Recent Advances in In Vivo Neurochemical Monitoring
Abstract
1. Introduction
1.1. Neurochemicals
1.2. In Vivo Neurochemical Sensing Challenges
1.2.1. Quantitative Analysis
1.2.2. The Inflammatory Response
2. Electrochemical Sensors
2.1. Electrochemical Detection Methods
2.1.1. Chronoamperometry
2.1.2. Differential Pulse Voltammetry
2.1.3. Square Wave Voltammetry
2.1.4. Fast Scan Cyclic Voltammetry
2.2. Materials for Electrochemical Sensors
2.2.1. Carbon Based Electrodes
2.2.2. Metal Electrodes
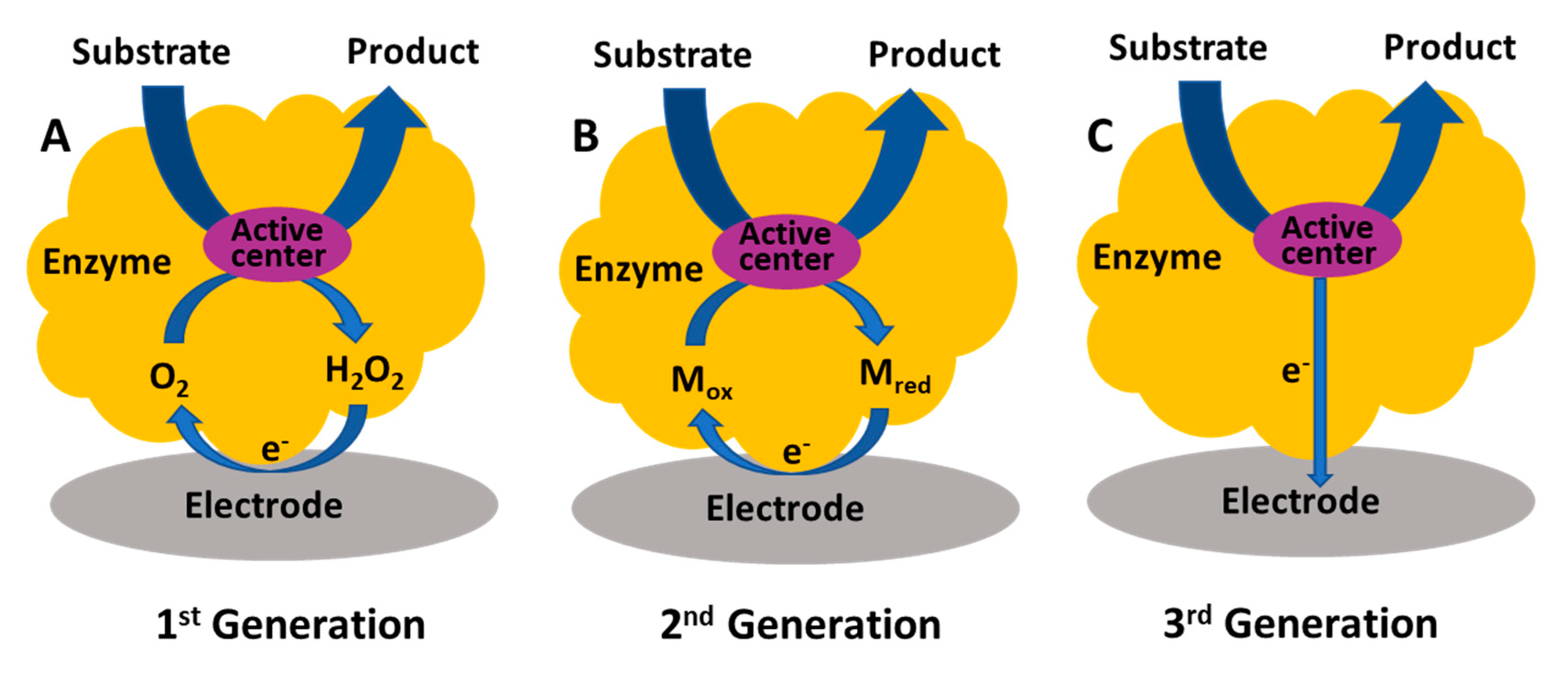
2.2.3. Enzyme Biosensors
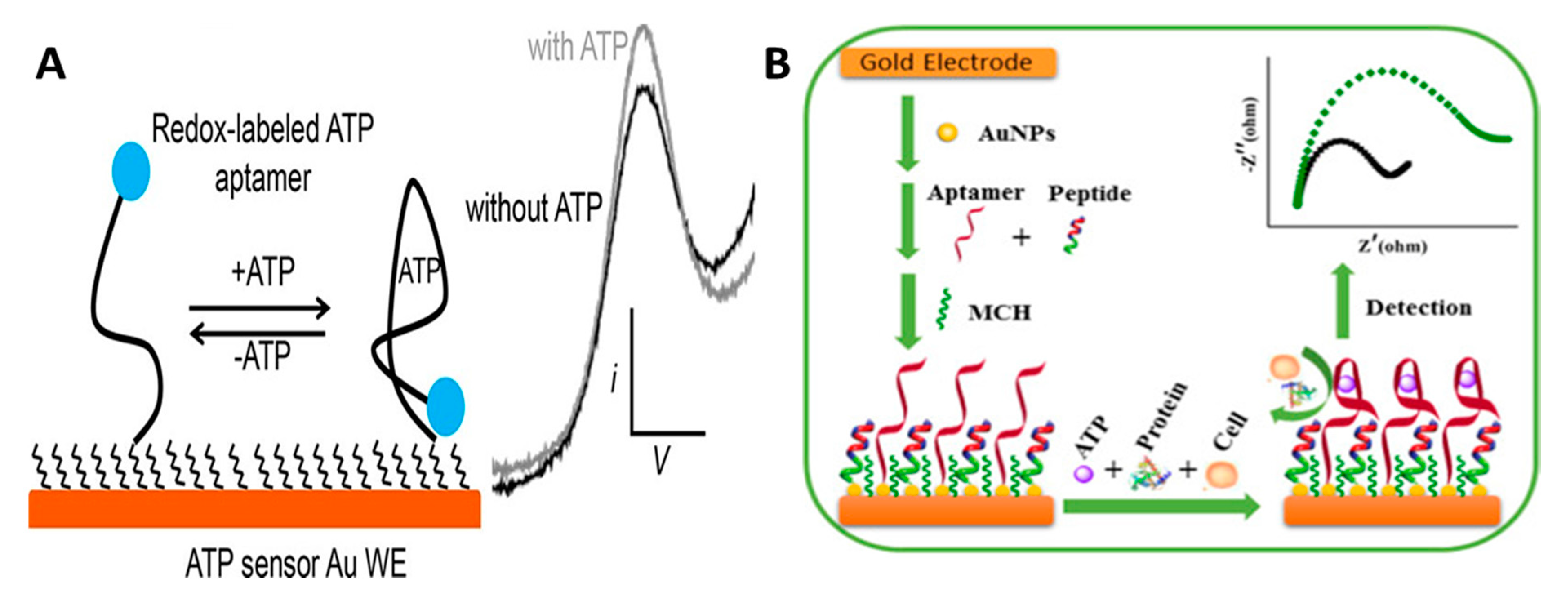
2.2.4. Aptamer Biosensors
2.3. In Vivo Challenges of Electrochemical Sensors
3. Optical Sensors
3.1. Fluorescence Sensors
3.2. Chemiluminescence Sensors
3.3. In Vivo Challenges of Optical Sensors
4. Alternative Methods
5. Designing an In Vivo Sensing Experiment
5.1. Comparing In Vivo Neurochemical Sensing Techniques
5.2. Addressing the Inflammatory Responses
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef]
- Cragg, S.J. Variable Dopamine Release Probability and Short-Term Plasticity between Functional Domains of the Primate Striatum. J. Neurosci. 2003, 23, 4378–4385. [Google Scholar] [CrossRef]
- Di Chiara, G.; Bassareo, V. Reward system and addiction: What dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007, 7, 69–76. [Google Scholar] [CrossRef]
- Kawagoe, R.; Takikawa, Y.; Hikosaka, O. Reward-Predicting Activity of Dopamine and Caudate Neurons—A Possible Mechanism of Motivational Control of Saccadic Eye Movement. J. Neurophysiol. 2004, 91, 1013–1024. [Google Scholar] [CrossRef]
- Horn, A.S. The Neurobiology of Dopamine; Academic Press: London, UK, 1979. [Google Scholar]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; Wade-Martins, R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef]
- Mikell, C.B.; McKhann, G.M. Regulation of parkinsonian motor behaviors by optogenetic control of Basal Ganglia circuitry. Neurosurgery 2010, 67, 28–29. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Zhao, H.; Xie, J.; Xu, S.; Gao, F.; Wang, M.; Xing, G. Microelectrode arrays modified with nanocomposites for monitoring dopamine and spike firings under deep brain stimulation in rat models of parkinson’s disease. ACS Sens. 2019, 4, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.B.; Mukhopadhyay, C.K.; Ghosh, M.K. Vitamin C: A potential saviour against free radical-induced oxidative damage. Curr. Sci. 1995, 747–751. [Google Scholar]
- Abdul-Muneer, P.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Sardinha, V.M.; Guerra-Gomes, S.; Araque, A.; Sousa, N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015, 38, 535–549. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.-C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond Black-and-White. J. Neurosci. 2018, 38, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R. Lactate transport and metabolism in the human brain: Implications for the astrocyte-neuron lactate shuttle hypothesis. J. Neurosci. 2011, 31, 4768–4770. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.L.H.; Ejalloh, I.; Hutchinson, P.J. Glycolysis and the significance of lactate in traumatic brain injury. Front. Neurosci. 2015, 9, 112. [Google Scholar] [CrossRef]
- Patet, C.; Suys, T.; Carteron, L.; Oddo, M. Cerebral Lactate Metabolism after Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Hadjihambi, A.; Karagiannis, A.; Theparambil, S.M.; Ackland, G.L.; Gourine, A.V. The effect of general anaesthetics on brain lactate release. Eur. J. Pharmacol. 2020, 881, 173188. [Google Scholar] [CrossRef]
- Rogers, M.L.; Boutelle, M.G. Real-Time Clinical Monitoring of Biomolecules. Annu. Rev. Anal. Chem. 2013, 6, 427–453. [Google Scholar] [CrossRef]
- Lippert, R.N.; Cremer, A.L.; Thanarajah, S.E.; Korn, C.; Jahans-Price, T.; Burgeno, L.M.; Tittgemeyer, M.; Brüning, J.C.; Walton, M.E.; Backes, H. Time-dependent assessment of stimulus-evoked regional dopamine release. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.; Wightman, R.M. Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Ferapontova, E.E. Electrochemical Analysis of Dopamine: Perspectives of Specific In Vivo Detection. Electrochim. Acta 2017, 245, 664–671. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Cai, X.; Xie, Y.; Wang, T.; Cheng, D.; Li, L.; Li, R.; Deng, Y.; Ding, H. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection. Microsyst. Nanoeng. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Qi, L.; Thomas, E.; White, S.H.; Smith, S.K.; Lee, C.A.; Wilson, L.R.; Sombers, L.A. Unmasking the Effects of L-DOPA on Rapid Dopamine Signaling with an Improved Approach for Nafion Coating Carbon-Fiber Microelectrodes. Anal. Chem. 2016, 88, 8129–8136. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef] [PubMed]
