Effect of Quenching Temperature on Microstructure and Hydrogen-Induced Cracking Susceptibility in S355 Steel
Abstract
:1. Introduction
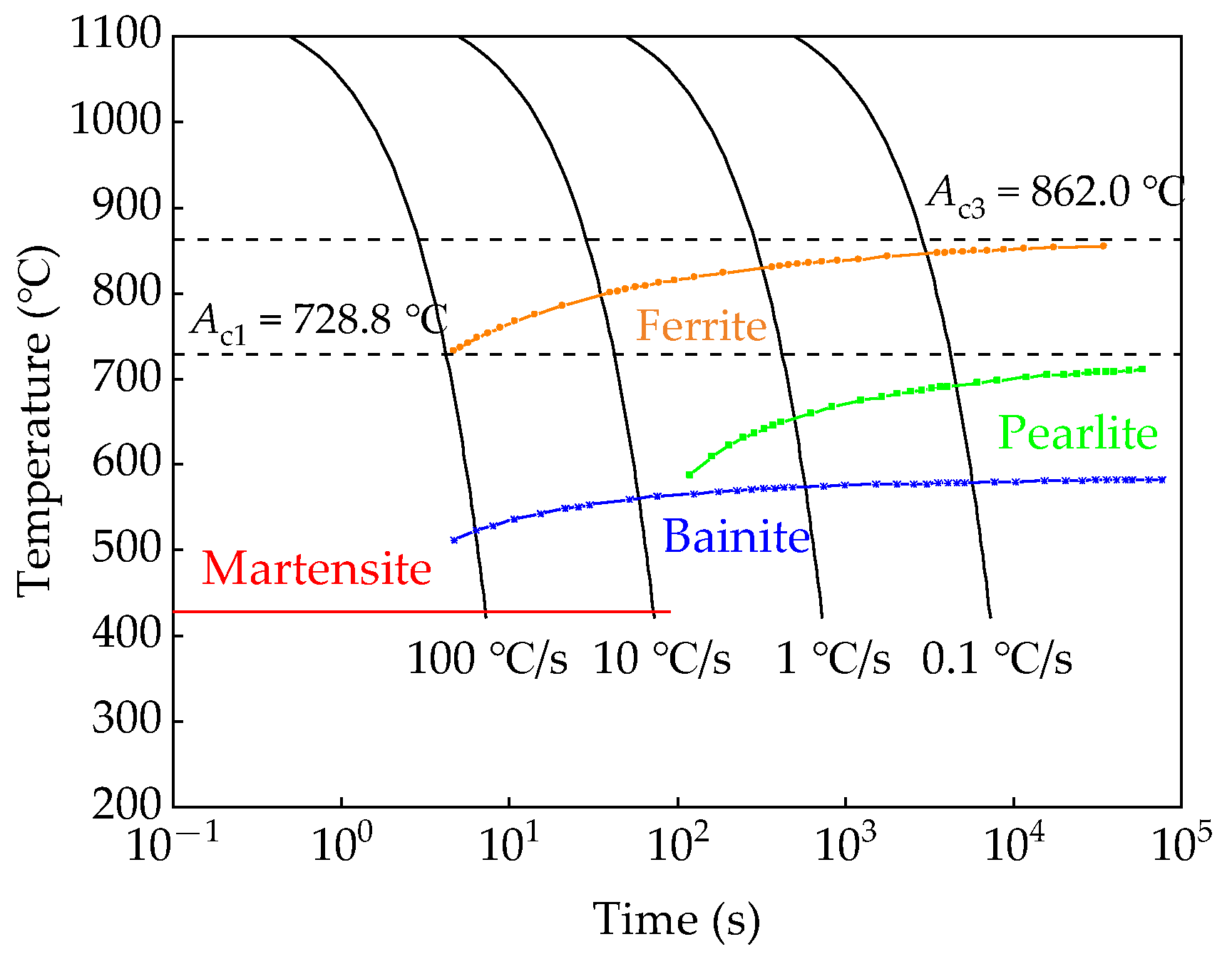
2. Materials and Methods
2.1. Experimental Materials
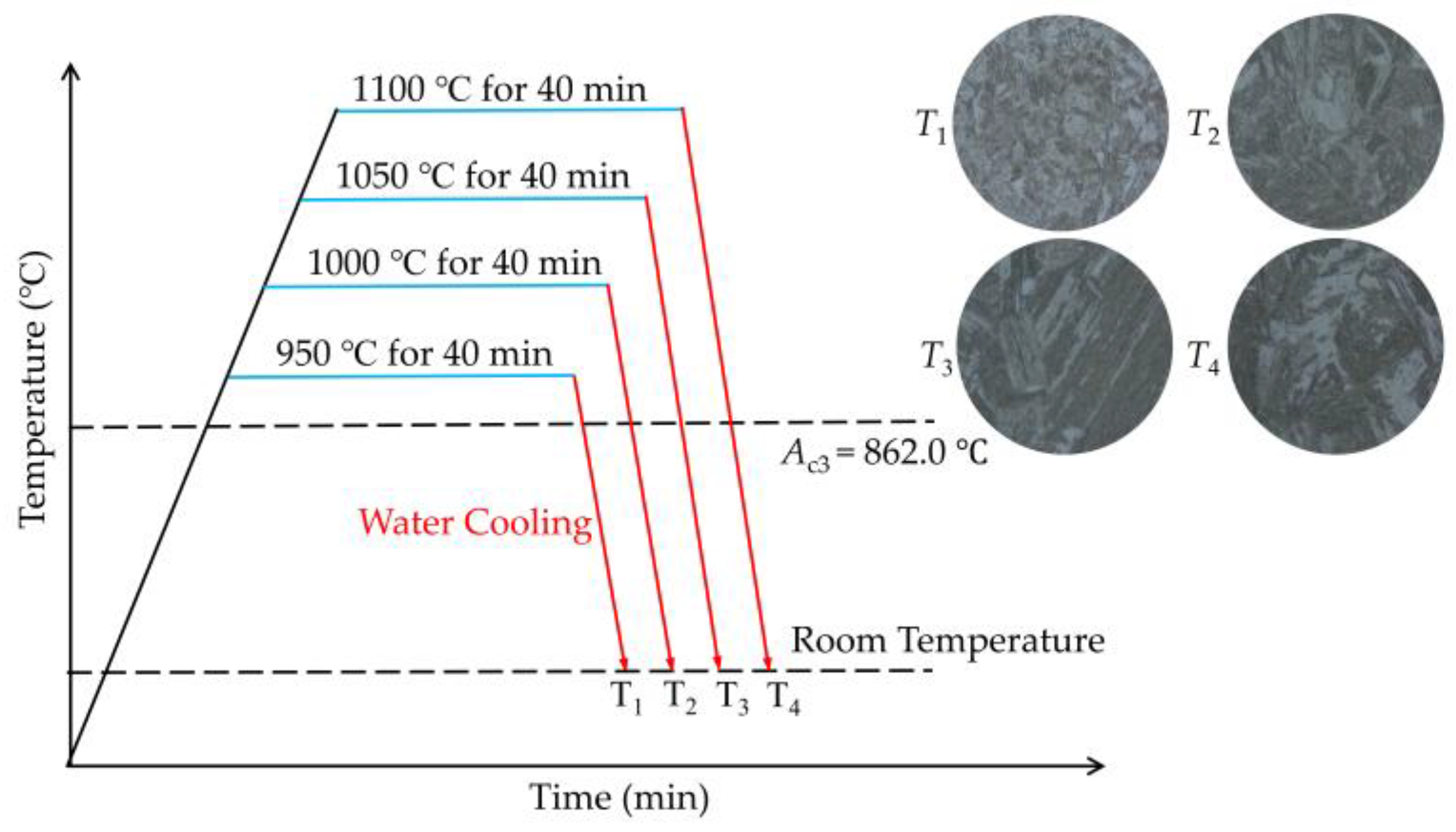
2.2. Microstructure Observation and Hardness Test
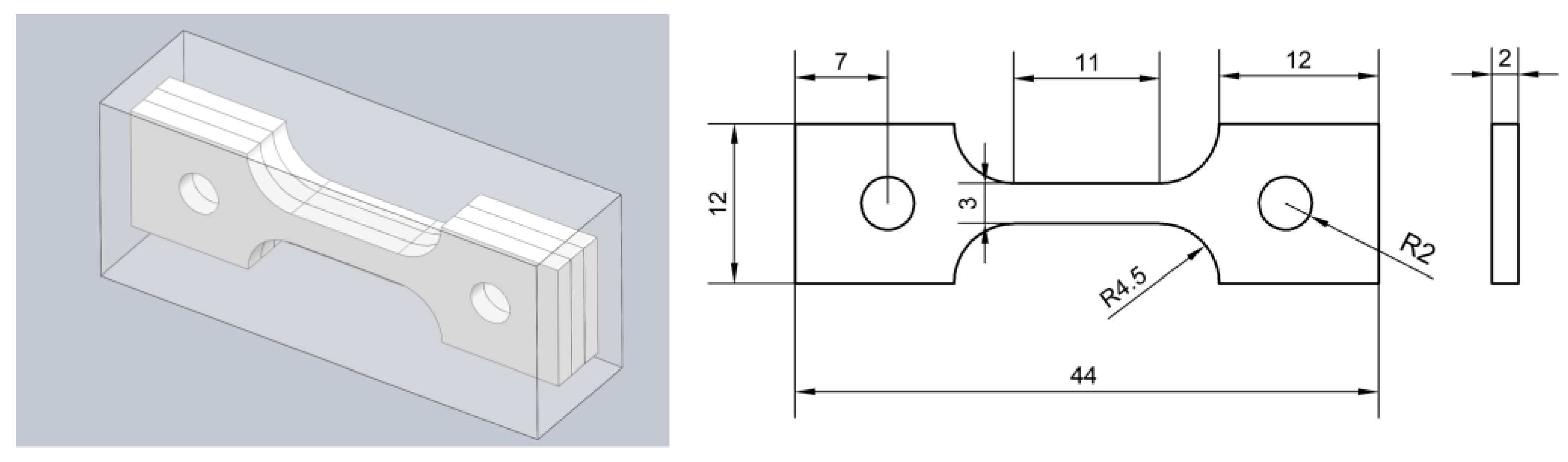
2.3. Pre-Charged Hydrogen Slow Strain Rate Tensile Test
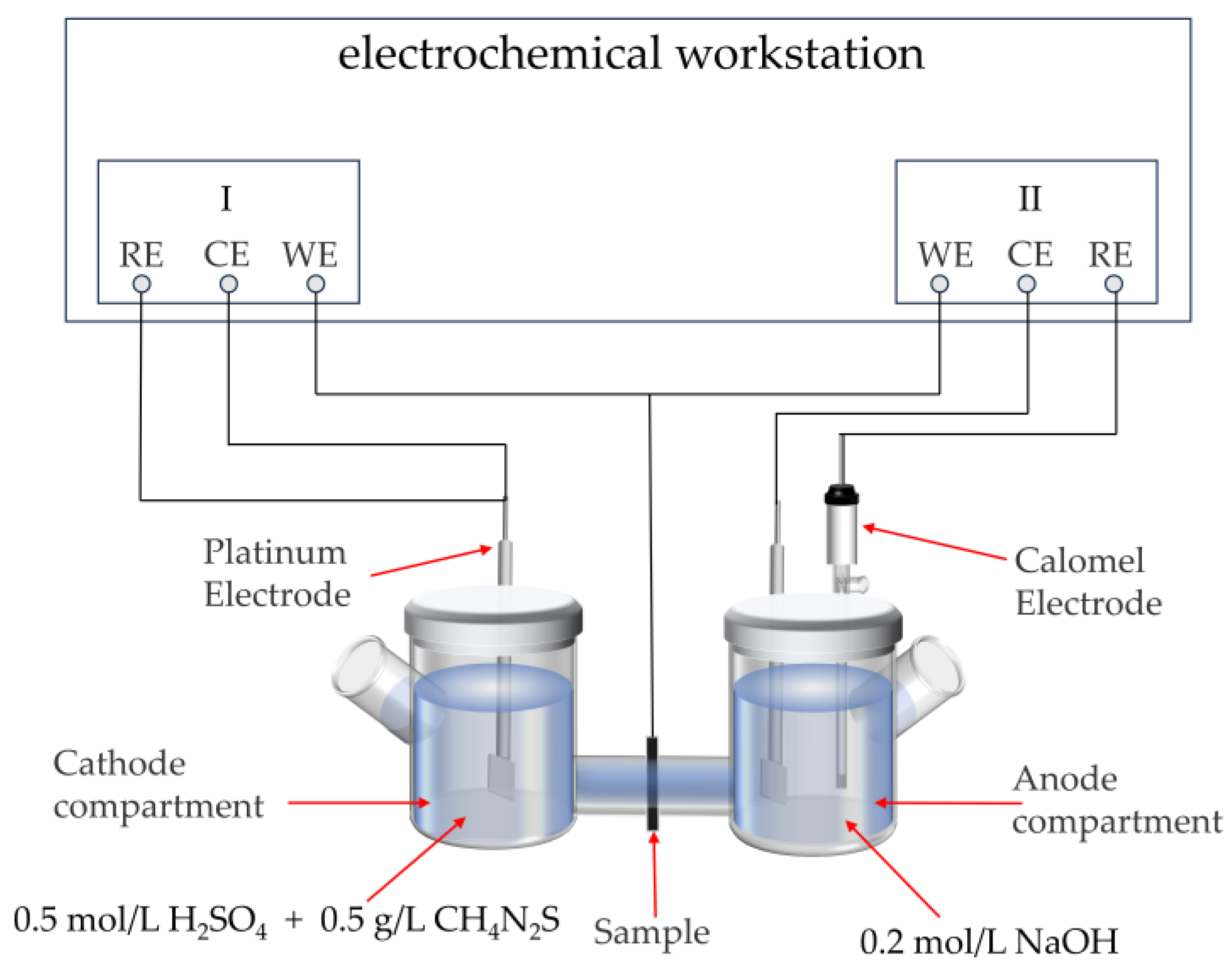
2.4. Hydrogen Permeation Test
2.5. Hydrogen Microprint Technique Test
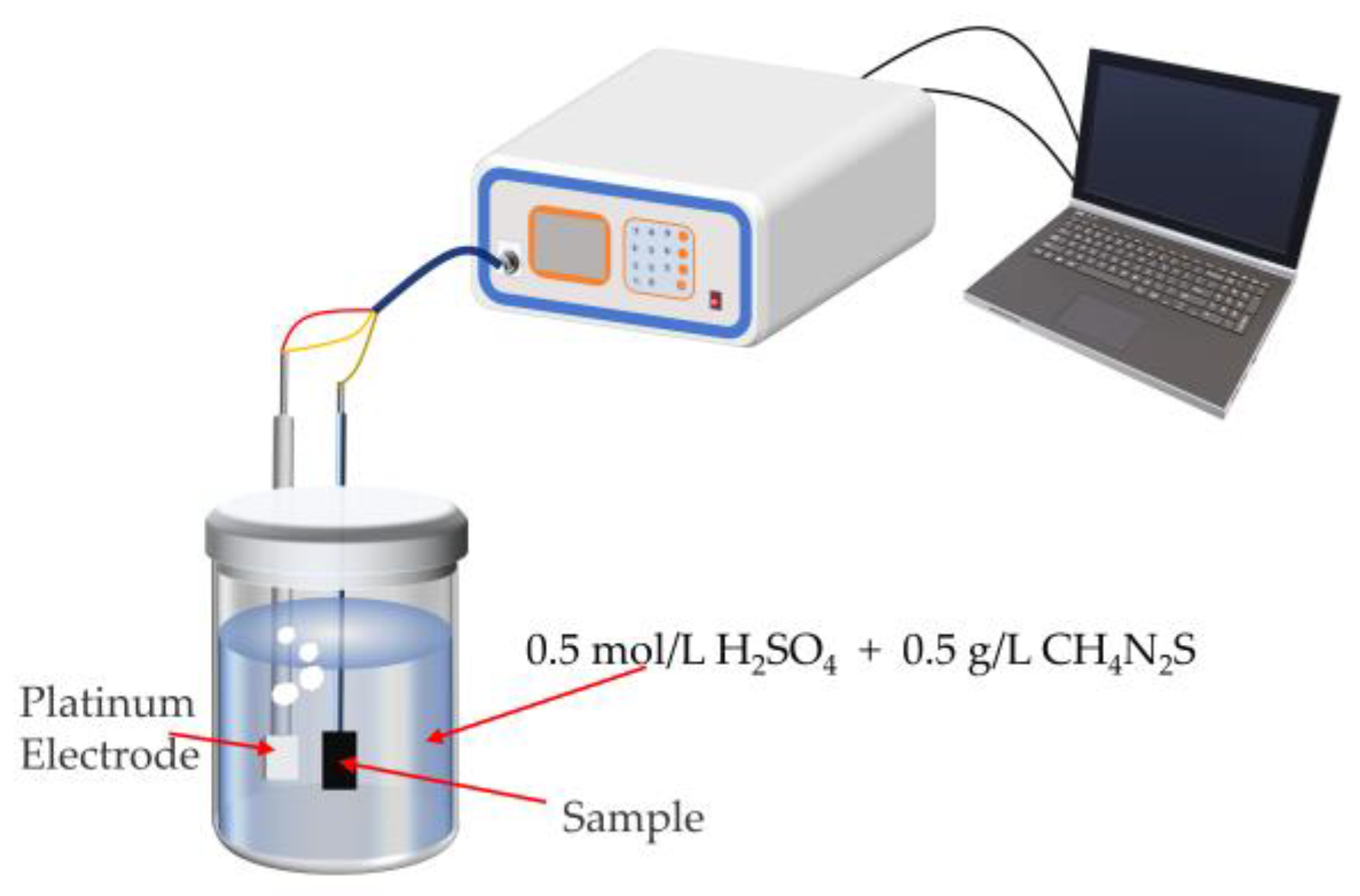
2.6. Hydrogen-Charged Cracking Test
3. Results
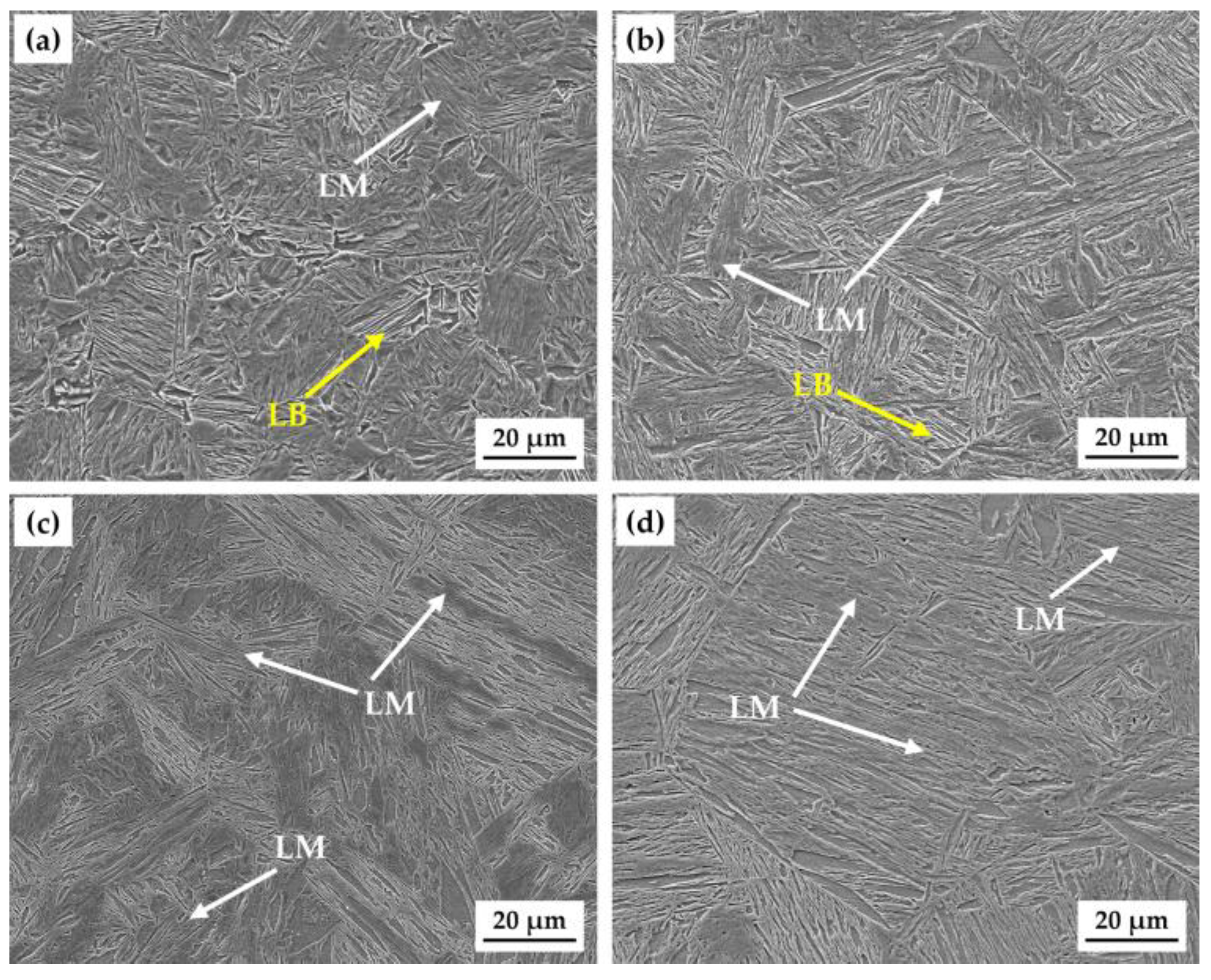
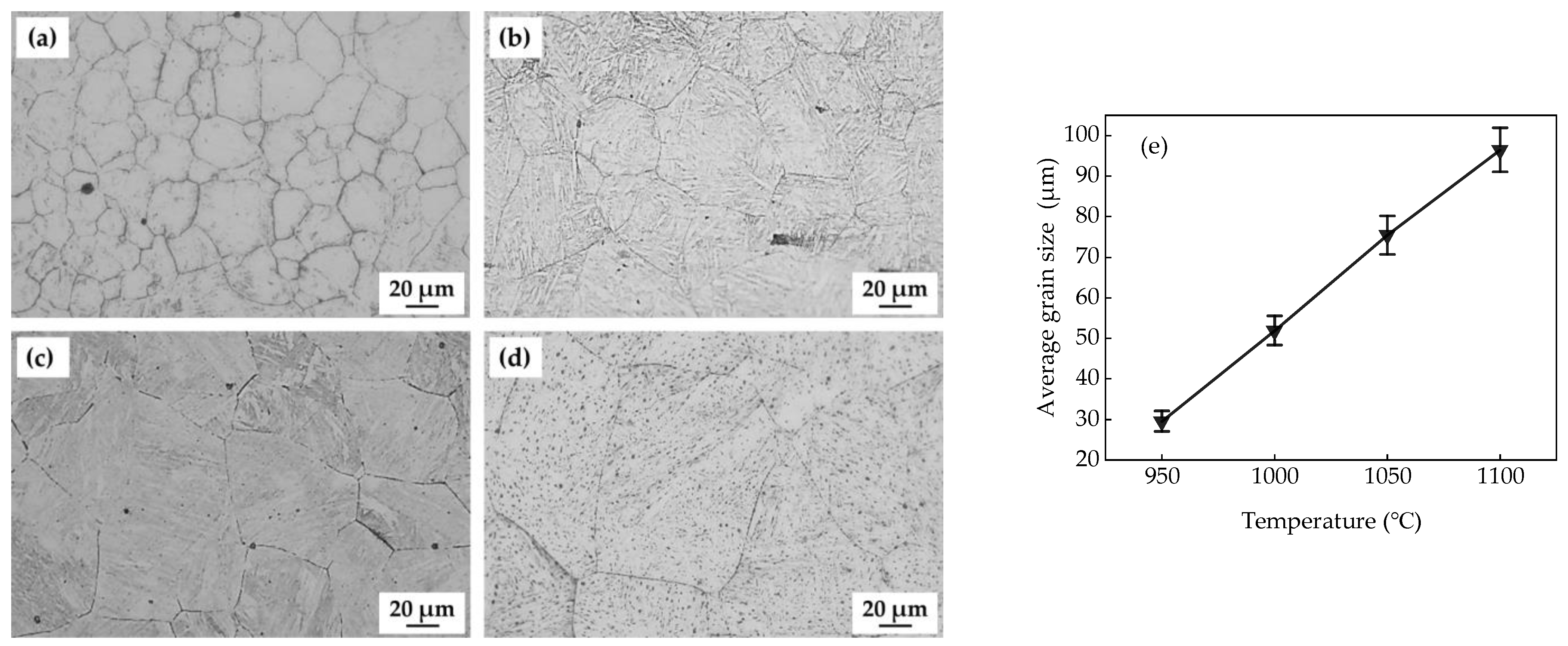
3.1. Microstructure and Hardness
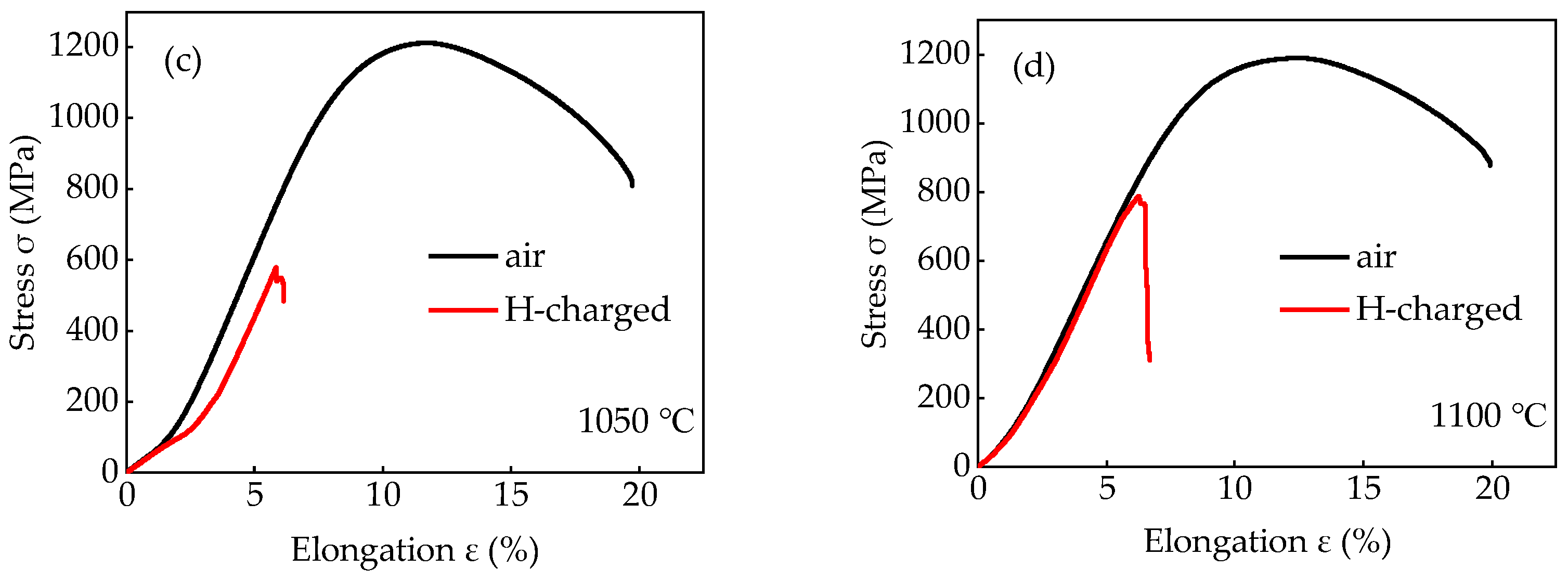
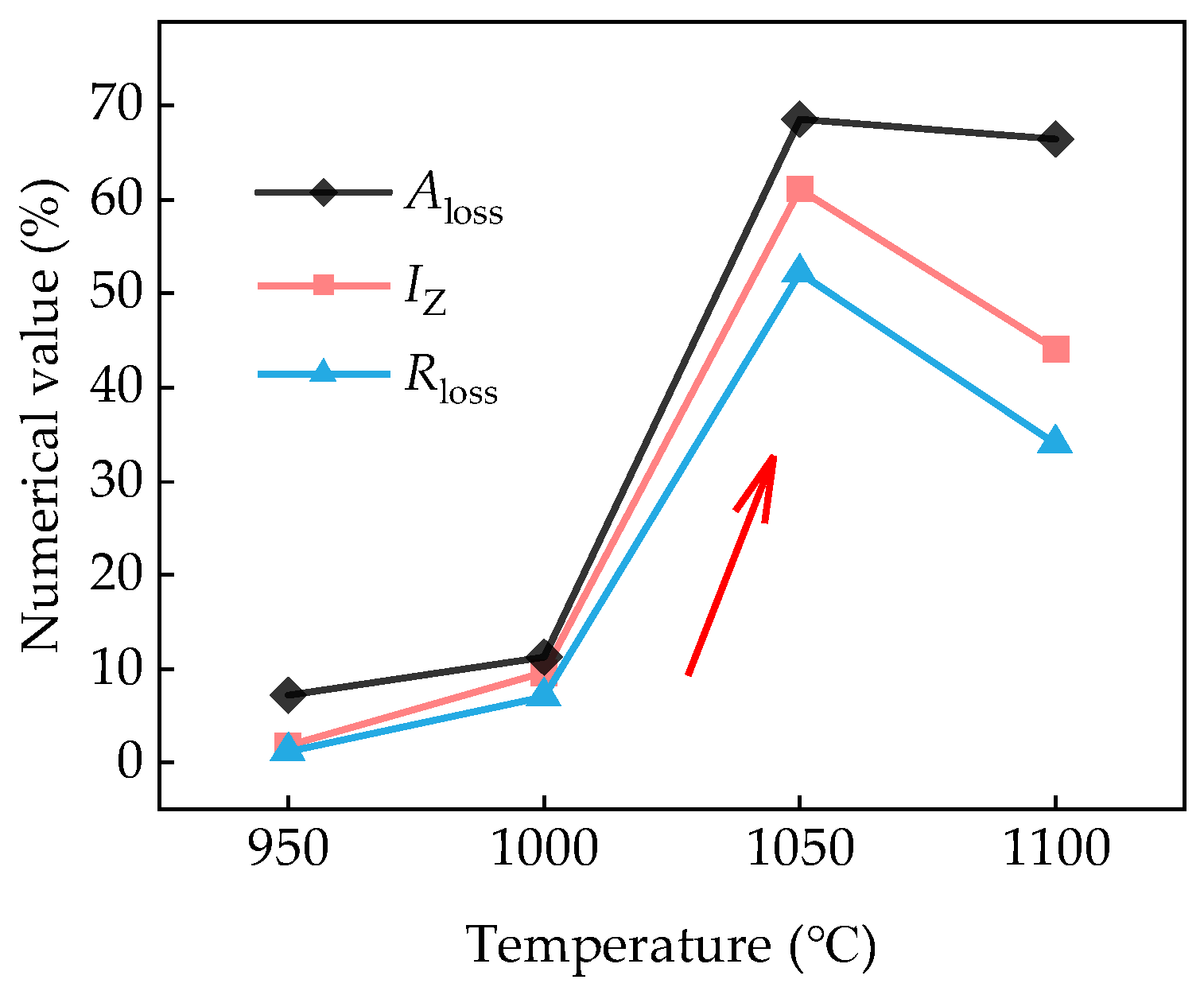
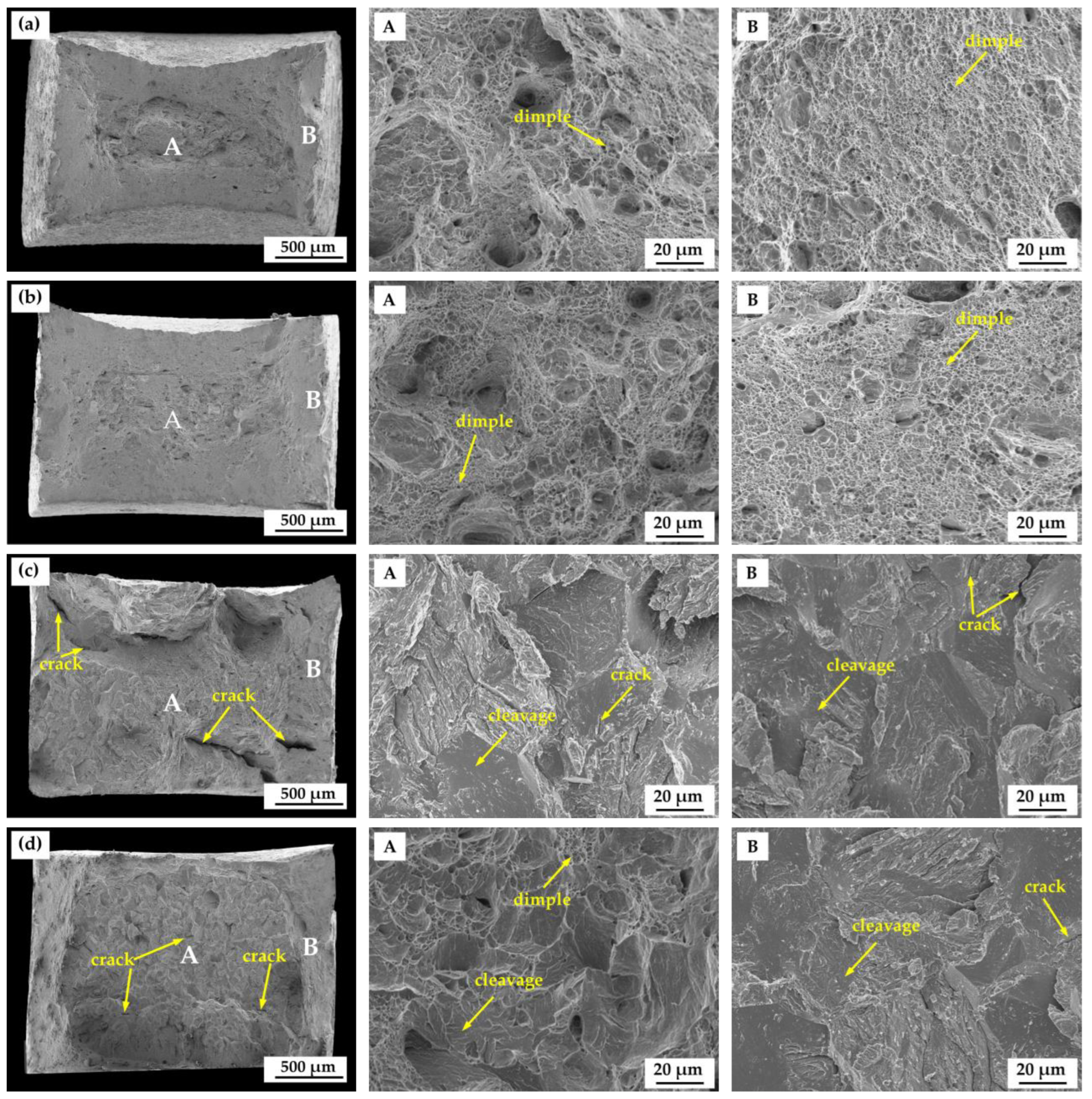
3.2. Pre-Charged Hydrogen Slow Strain Rate Tensile Test
3.3. Hydrogen Permeation
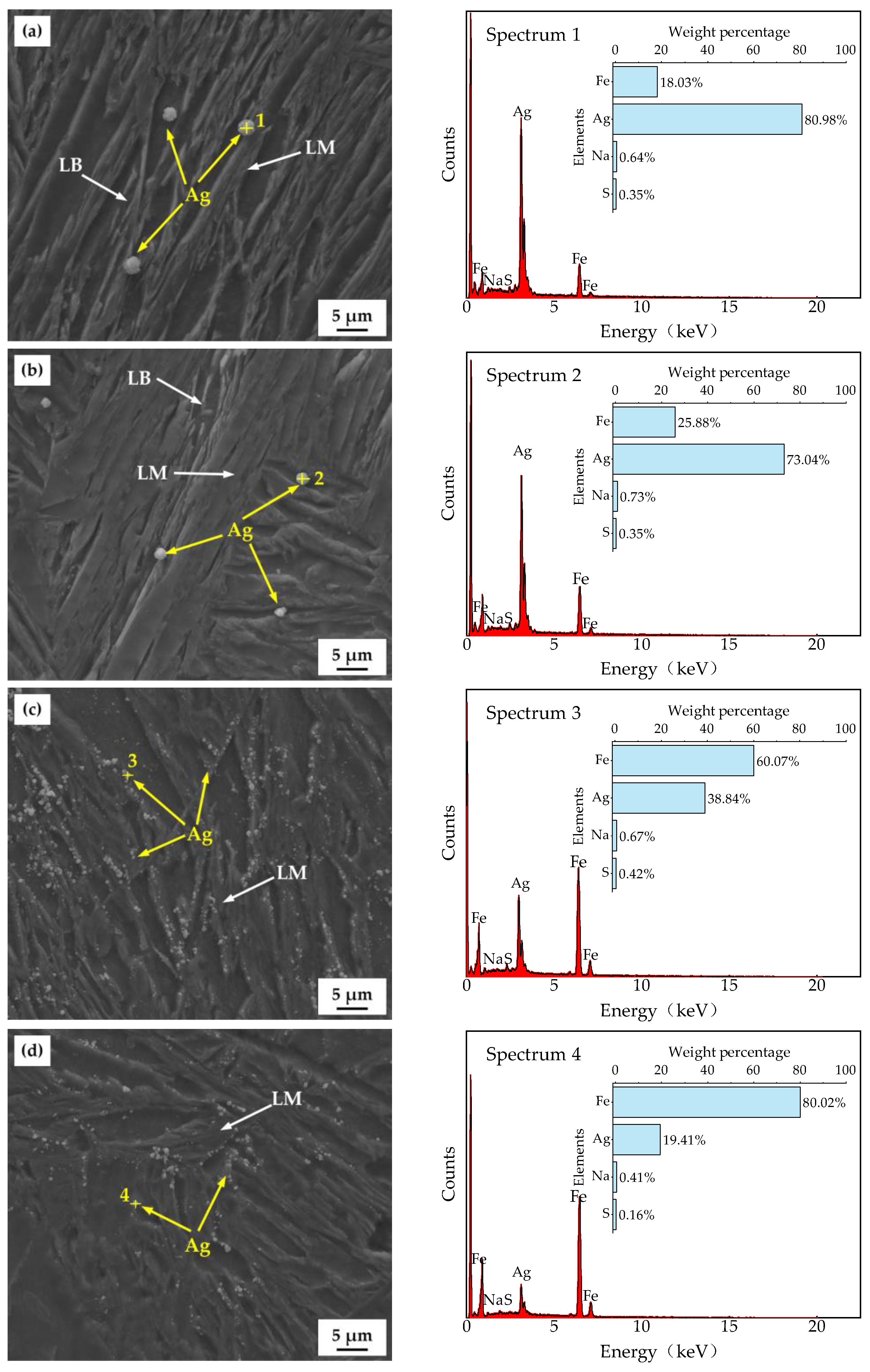
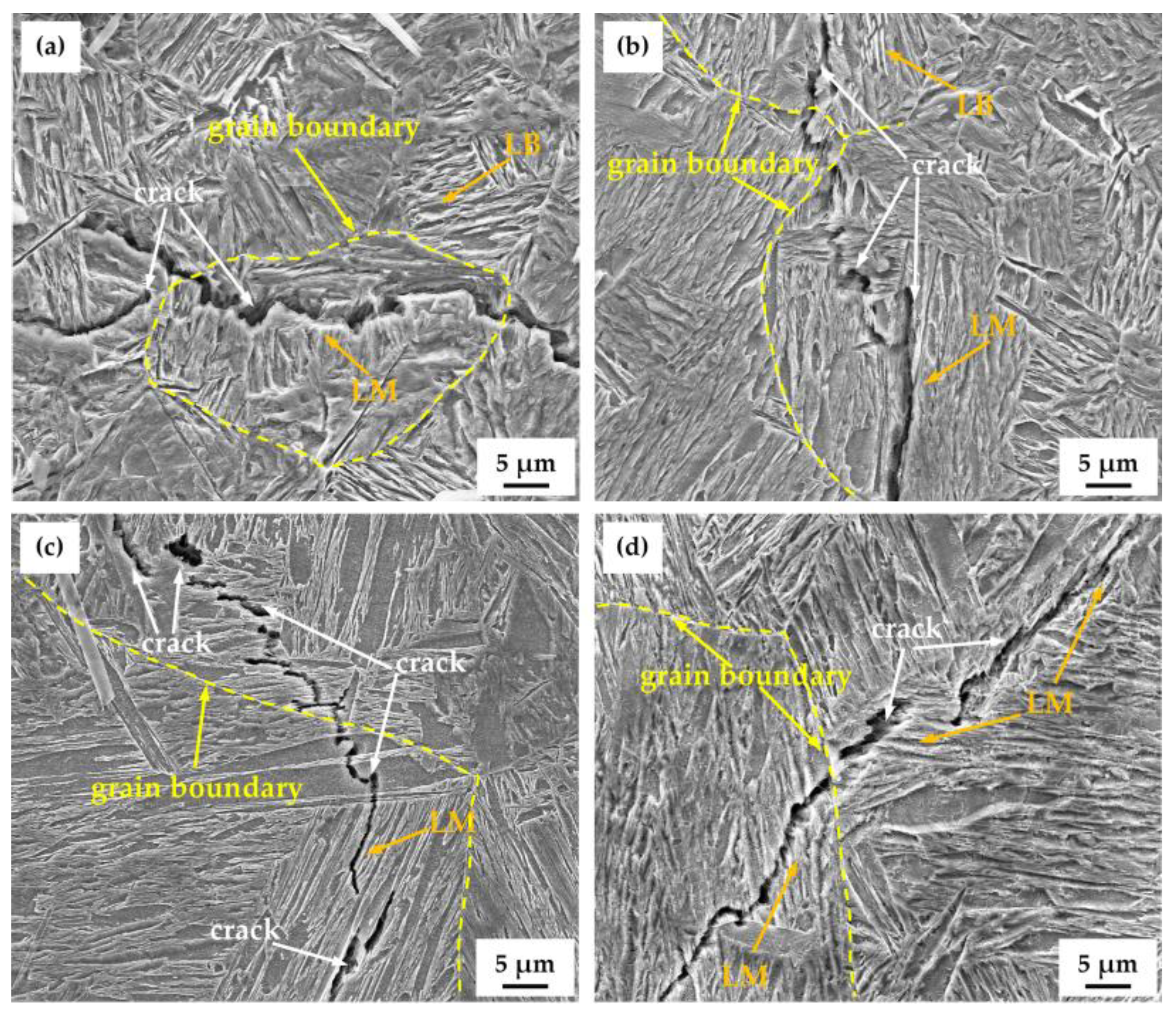
3.4. Hydrogen Microprint Technique and Hydrogen-Charged Cracking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Ilyushechkin, A.; Schoeman, L.; Carter, L.; Hla, S.S. Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale. Hydrogen 2023, 4, 599–619. [Google Scholar] [CrossRef]
- Johnson, W.H. On Some Remarkable Changes Produced in Iron and Steel by the Action of Hydrogen and Acids. Nature 1875, 11, 393. [Google Scholar] [CrossRef]
