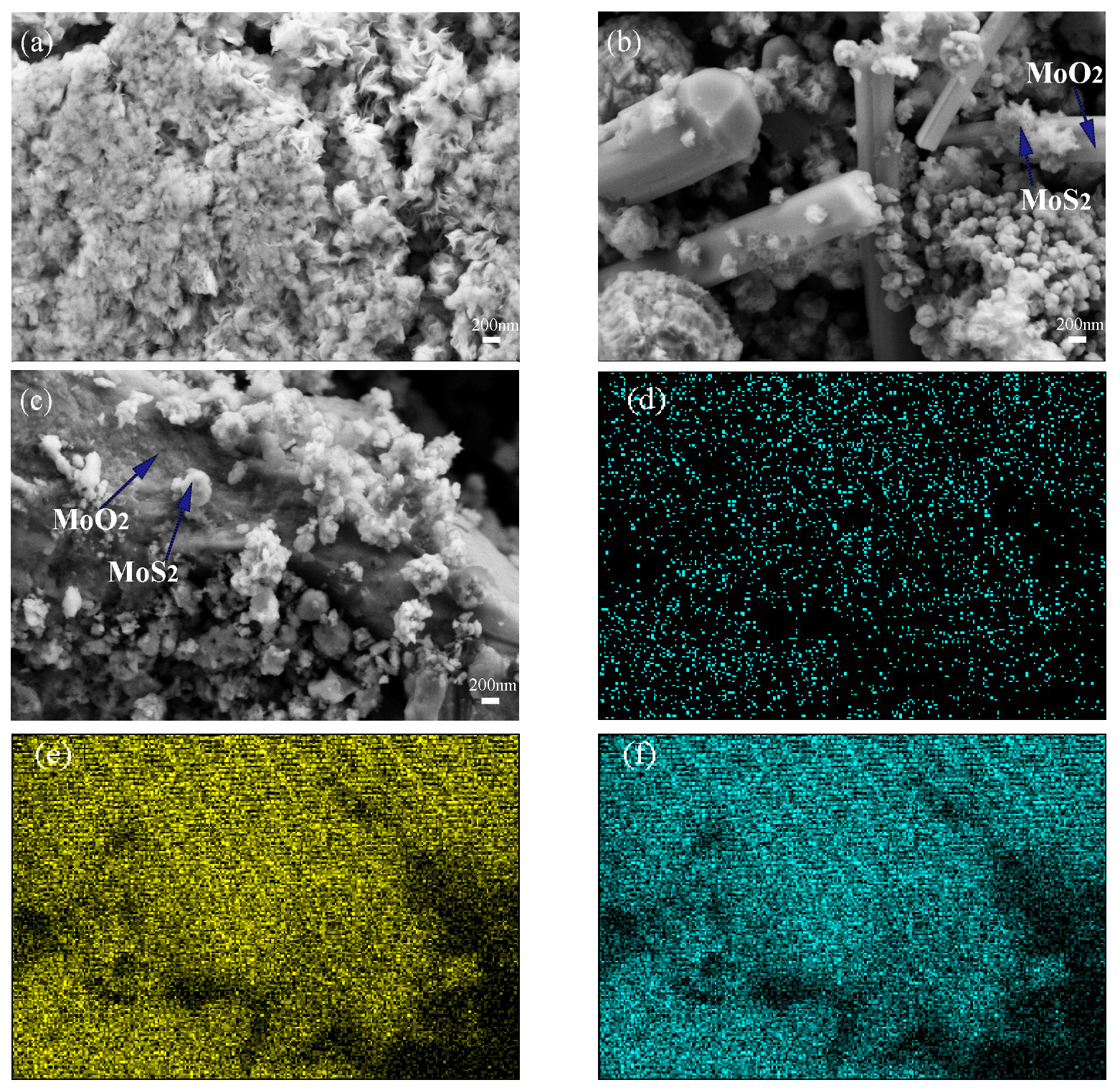
3.1.2. Morphology Analysis
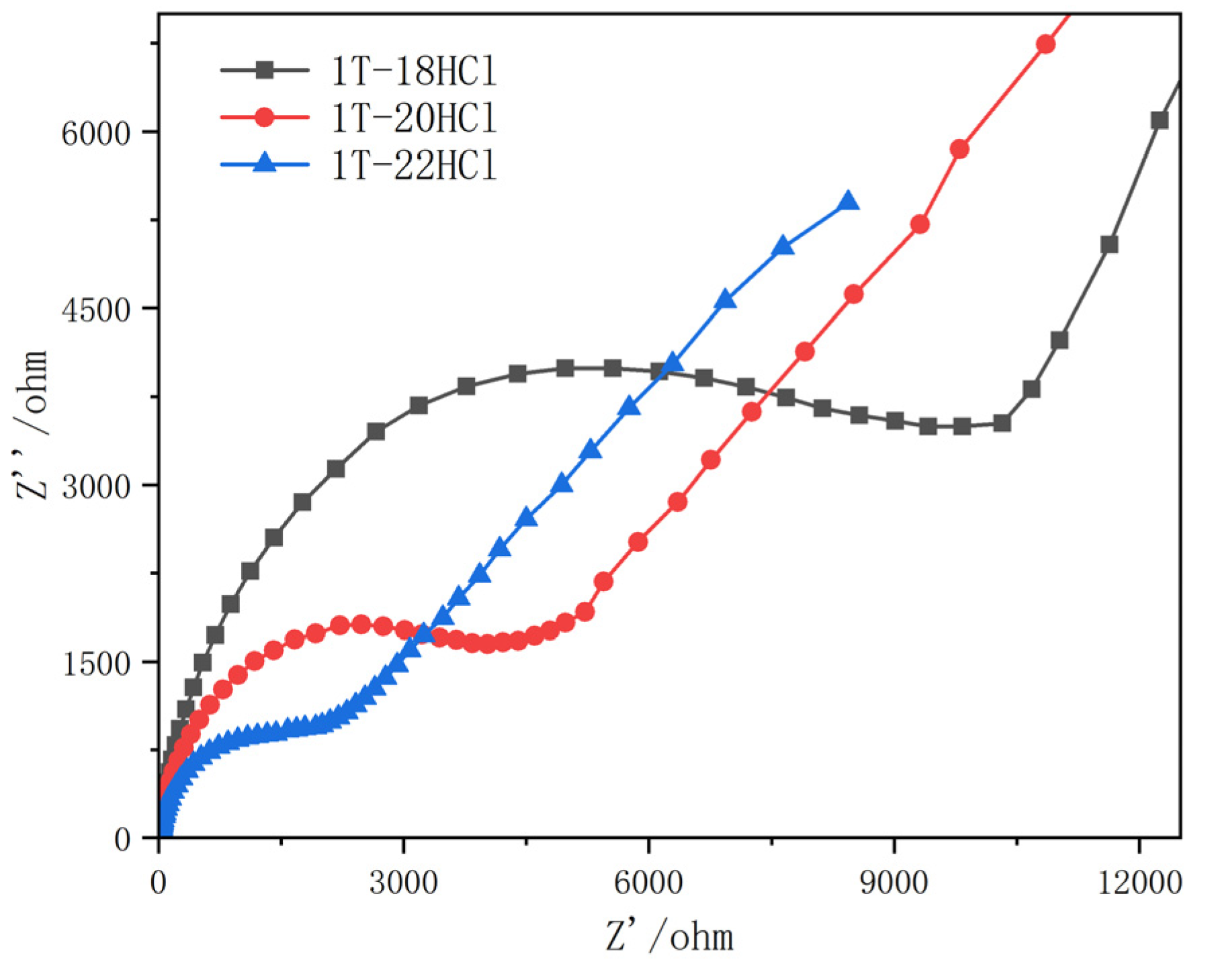
3.1.3. Electrochemical Impedance Measurement
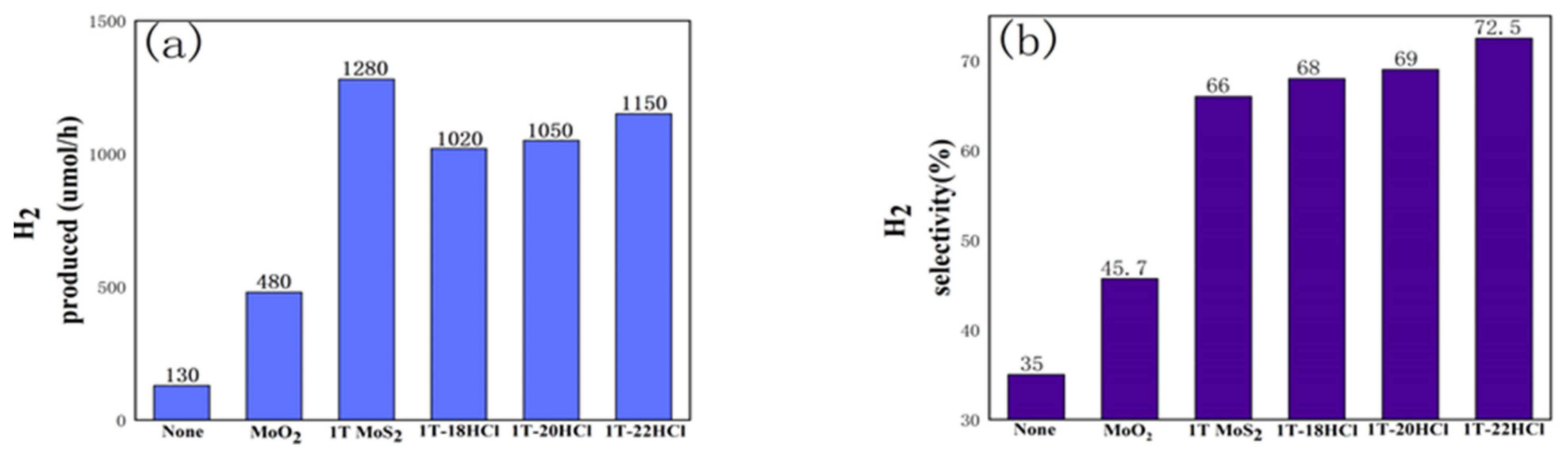
3.1.4. Photocatalytic Performance
3.2. Characterization and Properties of 2H-MoS2/MoO2
3.2.1. XRD Characterization
3.2.2. Morphology Analysis of 2H-MoS2/MoO2
3.2.3. BET Measurements
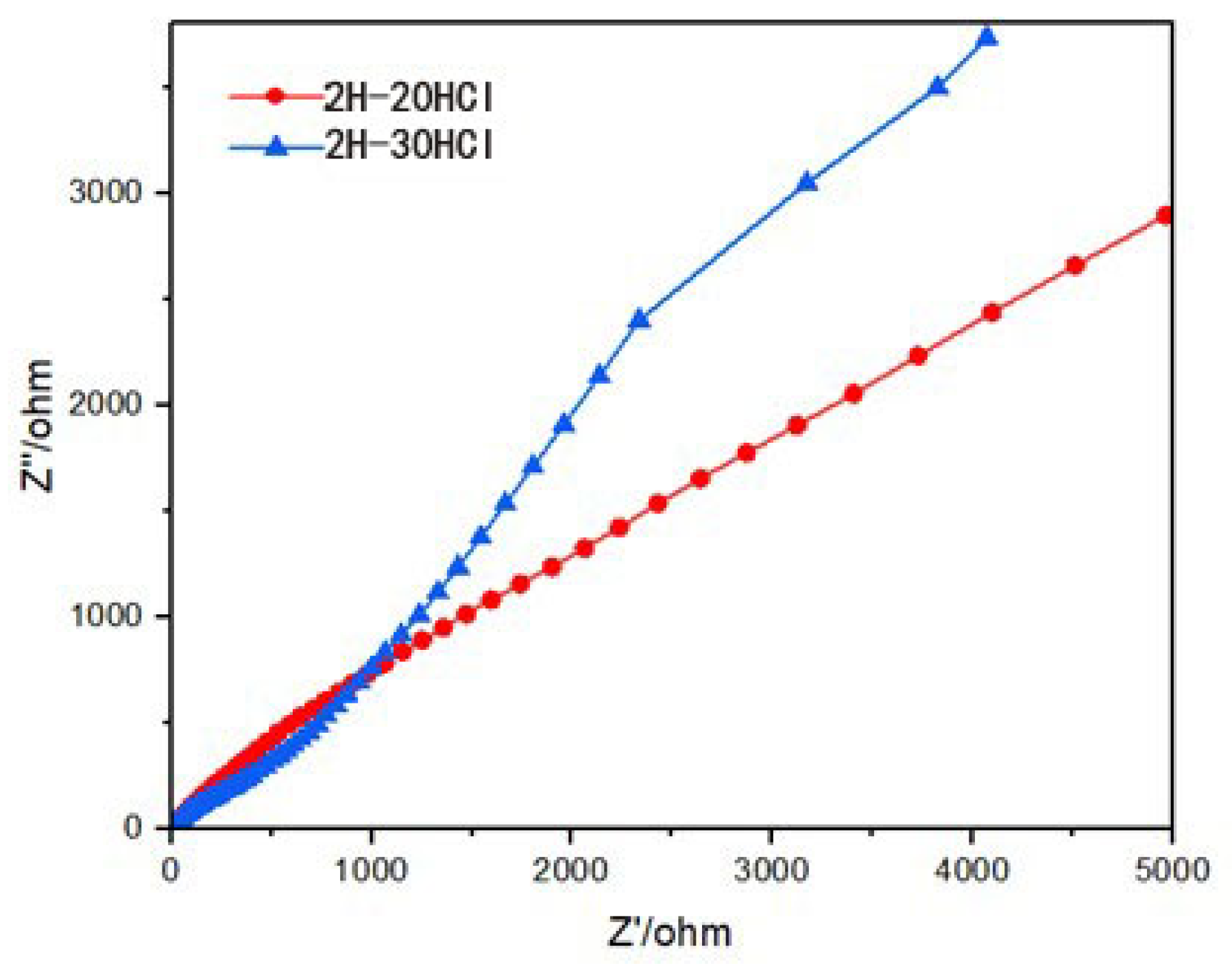
3.2.4. Electrochemical Impedance Measurement
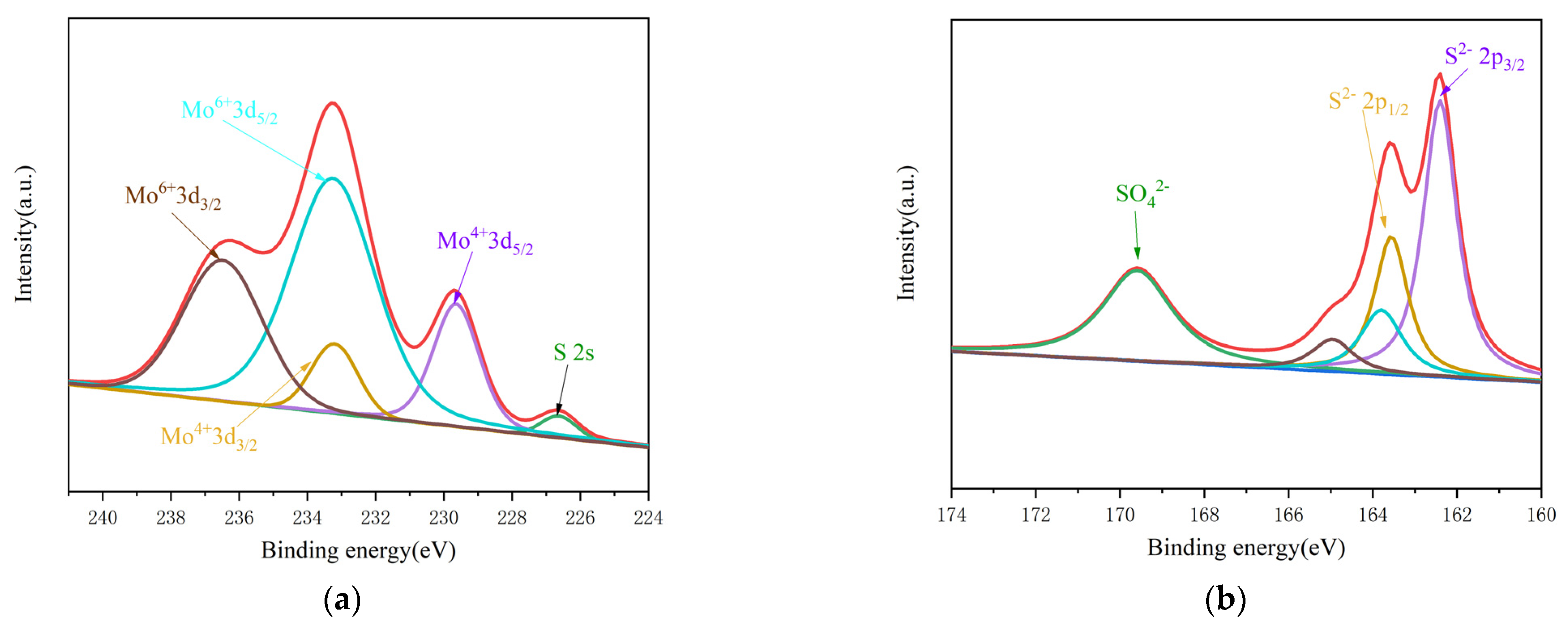
3.2.5. X-ray Photoelectronic Spectrum Analysis of 2H-MoS2/MoO2
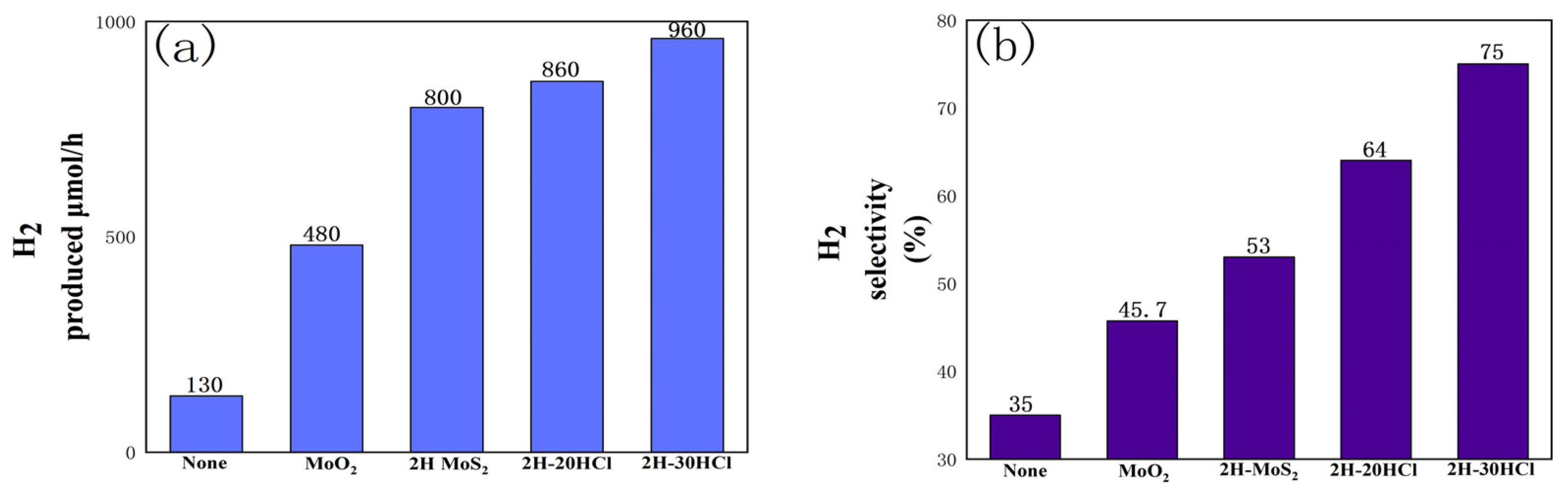
3.2.6. Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.D.; Wang, L.; Yu, P.; Zhao, L.; Tian, C.U.; Zhou, W.; Zhang, L.; Fu, H.G. From graphite to porous graphene-like nanosheets for high rate lithium-ion batteries. Nano Res. 2015, 8, 2998–3010. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. Spent rechargeable lithium batteries in e-waste: Composition and its implications. Front. Environ. Sci. Eng. 2014, 8, 792–796. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, D.X.; Yin, H.; Qu, Y.P.; Zhou, L.L.; Chen, C.; Wu, H.; Wang, P. Noble-Metal-Free Ni-W-O-Derived Catalysts for High-Capacity Hydrogen Production from Hydrazine Monohydrate. ACS Sustain. Chem. Eng. 2020, 8, 5595–5603. [Google Scholar] [CrossRef]
- Qiu, Y.P.; Shi, Q.; Zhou, L.L.; Chen, M.H.; Chen, C.; Tang, P.P.; Walker, G.S.; Wang, P. Ni Pt Nanoparticles Anchored onto Hierarchical Nanoporous N-Doped Carbon as an Efficient Catalyst for Hydrogen Generation from Hydrazine Monohydrate. ACS Appl. Mater. Interfaces 2020, 12, 18617–18624. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Lup, W.J.; Kuan, Y.D. Preparation of Catalyst for Hydrogen Production Reaction of Sodium Borohydride and Its Effectiveness. Sens. Mater. 2020, 32, 3659–3668. [Google Scholar] [CrossRef]
- Ghodke, N.P.; Rayaprol, S.; Bhoraskar, S.V.; Mathe, V.L. Catalytic hydrolysis of sodium borohydride solution for hydrogen production using thermal plasma synthesized nickel nanoparticles. Int. J. Hydrog. Energy 2020, 45, 16591–16605. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.B. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.M.; He, H.Y.; Huang, F.Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew Chem. Int. Ed. Engl. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Shaybanizadeh, S.; Chermahini, A.N.; Luque, R. Boron nitride nanosheets supported highly homogeneous bimetallic AuPd alloy nanoparticles catalyst for hydrogen production from formic acid. Nanotechnology 2022, 33, 27. [Google Scholar] [CrossRef]
- Wang, G.Z.; Chang, J.L.; Tang, W.Y.; Xie, W.J.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D-Appl. Phys. 2022, 55, 29. [Google Scholar] [CrossRef]
- He, Y.B.; Li, G.R.; Wang, Z.L.; Su, C.Y.; Tong, Y.X. Single-crystal ZnO nanorod/amorphous and nanoporous metal oxide shell composites: Controllable electrochemical synthesis and enhanced supercapacitor performances. Energy Environ. Sci. 2011, 4, 1288–1292. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.S.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.Y.; Ye, H.C.; Chen, S.M.; Ju, H.X.; Liu, D.B.; Lin, Y.; Ye, W.; Wang, C.M.; Xu, Q.; et al. Oxide Defect Engineering Enables to Couple Solar Energy into Oxygen Activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Paik, T.; Cargnello, M.; Gordon, T.R.; Zhang, S.; Yun, H.; Lee, J.D.; Woo, H.Y.; Oh, S.J.; Kagan, C.R.; Fornasiero, P.; et