- Heien, M.L.; Khan, A.S.; Ariansen, J.L.; Cheer, J.F.; Phillips, P.E.; Wassum, K.M.; Wightman, R.M. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. USA 2005, 102, 10023–10028. [Google Scholar] [CrossRef]
- Sombers, L.A.; Beyene, M.; Carelli, R.M.; Wightman, R.M. Synaptic Overflow of Dopamine in the Nucleus Accumbens Arises from Neuronal Activity in the Ventral Tegmental Area. J. Neurosci. 2009, 29, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.; Martig, A.K.; Deisseroth, K.; Witten, I.B.; Moghaddam, B. Dopamine Modulation of Prefrontal Cortex Activity Is Manifold and Operates at Multiple Temporal and Spatial Scales. Cell Rep. 2019, 27, 99–114. [Google Scholar] [CrossRef]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef]
- Gifford, R.; Batchelor, M.M.; Lee, Y.; Gokulrangan, G.; Meyerhoff, M.E.; Wilson, G.S. Mediation ofin vivo glucose sensor inflammatory response via nitric oxide release. J. Biomed. Mater. Res. Part A 2005, 75, 755–766. [Google Scholar] [CrossRef]
- Takmakov, P.; Ruda, K.; Phillips, K.S.; Isayeva, I.S.; Krauthamer, V.; Welle, C.G. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. J. Neural Eng. 2015, 12, 026003. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.-X.; Zhang, Y.; Zhai, L.-F.; Sun, M. Corrosion of graphite electrode in electrochemical advanced oxidation processes: Degradation protocol and environmental implication. Chem. Eng. J. 2018, 344, 410–418. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef]
- Huang, X.; Deng, X.; Qi, W.; Wu, D. Highly sensitive luminol electrochemiluminescence immunosensor based on platinum-gold alloy hybrid functionalized zinc oxide nanocomposites for catalytic amplification. Sens. Actuators B Chem. 2018, 273, 466–472. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Tian, Y. Electrochemical in-vivo sensors using nanomaterials made from carbon species, noble metals, or semiconductors. Microchim. Acta 2014, 181, 1471–1484. [Google Scholar] [CrossRef]
- Coustan, L.; Shul, G.; Belanger, D. Electrochemical behavior of platinum, gold and glassy carbon electrodes in water-in-salt electrolyte. Electrochem. Commun. 2017, 77, 89–92. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, K.; Xu, Y.; Li, L.; Luo, J.; Wang, C. Facile development of Au-ring microelectrode for in vivo analysis using non-toxic polydopamine as multifunctional material. Biosens. Bioelectron. 2016, 78, 274–280. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Dong, H.; Yu, Q.; Zhang, S.; Chen, H. Sensitive detection of dopamine using a platinum microelectrode modified by reduced graphene oxide and gold nanoparticles. J. Electroanal. Chem. 2019, 848, 113–244. [Google Scholar] [CrossRef]
- Suzuki, A.; Ivandini, T.A.; Yoshimi, K.; Fujishima, A.; Oyama, G.; Nakazato, T.; Hattori, N.; Kitazawa, S.; Einaga, Y. Fabrication, Characterization, and Application of Boron-Doped Diamond Microelectrodes for in Vivo Dopamine Detection. Anal. Chem. 2007, 79, 8608–8615. [Google Scholar] [CrossRef]
- Scoggin, J.L.; Tan, C.; Nguyen, N.H.; Kansakar, U.; Madadi, M.; Siddiqui, S.; Arumugam, P.U.; DeCoster, M.A.; Murray, T.A. An enzyme-based electrochemical biosensor probe with sensitivity to detect astrocytic versus glioma uptake of glutamate in real time in vitro. Biosens. Bioelectron. 2019, 126, 751–757. [Google Scholar] [CrossRef]
- Tan, C.; Doughty, P.T.; Magee, K.; Murray, T.A.; Siddiqui, S.; Arumugam, P.U. Effect of Process Parameters on Electro-chemical Performance of a Glutamate Microbiosensor. J. Electrochem. Soc. 2020, 167, 027528. [Google Scholar] [CrossRef]
- Njagi, J.; Chernov, M.M.; Leiter, J.C.; Andreescu, S. Amperometric Detection of Dopamine in Vivo with an Enzyme Based Carbon Fiber Microbiosensor. Anal. Chem. 2010, 82, 989–996. [Google Scholar] [CrossRef]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.A.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbicacid. Biosens. Bioelectron. 2013, 42, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zeng, C.; Ye, J.; Sun, Y. Simultaneous in vivo voltammetric determination of dopamine and 5-Hydroxytryptamine in the mouse brain. Appl. Surf. Sci. 2018, 455, 646–652. [Google Scholar] [CrossRef]