- Mousavi Anijdan, S.H.; Arab, G.; Sabzi, M.; Sadeghi, M.; Eivani, A.R.; Jafarian, H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an API X65 pipeline steel in H2S containing environment: Influence of heat treatment and its subsequent microstructural changes. J. Mater. Res. Technol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Fernández-Sousa, R.; Betegón, C.; Martínez-Pañeda, E. Analysis of the influence of microstructural traps on hydrogen assisted fatigue. Acta Mater. 2020, 199, 253–263. [Google Scholar] [CrossRef]
- Woodtli, J.; Kieselbach, R. Damage due to hydrogen embrittlement and stress corrosion cracking. Eng. Fail. Anal. 2000, 7, 427–450. [Google Scholar] [CrossRef]
- Meda, U.S.; Bhat, N.; Pandey, A.; Subramanya, K.N.; Lourdu Antony Raj, M.A. Challenges associated with hydrogen storage systems due to the hydrogen embrittlement of high strength steels. Int. J. Hydrogen Energy 2023, 48, 17894–17913. [Google Scholar] [CrossRef]
- Beidokhti, B.; Dolati, A.; Koukabi, A.H. Effects of alloying elements and microstructure on the susceptibility of the welded HSLA steel to hydrogen-induced cracking and sulfide stress cracking. Mater. Sci. Eng. A 2009, 507, 167–173. [Google Scholar] [CrossRef]
- Laureys, A.; Depover, T.; Petrov, R.; Verbeken, K. Microstructural characterization of hydrogen induced cracking in TRIP-assisted steel by EBSD. Mater. Charact. 2016, 112, 169–179. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Szpunar, J.A. Texture, local misorientation, grain boundary and recrystallization fraction in pipeline steels related to hydrogen induced cracking. Mater. Sci. Eng. A 2015, 620, 97–106. [Google Scholar] [CrossRef]