al. Photocatalytic Hydrogen Evolution from Substoichiometric Colloidal WO3-x Nanowires. ACS Energy Lett. 2018, 3, 1904–1910. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.L.; Guan, N.J.; Li, L.D.; Gong, J.L. Tungsten Oxide Single Crystal Nanosheets for Enhanced Multichannel Solar Light Harvesting. Adv. Mater. 2015, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Liu, L.; Xi, X.X.; Li, Y.M.; Geng, Z.L.; Jiang, G.Y.; Zhao, Z. Novel metal doped carbon quantum dots/CdS composites for efficient photocatalytic hydrogen evolution. Nanoscale 2019, 11, 1618–1625. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.Y.; Hong, G.S.; Dai, H.J. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Jia, F.; Jia, F.F.; Song, S.X. Adsorption of heavy metals on molybdenum disulfide in water: A critical review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide TS2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, Y.X.; Yang, Z.Y.; Yan, Y.M.; Sun, K.N. Synthesis of MoS2 and MoO2 for their applications in H2 generation and lithium ion batteries: A review. Sci. Technol. Adv. Mater. 2013, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Y.; Zhang, H.Y.; Li, Z.H. Synthesis of Double-Layer Nitrogen-Doped Microporous Hollow Carbon@MoS2/MoO2 Nanospheres for Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 29511–29520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Shao, J.; Li, J.; Zhang, L.; Qu, Q.T.; Zheng, H.H. Ordered mesoporous MoO2 as a high-performance anode material for aqueous supercapacitors. J. Power Sour. 2013, 237, 80–83. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.M.; Yang, X.F.; Jiao, M.G.; Zhou, Z.; Zhang, M.H.; Wang, D.H.; Bu, X.H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xin, X.; Song, Y.R.; Yang, L.; Wang, B.L.; Tan, L.L.; Li, X.H. Plasmonic MoO2 as co-catalyst of MoS2 for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 504, 144291. [Google Scholar] [CrossRef]
- Wang, W.; Yao, Q.; Ma, J.; Xu, Y.; Jiang, J.Q.; Liu, X.E.; Li, Z.C. Engineering MoS2 nanostructures from various MoO3 precursors towards hydrogen evolution reaction. Crystengcomm 2020, 22, 2258–2267. [Google Scholar] [CrossRef]