- Tan, C.; Dutta, G.; Yin, H.; Siddiqui, S.; Arumugam, P.U. Detection of neurochemicals with enhanced sensitivity and selectivity via hybrid multiwall carbon nanotube-ultrananocrystalline diamond microelectrodes. Sens. Actuators B Chem. 2018, 258, 193–203. [Google Scholar] [CrossRef]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.-Z. Probing the electrochemical properties of graphene nanosheets for biosensing applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Hočevar, S.B.; Wang, J.; Deo, R.P.; Musameh, M.; Ogorevc, B. Carbon Nanotube Modified Microelectrode for Enhanced Voltammetric Detection of Dopamine in the Presence of Ascorbate. Electroanalisys 2005, 17, 417–422. [Google Scholar] [CrossRef]
- Njagi, J.; Ball, M.; Best, M.; Wallace, K.N.; Andreescu, S. Electrochemical quantification of serotonin in the live embryonic zebrafish intestine. Anal. Chem. 2010, 82, 1822–1830. [Google Scholar] [CrossRef]
- Osteryoung, J.G.; Osteryoung, R.A. Square wave voltammetry. Anal. Chem. 1985, 57, 101–110. [Google Scholar] [CrossRef]
- Oh, Y.; Heien, M.L.; Park, C.; Kang, Y.M.; Kim, J.; Boschen, S.L.; Shin, H.; Cho, H.U.; Blaha, C.D.; Bennet, K.E.; et al. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens. Bioelectron. 2018, 121, 174–182. [Google Scholar] [CrossRef]
- Park, J.; Takmakov, P.; Wightman, R.M. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J. Neurochem. 2011, 119, 932–944. [Google Scholar] [CrossRef]
- Spanos, M.; Gras-Najjar, J.; Letchworth, J.M.; Sanford, A.L.; Toups, J.V.; Sombers, L.A. Quantitation of Hydrogen Peroxide Fluctuations and Their Modulation of Dopamine Dynamics in the Rat Dorsal Striatum Using Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 2013, 4, 782–789. [Google Scholar] [CrossRef]
- Bennet, K.E.; Tomshine, J.R.; Min, H.-K.; Manciu, F.S.; Marsh, M.P.; Paek, S.B.; Settell, M.L.; Nicolai, E.N.; Blaha, C.D.; Kouzani, A.Z.; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci. 2016, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Garris, P.A.; Wightman, R.M.; Boulton, A.A.; Baker, G.B.; Adams, R. Regional Differences in Dopamine Release, Uptake, and Diffusion Measured by Fast-Scan Cyclic Voltammetry. Voltammetric Methods Brain Syst. 2003, 27, 179–220. [Google Scholar] [CrossRef]
- Park, C.; Oh, Y.; Shin, H.; Kim, J.; Kang, Y.M.; Sim, J.; Cho, H.U.; Lee, H.K.; Jung, S.J.; Blaha, C.D.; et al. Fast Cyclic Square-Wave Voltammetry to Enhance Neurotransmitter Selectivity and Sensitivity. Anal. Chem. 2018, 90, 13348–13355. [Google Scholar] [CrossRef] [PubMed]
- Keithley, R.B.; Takmakov, P.; Bucher, E.S.; Belle, A.M.; Owesson-White, C.A.; Park, J.; Wightman, R.M. Higher Sensitivity Dopamine Measurements with Faster-Scan Cyclic Voltammetry. Anal. Chem. 2011, 83, 3563–3571. [Google Scholar] [CrossRef]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef]
- Puthongkham, P.; Rocha, J.; Borgus, J.R.; Ganesana, M.; Wang, Y.; Chang, Y.; Gahlmann, A.; Venton, B.J. Structural Similarity Image Analysis for Detection of Adenosine and Dopamine in Fast-Scan Cyclic Voltammetry Color Plots. Anal. Chem. 2020, 92, 10485–10494. [Google Scholar] [CrossRef]
- Puthongkham, P.; Lee, S.T.; Venton, B.J. Mechanism of histamine oxidation and electropolymerization at carbon electrodes. Anal. Chem. 2019, 91, 8366–8373. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Panda, S.; Schmidt, A.C.; Sombers, L.A. Selective and Mechanically Robust Sensors for Electrochemical Measurements of Real-Time Hydrogen Peroxide Dynamics in Vivo. Anal. Chem. 2017, 90, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Woeppel, K.; Golabchi, A.; McGuier, M.; Chodapaneedi, N.; Metro, J.; Taylor, I.M.; Cui, X.T. Electro-chemical detection of exogenously administered melatonin in the brain. Analyst 2020, 145, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Ammam, M.; Fransaer, J. Highly sensitive and selective glutamate microbiosensor based on cast polyure-thane/AC-electrophoresis deposited multiwalled carbon nanotubes and then glutamate oxidase/electrosynthesized polypyrrole/Pt electrode. Biosens. Bioelectron. 2010, 25, 1597–1602. [Google Scholar] [CrossRef]
- Meunier, C.J.; Roberts, J.G.; McCarty, G.S.; Sombers, L.A. Background Signal as an in Situ Predictor of Dopamine Oxidation Potential: Improving Interpretation of Fast-Scan Cyclic Voltammetry Data. ACS Chem. Neurosci. 2017, 8, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kado, Y.; Soneda, Y.; Hatori, H.; Kodama, M. Advanced carbon electrode for electrochemical capacitors. J. Solid State Electrochem. 2019, 23, 1061–1081. [Google Scholar] [CrossRef]