- Wu, C.; Yan, C.; Zhang, S.; Zhou, L.; Shen, M.; Tian, Z. Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments. Materials 2024, 17, 1953. [Google Scholar] [CrossRef] [PubMed]
- Yanachkov, B.; Mourdjeva, Y.; Valuiska, K.; Dyakova, V.; Kolev, K.; Kaleicheva, J.; Lazarova, R.; Katzarov, I. Effect of Hydrogen Content on the Microstructure, Mechanical Properties, and Fracture Mechanism of Low-Carbon Lath Martensite Steel. Metals 2024, 14, 1340. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Fu, L.; Li, G.; Li, W.; Yan, L. Mechanism of the Interaction Between Hydrogen, Microstructure, and Mechanical Properties in Low-Alloy High-Strength Marine Steel. J. Mater. Eng. Perform. 2024, 33, 9557–9569. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Du, Y.; Du, L.; Wang, H.; Liu, Z.; Su, G. Effect of microstructure refinement on hydrogen-induced damage behavior of low alloy high strength steel for flexible riser. Mater. Sci. Eng. A 2019, 765, 138278. [Google Scholar] [CrossRef]
- Sun, J.; Hensel, J.; Klassen, J.; Nitschke-Pagel, T.; Dilger, K. Solid-state phase transformation and strain hardening on the residual stresses in S355 steel weldments. J. Mater. Process. Technol. 2019, 265, 173–184. [Google Scholar] [CrossRef]
- Gu, B.; Gu, K.; Xu, G.; Gao, L.; Yu, Y. Fracture and electrochemical behavior of S355 steel welded joint under High-Frequency vibration. Eng. Fract. Mech. 2024, 306, 110208. [Google Scholar] [CrossRef]