- Kang, H.; Youn, J.-S.; Oh, I.; Manavalan, K.; Jeon, K.J. Controllable atomic-ratio of CVD-grown MoS2-MoO2 hybrid catalyst by soft annealing for enhancing hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 1399–1408. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, Y.Y.; Luo, X.N.; Wu, Z.Z.; Wang, Y.J.; Fang, B.Z. Swollen Ammoniated MoS2 with 1T/2H Hybrid Phases for High -Rate Electrochemical Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 2509–2515. [Google Scholar] [CrossRef]
- Toby, B.H.; Von dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Von Dreele, R.B. Small-angle scattering data analysis in GSAS-II. J. Appl. Crystallogr. 2014, 47, 1784–1789. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Z.J.; Luo, X.N.; Sun, A.K.; Wu, Z.Z.; Wang, D.Z. Template-free fabrication of hierarchical MoS2/MoO2 nanostructures as efficient catalysts for hydrogen production. Appl. Surf. Sci. 2018, 433, 723–729. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Sharma, R.K.; Reddy, G.S. A study on role of partial pressure in controlled synthesis of core-shell MoO2/MoS2 nanoflakes. Mater. Chem. Phys. 2016, 178, 6–11. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, B.C.; Zhou, J.F.; Fang, Z.T.; Li, K.Z.; Ma, H.Y.; Dai, J.M.; Zhu, X.B.; Sun, Y.P. Improved Electrochemical Performance of Ultrathin MoS2 Nanosheet/Co Composites for Lithium-Ion Battery Anodes. Chemelectrochem 2019, 6, 1930–1938. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.M.; Chen, Y.F.; Chen, Y.F.; Li, M.X.; Liu, P.Y.; Wang, C.C.; Wang, P.; Lu, H.B. Designing vertical channels with expanded interlayers for Li-ion batteries. Chem. Commun. 2019, 55, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Modak, A.; Pant, K.; Sinhamahapatra, A.; Biswas, P. MoS2-Nanosheets-Based Catalysts for Photocatalytic CO2 Reduction: A Review. ACS Appl. Nano Mater. 2021, 4, 8644–8667. [Google Scholar] [CrossRef]
- Tian, J.; Yang, C.; Liu, Z.; Li, F.N.; He, X.; Chen, W.; Xia, N.; Lin, C. Construction of MoO2@MoS2 heterostructures in situ on carbon cloth for the hydrogen evolution reaction. New J. Chem. 2021, 45, 19826–19830. [Google Scholar] [CrossRef]
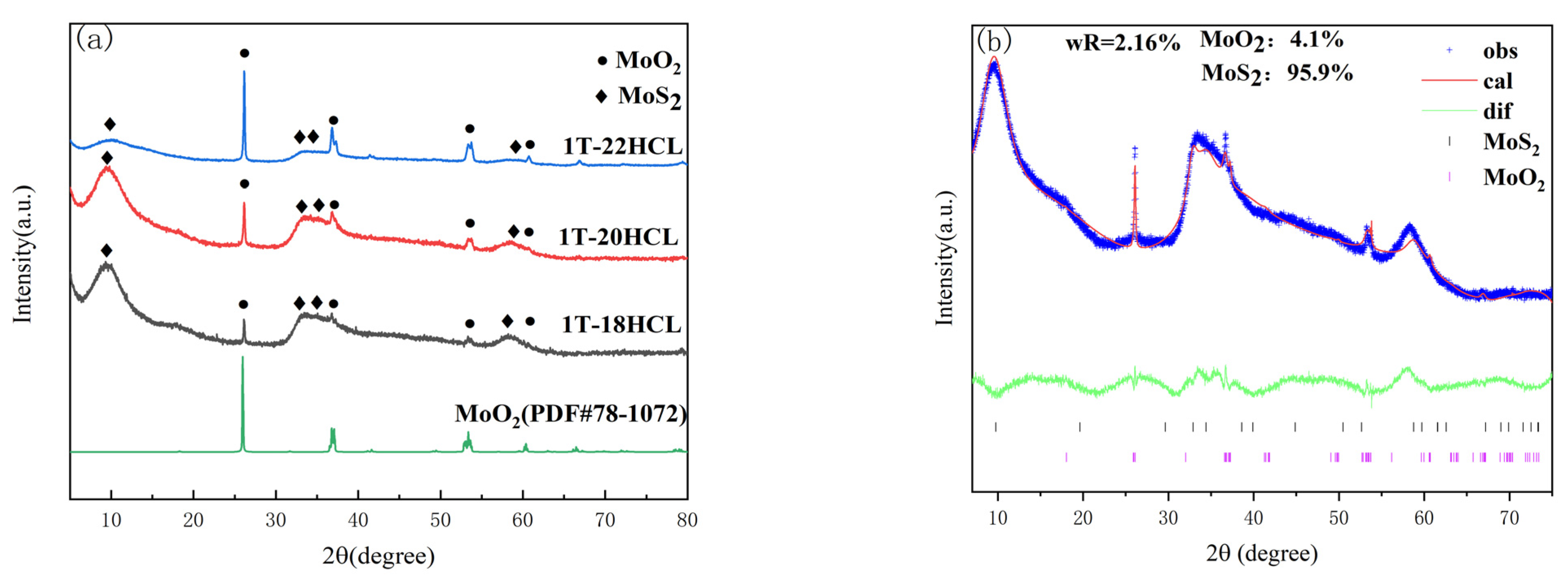
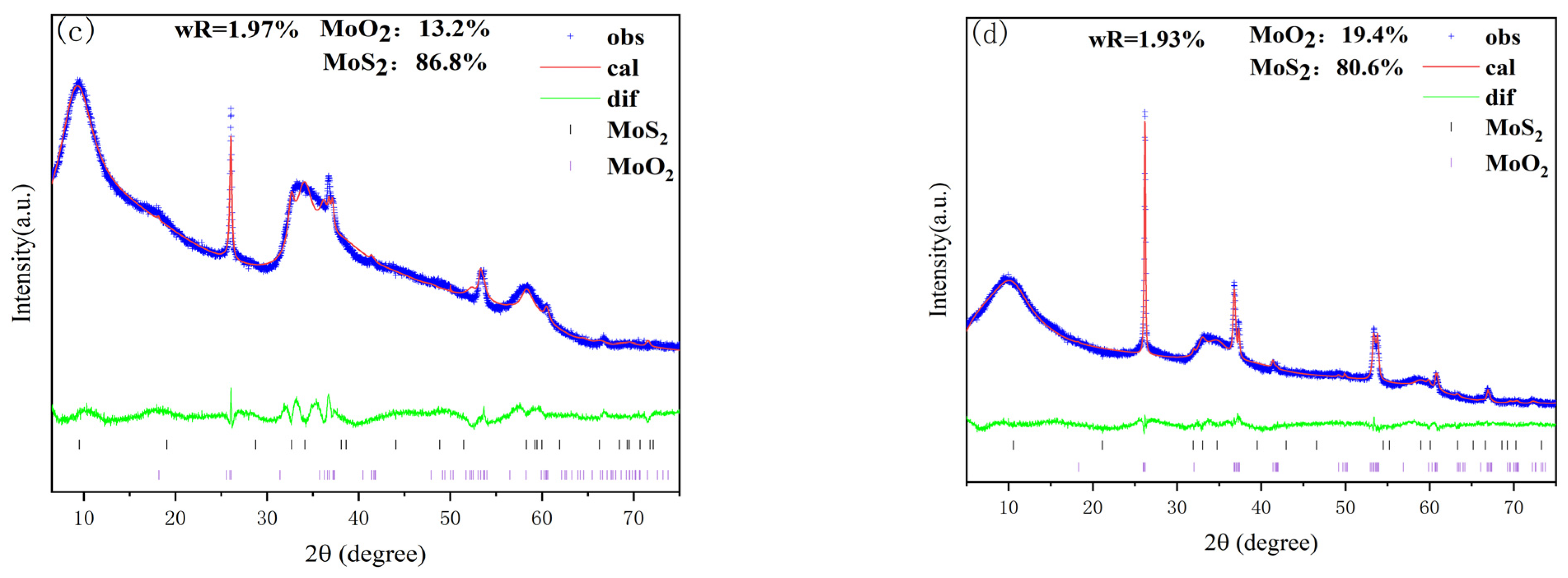
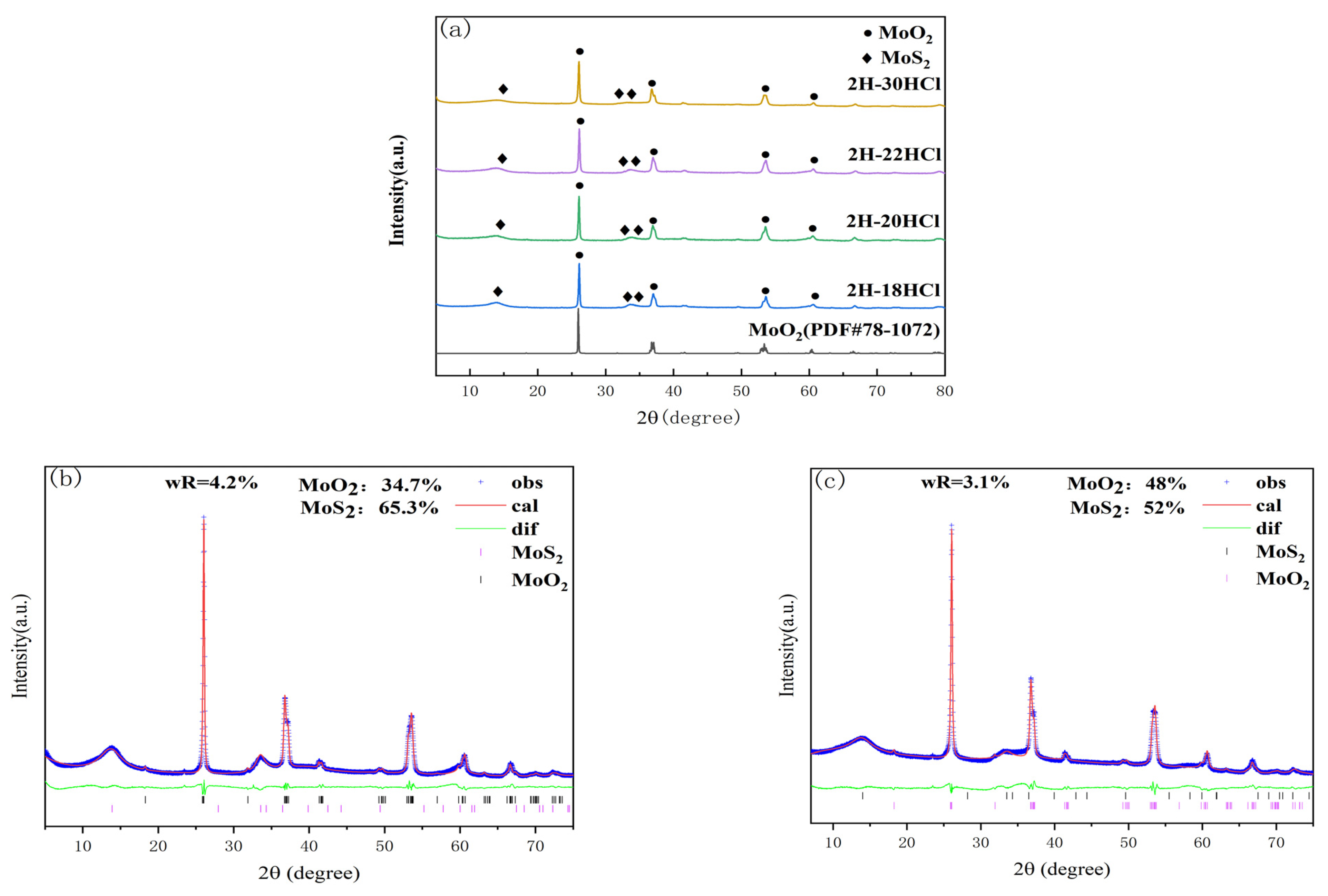