- Haddon, R.C. Carbon Nanotubes; ACS Publications: Washington, DC, USA, 2002. [Google Scholar]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Ewing, A.G.; Bigelow, J.C.; Wightman, R.M. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science 1983, 221, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Zhang, F.; Adamantidis, A.; Stuber, G.D.; Bonci, A.; De Lecea, L.; Deisseroth, K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef]
- Howe, M.W.; Tierney, P.L.; Sandberg, S.G.; Phillips, P.E.M.; Graybiel, A.M. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nat. Cell Biol. 2013, 500, 575–579. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Petrovic, J.; Keithley, R.B.; Wightman, R.M. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal. Chem. 2009, 81, 9462–9471. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Wood, K.M.; Ambrose, R.E.; Wightman, R.M. In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J. Neurochem. 2011, 118, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, S.; Abdalla, A.; Robke, R.; Wood, K.M.; Zeqja, A.; Hashemi, P. In vivo histamine voltammetry in the mouse premammillary nucleus. Anal. Chem. 2015, 140, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Venton, B.J. Sawhorse Waveform Voltammetry for Selective Detection of Adenosine, ATP, and Hydrogen Peroxide. Anal. Chem. 2014, 86, 7486–7493. [Google Scholar] [CrossRef]
- Hensley, A.L.; Colley, A.R.; Ross, A.E. Real-Time Detection of Melatonin Using Fast-Scan Cyclic Voltammetry. Anal. Chem. 2018, 90, 8642–8650. [Google Scholar] [CrossRef]
- Forderhase, A.G.; Styers, H.C.; Lee, C.A.; Sombers, L.A. Simultaneous voltammetric detection of glucose and lactate fluctuations in rat striatum evoked by electrical stimulation of the midbrain. Anal. Bioanal. Chem. 2020, 412, 6611–6624. [Google Scholar] [CrossRef]
- Calhoun, S.E.; Meunier, C.J.; Lee, C.A.; Mccarty, G.S.; Sombers, L.A. Characterization of a Multiple-Scan-Rate Voltammetric Waveform for Real-Time Detection of Met-Enkephalin. ACS Chem. Neurosci. 2019, 10, 2022–2032. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Woeppel, K.M.; McGuier, M.; Golabchi, A.; Taylor, I.M.; Michael, A.C.; Cui, X.T. Real-Time Fast Scan Cyclic Voltammetry Detection and Quantification of Exogenously Administered Melatonin in Mice Brain. Front. Bioeng. Biotechnol. 2020, 8, 1343. [Google Scholar] [CrossRef]
- Holloway, A.F.; Wildgoose, G.G.; Compton, R.G.; Shao, L.; Green, M.L. The influence of edge-plane defects and oxygen containing surface groups on the voltammetry of acid-treated, annealed and “super-annealed” multiwalled carbon nanotubes. J. Solid State Electrochem. 2008, 12, 1337. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Lee, J.-J.; Rahman, A. Electrochemical Sensors Based on Carbon Nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef]
- Yang, C.; Trikantzopoulos, E.; Nguyen, M.D.; Jacobs, C.B.; Wang, Y.; Mahjouri-Samani, M.; Ivanov, I.N.; Venton, B.J. Laser treated carbon nanotube yarn microelectrodes for rapid and sensitive detection of dopamine in vivo. ACS Sens. 2016, 1, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Asrat, T.; Liu, F.; Wonnenberg, P.; Zestos, A.G. Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry. Sensors 2020, 20, 1173. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, W.; Burugapalli, K.; Moussy, F.; Li, Y.-L.; Zhong, X.-H. Nano-yarn carbon nanotube fiber based enzymatic glucose biosensor. Nanotechnology 2010, 21, 165501. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gómez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef]
- Lourenção, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Simultaneous Differential Pulse Voltammetric Determination of Ascorbic Acid and Caffeine in Pharmaceutical Formulations Using a Boron-Doped Diamond Electrode. Electroanalisys 2010, 22, 1717–1723. [Google Scholar] [CrossRef]
- Deng, Z.; Long, H.; Wei, Q.; Yu, Z.; Zhou, B.; Wang, Y.; Zhang, L.; Li, S.; Ma, L.; Xie, Y.; et al. High-performance non-enzymatic glucose sensor based on nickel-microcrystalline graphite-boron doped diamond complex electrode. Sens. Actuators B Chem. 2017, 242, 825–834. [Google Scholar] [CrossRef]
- Puthongkham, P.; Venton, B.J. Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes. ACS Sens. 2019, 4, 2403–2411. [Google Scholar] [CrossRef]
- Yuan, L.; Li, T.; Saito, K. Growth mechanism of carbon nanotubes in methane diffusion flames. Carbon 2003, 41, 1889–1896. [Google Scholar] [CrossRef]
- Rivera-Serrano, N.; Pagan, M.; Colón-Rodríguez, J.; Fuster, C.; Vélez, R.N.; Almodovar-Faria, J.; Jiménez-Rivera, C.; Cunci, L. Static and Dynamic Measurement of Dopamine Adsorption in Carbon Fiber Microelectrodes Using Electrochemical Impedance Spectroscopy. Anal. Chem. 2018, 90, 2293–2301. [Google Scholar] [CrossRef]