- Gu, B.; Chu, J.; Zhang, H.; Gao, L.; Xu, G.; Yue, C. Effects of ultrasonic impact on surface characterization of S355 steel welded joint. Mater. Today Commun. 2024, 40, 109878. [Google Scholar] [CrossRef]
- Niklas, K.; Bera, A.; Garbatov, Y. Impact of steel grade on a ship colliding with an offshore wind turbine monopile supporting structure. Ocean Eng. 2023, 287, 115899. [Google Scholar] [CrossRef]
- Zurnadzhy, V.; Stavrovskaia, V.; Chabak, Y.; Petryshynets, I.; Efremenko, B.; Wu, K.; Efremenko, V.; Brykov, M. Enhancing the Tensile Properties and Ductile-Brittle Transition Behavior of the EN S355 Grade Rolled Steel via Cost-Saving Processing Routes. Materials 2024, 17, 1958. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Liang, J.; Su, J.; Yang, Z.; Zhang, B.; Sheng, G. Correlation between the microstructure and hydrogen embrittlement resistance in a precipitation-hardened martensitic stainless steel. Corros. Sci. 2021, 182, 109260. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z.; Tompkins, F.C. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1997, 270, 90–102. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Li, W.; Fu, H.; Liu, S.; Wang, Y.; Li, J. Effect of hydrogen trapping on hydrogen permeation in a 2205 duplex stainless steel: Role of austenite–ferrite interface. Corros. Sci. 2022, 202, 110332. [Google Scholar] [CrossRef]
- Yao, C.; Ming, H.; Chen, J.; Wang, J.; Han, E.-H. Effect of cold deformation on the hydrogen permeation behavior of X65 pipeline steel. Coatings 2023, 13, 280. [Google Scholar] [CrossRef]