| Sample | 1T-MoS2/% | MoO2/% |
|---|---|---|
| 1T-18HCl | 95.9 | 4.1 |
| 1T-20HCl | 86.8 | 13.2 |
| 1T-22HCl | 80.6 | 19.4 |
| Sample | 2H-MoS2/% | MoO2/% |
|---|---|---|
| 2H-20HCl | 65.3 | 34.7 |
| 2H-30HCl | 52 | 48 |
| Element | Element wt% |
|---|---|
| O | 28.18 |
| S | 41.82 |
| Mo | 30 |
| Catalysts | BET Surface Area (m2/g) | Average Pore Width (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| 2H-20HCl | 6.632 | 24.34 | 0.040 |
| 2H-30HCl | 6.019 | 14.97 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Yan, W.; Tao, Y.; Liu, Y.; Lu, Y.; Pan, Z. Preparation and Photocatalytic Performance of MoS2/MoO2 Composite Catalyst. Materials 2023, 16, 4030. https://doi.org/10.3390/ma16114030
Dong D, Yan W, Tao Y, Liu Y, Lu Y, Pan Z. Preparation and Photocatalytic Performance of MoS2/MoO2 Composite Catalyst. Materials. 2023; 16(11):4030. https://doi.org/10.3390/ma16114030
Chicago/Turabian StyleDong, Daoyu, Weitao Yan, Yaqiu Tao, Yunfei Liu, Yinong Lu, and Zhigang Pan. 2023. "Preparation and Photocatalytic Performance of MoS2/MoO2 Composite Catalyst" Materials 16, no. 11: 4030. https://doi.org/10.3390/ma16114030