- Siddiqui, S.; Arumugam, P.U.; Chen, H.; Li, J.; Meyyappan, M. Characterization of Carbon Nanofiber Electrode Arrays Using Electrochemical Impedance Spectroscopy: Effect of Scaling Down Electrode Size. ACS Nano 2010, 4, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Garg, R.; Rastogi, S.; Cohen-Karni, T.; Cui, X.T. 3D Fuzzy Graphene Microelectrode Array for Neurotransmitter Sensing at Sub-cellular Spatial Resolution. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Taylor, I.M.; Du, Z.; Bigelow, E.T.; Eles, J.R.; Horner, A.R.; Catt, K.A.; Weber, S.G.; Jamieson, B.G.; Cui, X.T. Ap-tamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B 2017, 5, 2445–2458. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Q.; Zhu, F.; Xu, H.; Zhang, Y.; Xu, W.; Liao, X. An Amperometric Hydrogen Peroxide Sensor Based on Reduced Graphene Oxide/Carbon Nanotubes/Pt NPs Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2020, 15, 8771–8785. [Google Scholar] [CrossRef]
- Negahdary, M. Electrochemical aptasensors based on the gold nanostructures. Talanta 2020, 216, 120999. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Chen, S.-M.; Hung, Y.-T.; Ali, M.A.; Al-Hemaid, F.M. Electrochemical oxidation and determination of norepinephrine in the presence of acetaminophen using MnO2 nanoparticle decorated reduced graphene oxide sheets. Anal. Methods 2014, 6, 6504–6513. [Google Scholar] [CrossRef]
- Li, H.-H.; Wang, H.-H.; Li, W.-T.; Fang, X.-X.; Guo, X.-C.; Zhou, W.-H.; Cao, X.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. A novel electrochemical sensor for epinephrine based on three dimensional molecularly imprinted polymer arrays. Sens. Actuators B Chem. 2016, 222, 1127–1133. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zeng, X.; Liu, X.; Kong, B.; Wei, W.; Luo, S. Selective and sensitive detection of dopamine in the presence of ascorbic acid by molecular sieve/ionic liquids composite electrode. Electrochim. Acta 2011, 56, 2730–2734. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2012, 405, 3753–3771. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugama, A.; Kojima, N. Micromachined Clark oxygen electrode. Sens. Actuators B Chem. 1993, 10, 91–98. [Google Scholar] [CrossRef]
- Rivas, L.; Dulay, S.; Miserere, S.; Pla, L.; Marin, S.B.; Parra, J.; Eixarch, E.; Gratacós, E.; Illa, M.; Mir, M. Micro-needle implantable electrochemical oxygen sensor: Ex-vivo and in-vivo studies. Biosens. Bioelectron. 2020, 153, 112028. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Clark, E.W. A personalized history of the Clark oxygen electrode. Int. Anesthesiol. Clin. 1987, 25, 1–29. [Google Scholar] [CrossRef]
- Stewart, C.; Haitsma, I.; Zador, Z.; Morabito, D.; Manley, G.; Rosenthal, G.; Hemphill, J.C., III. The new Licox combined brain tissue oxygen and brain temperature monitor: Assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery 2008, 63, 1159–1165. [Google Scholar] [CrossRef]
- Ben-Amor, S.; Vanhove, E.; Belaïdi, F.S.; Charlot, S.; Colin, D.; Rigoulet, M.; Devin, A.; Sojic, N.; Launay, J.; Tem-ple-Boyer, P. Enhanced detection of hydrogen peroxide with platinized microelectrode arrays for analyses of mito-chondria activities. Electrochim. Acta 2014, 126, 171–178. [Google Scholar] [CrossRef]
- Griveau, S.; Dumézy, C.; Seguin, J.; Chabot, G.G.; Scherman, D.; Bedioui, F. In Vivo Electrochemical Detection of Nitric Oxide in Tumor-Bearing Mice. Anal. Chem. 2007, 79, 1030–1033. [Google Scholar] [CrossRef]
- Kobos, R. Enzyme-based electrochemical biosensors. TrAC Trends Anal. Chem. 1987, 6, 6–9. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Davis, V.A.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Glutaraldehyde cross-linked gluta-mate oxidase coated microelectrode arrays: Selectivity and resting levels of glutamate in the CNS. ACS Chem. Neurosci. 2013, 4, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, H.; Midorikawa, Y.; Fujishima, T.; Kuninaka, A.; Yoshino, H. Purification and properties of a new enzyme, L-glutamate oxidase, from Streptomyces sp. X-119-6 grown on wheat bran. Agric. Biol. Chem. 1983, 47, 1323–1328. [Google Scholar] [CrossRef]
- Zhang, M.; Mullens, C.; Gorski, W. Amperometric glutamate biosensor based on chitosan enzyme film. Electrochim. Acta 2006, 51, 4528–4532. [Google Scholar] [CrossRef]
- Day, B.; Pomerleau, F.; Burmeister, J.; Huettl, P.; Gerhardt, G. Microelectrode array studies of basal and potassium evoked release of l-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Kieninger, J.; Enderle, B.; Gellner, A.-K.; Fritsch, B.; Urban, G.A. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectron. 2014, 61, 192–199. [Google Scholar] [CrossRef]