- Ovejero-García, J. Hydrogen microprint technique in the study of hydrogen in steels. J. Mater. Sci. 1985, 20, 2623–2629. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. 21—Physical metallurgy of steels. In Physical Metallurgy, 5th ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Oxford, UK, 2014; pp. 2157–2214. ISBN 978-0-444-53770-6. [Google Scholar]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 1999, 273–275, 40–57. [Google Scholar] [CrossRef]
- De-Castro, D.; Eres-Castellanos, A.; Vivas, J.; Caballero, F.G.; San-Martín, D.; Capdevila, C. Morphological and crystallographic features of granular and lath-like bainite in a low carbon microalloyed steel. Mater. Charact. 2022, 184, 111703. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Jiang, W.; Wang, Y.; Yang, M. Numerical Simulation of Hydrogen Diffusion in X80 Welded Joint Under the Combined Effect of Residual Stress and Microstructure Inhomogeneity. Acta Metall. Sin. 2019, 55, 258–266. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Rahman, K.M.M.; Ouellet, R.; Szpunar, J.A. An extensive study of hydrogen-induced cracking susceptibility in an API X60 sour service pipeline steel. Int. J. Hydrogen Energy 2016, 41, 4185–4197. [Google Scholar] [CrossRef]
- Yazdipour, N.; Haq, A.J.; Muzaka, K.; Pereloma, E.V. 2D modelling of the effect of grain size on hydrogen diffusion in X70 steel. Comput. Mater. Sci. 2012, 56, 49–57. [Google Scholar] [CrossRef]
- Jo, J.W.; Seo, H.J.; Jung, B.-I.; Choi, S.; Lee, C.S. Effect of bainite fraction on hydrogen embrittlement of bainite/martensite steel. Mater. Sci. Eng. A 2021, 814, 141226. [Google Scholar] [CrossRef]
- Lee, Y.; Gangloff, R.P. Measurement and Modeling of Hydrogen Environment–Assisted Cracking of Ultra-High-Strength Steel. Metall. Mater. Trans. A 2007, 38, 2174–2190. [Google Scholar] [CrossRef]
- Yao, J.; Cahoon, J.R. Experimental studies of grain boundary diffusion of hydrogen in metals. Acta Metall. Mater. 1991, 39, 119–126. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Sharma, L.; Chhibber, R. Mechanical properties and hydrogen induced cracking behaviour of API X70 SAW weldments. Int. J. Press. Vessels Pip. 2018, 165, 193–207. [Google Scholar] [CrossRef]
- Masoumi, M.; Santos, L.P.M.; Bastos, I.N.; Tavares, S.S.M.; da Silva, M.J.G.; de Abreu, H.F.G. Texture and grain boundary study in high strength Fe–18Ni–Co steel related to hydrogen embrittlement. Mater. Des. 2016, 91, 90–97. [Google Scholar] [CrossRef]
- Tsay, L.W.; Lu, H.L.; Chen, C. The effect of grain size and aging on hydrogen embrittlement of a maraging steel. Corros. Sci. 2008, 50, 2506–2511. [Google Scholar] [CrossRef]
- Kimura, A.; Birnbaum, H.K. Hydrogen induced grain boundary fracture in high purity nickel and its alloys—Enhanced hydrogen diffusion along grain boundaries. Acta Metall. 1988, 36, 757–766. [Google Scholar] [CrossRef]
- Calder, R.D.; Elleman, T.S.; Verghese, K. Grain boundary diffusion of tritium in 304- and 316-stainless steels. J. Nucl. Mater. 1973, 46, 46–52. [Google Scholar] [CrossRef]