- Tian, F.; Gourine, A.V.; Huckstepp, R.T.; Dale, N. A microelectrode biosensor for real time monitoring of L-glutamate release. Anal. Chim. Acta 2009, 645, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wahono, N.; Qin, S.; Oomen, P.; Cremers, T.; De Vries, M.; Westerink, B. Evaluation of permselective membranes for optimization of intracerebral amperometric glutamate biosensors. Biosens. Bioelectron. 2012, 33, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Simultaneous measurements of ascorbate and glutamate in vivo in the rat brain using carbon fiber nanocomposite sensors and microbiosensor arrays. Bioelectrochemistry 2018, 121, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389. [Google Scholar] [CrossRef]
- Mascini, M.; Mazzei, F. Amperometric sensor for pyruvate with immobilized pyruvate oxidase. Anal. Chim. Acta 1987, 192, 9–16. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Loaiza, O.A.; Añorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabañero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351. [Google Scholar] [CrossRef]
- Batra, B.; Pundir, C. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carbox-ylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Mao, J.; Han, Y.; Wu, F.; Zhang, M.; Wang, D.; Mao, L.; Li, Y. Single-atom electrocatalysis: A new approach to in vivo electrochemical biosensing. Sci. China Ser. B Chem. 2019, 62, 1720–1724. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Xie, Y.; Qian, C.; Ma, W.; Wang, L.; Song, Y. Ratiometric electrochemical glucose sensor based on electroactive Schiff base polymers. Sens. Actuators B Chem. 2019, 285, 264–270. [Google Scholar] [CrossRef]
- Soylemez, S.; Kaya, H.Z.; Udum, Y.A.; Toppare, L. A multipurpose conjugated polymer: Electrochromic device and biosensor construction for glucose detection. Org. Electron. 2019, 65, 327–333. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. NPJ Flex. Electron. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Yamashita, Y.; Suzuki, N.; Hirose, N.; Kojima, K.; Tsugawa, W.; Sode, K. Mutagenesis Study of the Cytochrome c Subunit Responsible for the Direct Electron Transfer-Type Catalytic Activity of FAD-Dependent Glucose Dehydrogenase. Int. J. Mol. Sci. 2018, 19, 931. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Shimazaki, J.; Loew, N.; Hirose, N.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Construction and characterization of flavin adenine dinucleotide glucose dehydrogenase complex harboring a truncated electron transfer subunit. Electrochim. Acta 2018, 277, 276–286. [Google Scholar] [CrossRef]
- Lee, I.; Loew, N.; Tsugawa, W.; Ikebukuro, K.; Sode, K. Development of a third-generation glucose sensor based on the open circuit potential for continuous glucose monitoring. Biosens. Bioelectron. 2019, 124, 216–223. [Google Scholar] [CrossRef]
- Ito, Y.; Okuda-Shimazaki, J.; Tsugawa, W.; Loew, N.; Shitanda, I.; Lin, C.-E.; La Belle, J.; Sode, K. Third generation impedimetric sensor employing direct electron transfer type glucose dehydrogenase. Biosens. Bioelectron. 2019, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. FAD dependent glucose dehydrogenases–Discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Ibadullaeva, S.Z.; Appazov, N.O.; Tarahovsky, Y.S.; Zamyatina, E.A.; Fomkina, M.G.; Kim, Y.A. Amperometric Multi-Enzyme Biosensors: Development and Application, a Short Review. Biophysics 2019, 64, 696–707. [Google Scholar] [CrossRef]
- Guerrieri, A.; Palmisano, F. An acetylcholinesterase/choline oxidase-based amperometric biosensor as a liquid chroma-tography detector for acetylcholine and choline determination in brain tissue homogenates. Anal. Chem. 2001, 73, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A.; Dragulescu, S.; Cremisini, C.; Palleschi, G. Bienzyme amperometric probes for choline and choline esters assembled with nonconducting electrosynthesized polymers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 236–242. [Google Scholar] [CrossRef]
- Bruno, J.P.; Gash, C.; Martin, B.; Zmarowski, A.; Pomerleau, F.; Burmeister, J.; Huettl, P.; Gerhardt, G.A. Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur. J. Neurosci. 2006, 24, 2749–2757. [Google Scholar] [CrossRef]
- Mitchell, K.M. Acetylcholine and Choline Amperometric Enzyme Sensors Characterized in Vitro and in Vivo. Anal. Chem. 2004, 76, 1098–1106. [Google Scholar] [CrossRef]