- Prawoto, Y.; Jasmawati, N.; Sumeru, K. Effect of Prior Austenite Grain Size on the Morphology and Mechanical Properties of Martensite in Medium Carbon Steel. J. Mater. Sci. Technol. 2012, 28, 461–466. [Google Scholar] [CrossRef]
- Schaffner, T.; Hartmaier, A.; Kokotin, V.; Pohl, M. Analysis of hydrogen diffusion and trapping in ultra-high strength steel grades. J. Alloys Compd. 2018, 746, 557–566. [Google Scholar] [CrossRef]
- Hu, C.; Xia, S.; Li, H.; Liu, T.; Zhou, B.; Chen, W. Effect of grain boundary network on the intergranular stress corrosion cracking of 304 stainless steel. Acta Met. Sin 2011, 47, 939–945. [Google Scholar] [CrossRef]
- Venezuela, J.; Lim, F.Y.; Liu, L.; James, S.; Zhou, Q.; Knibbe, R.; Zhang, M.; Li, H.; Dong, F.; Dargusch, M.S.; et al. Hydrogen embrittlement of an automotive 1700 MPa martensitic advanced high-strength steel. Corros. Sci. 2020, 171, 108726. [Google Scholar] [CrossRef]
- Venezuela, J.; Gray, E.; Liu, Q.; Zhou, Q.; Tapia-Bastidas, C.; Zhang, M.; Atrens, A. Equivalent hydrogen fugacity during electrochemical charging of some martensitic advanced high-strength steels. Corros. Sci. 2017, 127, 45–58. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Nb | Al | Cr | Cu | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.150 | 0.310 | 1.340 | 0.007 | 0.003 | 0.003 | 0.032 | 0.021 | 0.016 | 0.009 | Balanced |
| Yield Strength/MPa | Tensile Strength/MPa | Elongation Rate/% | Impact Toughness at −20 °C/J |
|---|---|---|---|
| 355~400 | 470~510 | 22~25 | 47~87 |
| Quenching Temperature/°C | ||||
|---|---|---|---|---|
| 950 | 1000 | 1050 | 1100 | |