- Hossain, I.; Tan, C.; Doughty, P.T.; Dutta, G.; Murray, T.A.; Siddiqui, S.; Iasemidis, L.; Arumugam, P.U. A novel microbiosensor microarray for continuous ex vivo monitoring of gamma-aminobutyric acid in real-time. Front. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Price, D.A.; Pomerleau, F.; Huettl, P.; Quintero, J.E.; Gerhardt, G.A. Challenges of simultaneous measurements of brain extracellular GABA and glutamate in vivo using enzyme-coated microelectrode arrays. J. Neurosci. Methods 2020, 329, 108435. [Google Scholar] [CrossRef] [PubMed]
- Doughty, P.T.; Hossain, I.; Gong, C.; Ponder, K.A.; Pati, S.; Arumugam, P.U.; Murray, T.A. Novel microwire-based biosensor probe for simultaneous real-time measurement of glutamate and GABA dynamics in vitro and in vivo. Sci. Rep. 2020, 10, 12777. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.K. Aptasensors—The future of biosensing? Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Santos-Cancel, M.; Simpson, L.W.; Leach, J.B.; White, R.J. Direct, Real-Time Detection of Adenosine Triphosphate Release from Astrocytes in Three-Dimensional Culture Using an Integrated Electrochemical Aptamer-Based Sensor. ACS Chem. Neurosci. 2019, 10, 2070–2079. [Google Scholar] [CrossRef]
- Wang, G.; Su, X.; Xu, Q.; Xu, G.; Lin, J.; Luo, X. Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens. Bioelectron. 2018, 101, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, J.; Meng, X.; Xu, Z.; Zhang, J.; Fang, Y.; Guo, Y. Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal. Chim. Acta 2019, 1076, 55–63. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, C.; Chen, M.; Liu, Y.; Zhao, Z.; Wu, F.; Li, Z.; Weiss, P.S.; Andrews, A.M.; Zhou, C. Flexible Multiplexed In2O3 Nanoribbon Aptamer-Field-Effect Transistors for Biosensing. iScience 2020, 23, 101469. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Ozkaya, C.; Davis, F.; Walch, N.; Nabok, A. Electrochemical Aptasensor for Detection of Dopamine. Chemosensors 2020, 8, 28. [Google Scholar] [CrossRef]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA Aptamer-Based Electrochemical Biosensor for Selective and Label-Free Analysis of Dopamine. Anal. Chem. 2012, 85, 121–128. [Google Scholar] [CrossRef]
- Bahrami, S.; Abbasi, A.R.; Roushani, M.; Derikvand, Z.; Azadbakht, A. An electrochemical dopamine aptasensor incorporating silver nanoparticle, functionalized carbon nanotubes and graphene oxide for signal amplification. Talanta 2016, 159, 307–316. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Dai, J.; Li, C.; Zhu, M.; Li, H.; Lou, X.; Xia, F.; Plaxco, K.W. High frequency, calibration-free molecular measurements in situ in the living body. Chem. Sci. 2019, 10, 10843–10848. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Yang, K.; Arroyo-Currás, N.; Ploense, K.L.; Zhang, Y.; Gerson, J.; Kurnik, M.; Kippin, T.E.; Stojanovic, M.N.; Plaxco, K.W. Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS Sens. 2019, 4, 2832–2837. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Somerson, J.; Vieira, P.A.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 2017, 114, 645–650. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Zhu, M.; Arroyo-Currás, N.; Li, H.; Wang, Y.; Wang, Q.; Lou, X.; Kippin, T.E.; Wang, S. Hydrogel-coating improves the in-vivo stability of electrochemical aptamer-based biosensors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Hoggarth, D.A.; Maliniak, D.; Ploense, K.; White, R.J.; Woodward, N.; Hsieh, K.; Bonham, A.J.; Eisenstein, M.; Kippin, T.E.; et al. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci. Transl. Med. 2013, 5, 213ra165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Arroyo-Currás, N.; Kang, D.; Ricci, F.; Plaxco, K.W. Dual-Reporter Drift Correction To Enhance the Performance of Electrochemical Aptamer-Based Sensors in Whole Blood. J. Am. Chem. Soc. 2016, 138, 15809–15812. [Google Scholar] [CrossRef]
- Wei, H.; Wu, F.; Li, L.; Yang, X.; Xu, C.; Yu, P.; Ma, F.; Mao, L. Natural Leukocyte Membrane-Masked Microelectrodes with an Enhanced Antifouling Ability and Biocompatibility for In Vivo Electrochemical Sensing. Anal. Chem. 2020, 92, 11374–11379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, T.; Wu, F.; Shen, M.-Y.; Zhang, M.; Yu, H.-H.; Mao, L. Ultrathin Cell-Membrane-Mimic Phosphorylcholine Polymer Film Coating Enables Large Improvements for In Vivo Electrochemical Detection. Angew. Chem. 2017, 129, 11964–11968. [Google Scholar] [CrossRef]
- Peairs, M.J.; Ross, A.E.; Venton, B.J. Comparison of Nafion- and overoxidized polypyrrole-carbon nanotube electrodes for neurotransmitter detection. Anal. Methods 2011, 3, 2379–2386. [Google Scholar] [CrossRef]