| I∞/A cm−2 | 1.48 × 10−9 | 1.27 × 10−9 | 8.45 × 10−10 | 1.00 × 10−9 |
| J∞/mol·cm−2·s−1 | 1.53 × 10−9 | 1.32 × 10−9 | 8.76 × 10−10 | 1.04 × 10−9 |
| Deff/cm2·s−1 | 4.17 × 10−6 | 3.55 × 10−6 | 2.08 × 10−6 | 2.49 × 10−6 |
| C0/mol·cm−3 | 3.67 × 10−5 | 3.71 × 10−5 | 4.20 × 10−5 | 4.17 × 10−5 |
| NT/mol·cm−3 | 3.74 × 10−3 | 4.45 × 10−3 | 8.59 × 10−3 | 7.14 × 10−3 |
| Nr/mol·cm−3 | 2.04 × 10−3 | 2.72 × 10−3 | 5.74 × 10−3 | 3.89 × 10−3 |
| Nir/mol·cm−3 | 1.70 × 10−3 | 1.73 × 10−3 | 3.25 × 10−3 | 2.85 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Zhang, S.; Zhou, L.; Tian, Z.; Shen, M.; Liu, X. Effect of Quenching Temperature on Microstructure and Hydrogen-Induced Cracking Susceptibility in S355 Steel. Materials 2025, 18, 1161. https://doi.org/10.3390/ma18051161
Yan C, Zhang S, Zhou L, Tian Z, Shen M, Liu X. Effect of Quenching Temperature on Microstructure and Hydrogen-Induced Cracking Susceptibility in S355 Steel. Materials. 2025; 18(5):1161. https://doi.org/10.3390/ma18051161
Chicago/Turabian StyleYan, Chunyan, Shenglin Zhang, Lingchuan Zhou, Zhanpeng Tian, Mengdie Shen, and Xinyi Liu. 2025. "Effect of Quenching Temperature on Microstructure and Hydrogen-Induced Cracking Susceptibility in S355 Steel" Materials 18, no. 5: 1161. https://doi.org/10.3390/ma18051161
APA StyleYan, C., Zhang, S., Zhou, L., Tian, Z., Shen, M., & Liu, X. (2025). Effect of Quenching Temperature on Microstructure and Hydrogen-Induced Cracking Susceptibility in S355 Steel. Materials, 18(5), 1161. https://doi.org/10.3390/ma18051161