- Soldatkina, O.; Kucherenko, I.; Pyeshkova, V.; Alekseev, S.; Soldatkin, O.; Dzyadevych, S. Improvement of amperomet-ric transducer selectivity using nanosized phenylenediamine films. Nanoscale Res. Lett. 2017, 12, 594. [Google Scholar] [CrossRef]
- Dodevska, T.; Lazarova, Y.; Shterev, I. Amperometric Biosensors for Glucose and Lactate with Applications in Food Analysis: A Brief Review. Acta Chim. Slov. 2019, 66, 762–776. [Google Scholar] [CrossRef]
- Wang, J. Permselective Coatings for Amperometric Biosensing; American Chemical Society: Washington, DC, USA, 1992; pp. 125–132. [Google Scholar]
- Feng, T.; Ji, W.; Tang, Q.; Wei, H.; Zhang, S.; Mao, J.; Zhang, Y.; Mao, L.; Zhang, M. Low-Fouling Nanoporous Conductive Polymer-Coated Microelectrode for In Vivo Monitoring of Dopamine in the Rat Brain. Anal. Chem. 2019, 91, 10786–10791. [Google Scholar] [CrossRef]
- Patel, J.; Radhakrishnan, L.; Zhao, B.; Uppalapati, B.; Daniels, R.C.; Ward, K.R.; Collinson, M.M. Electrochemical proper-ties of nanostructured porous gold electrodes in biofouling solutions. Anal. Chem. 2013, 85, 11610–11618. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Wei, H.; Yao, L.; Sun, L.; Yu, P.; Su, B.; Mao, L. In Vivo Monitoring of Oxygen in Rat Brain by Carbon Fiber Microelectrode Modified with Antifouling Nanoporous Membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Zhang, E.; Kim, M.J.; Yoshida, T.; Stanwicks, L.; Amemori, S.; Dagdeviren, H.E.; Langer, R.; Cima, M.J.; Graybiel, A.M. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a ro-dent model. Commun. Biol. 2018, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.-L.; Ding, Z.; Zhan, D.; Long, Y.-T. Advanced electroanalytical chemistry at nanoelectrodes. Chem. Sci. 2017, 8, 3338–3348. [Google Scholar] [CrossRef]
- Li, X.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A.G. Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes. Angew. Chem. 2015, 127, 12146–12150. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Yu, Y.; Rotenberg, S.A.; Amatore, C.; Mirkin, M.V. Direct electrochemical measurements of reactive oxy-gen and nitrogen species in nontransformed and metastatic human breast cells. J. Am. Chem. Soc. 2017, 139, 13055–13062. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.-H.; Wang, C.; Zhang, W.; Cai, Y.-L.; Li, K.; Lin, Y.-Q. A Facile Strategy for Two-Step Fabrication of Gold Nanoe-lectrode for in Vivo Dopamine Detection. J. Electrochem. 2019, 25, 244. [Google Scholar]
- Ding, S.; Liu, Y.; Ma, C.; Zhang, J.; Zhu, A.; Shi, G. Development of Glass-sealed Gold Nanoelectrodes for in vivo Detec-tion of Dopamine in Rat Brain. Electroanalysis 2018, 30, 1041–1046. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Lourenço, C.F.; Santos, R.M.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Laranjinha, J. In Vivo Real-Time Measurement of Nitric Oxide in Anesthetized Rat Brain. In Methods in Enzymology; Academic Press: London, UK, 2008; pp. 351–367. [Google Scholar]
- Yang, F.; Chang, M.-Y.; Yang, C.-H.; Teng, C.-C.; Fan, L.-S. Flexible, high-density microphotodiode array with integrated sputtered iridium oxide electrodes for retinal stimulation. J. Micro/Nanolithogr. MEMS MOEMS 2016, 15, 015002. [Google Scholar] [CrossRef]
- Özel, R.E.; Wallace, K.N.; Andreescu, S. Chitosan coated carbon fiber microelectrode for selective in vivo detection of neurotransmitters in live zebrafish embryos. Anal. Chim. Acta 2011, 695, 89–95. [Google Scholar] [CrossRef]
- Niu, S.; Fang, Y.; Zhang, K.; Sun, J.; Wan, J. Determination of dopamine using the fluorescence quenching of 2, 3-diaminophenazine. Instrum. Sci. Technol. 2016, 45, 101–110. [Google Scholar] [CrossRef]
- Bera, K.; Das, A.K.; Rakshit, A.; Sarkar, B.; Rawat, A.; Maity, B.K.; Maiti, S. Fluorogenic detection of monoamine neurotransmitters in live cells. ACS Chem. Neurosci. 2017, 9, 469–474. [Google Scholar] [CrossRef]
- Kruss, S.; Landry, M.P.; Ende, E.V.; Lima, B.M.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter Detection Using Corona Phase Molecular Recognition on Fluorescent Single-Walled Carbon Nanotube Sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Beyene, A.G.; Delevich, K.; Del Bonis-O’Donnell, J.T.; Piekarski, D.J.; Lin, W.C.; Thomas, A.W.; Yang, S.J.; Kosillo, P.; Yang, D.; Prounis, G.S. Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent cat-echolamine nanosensor. Sci. Adv. 2019, 5, eaaw3108. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef] [PubMed]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum Dots for Live Cell and In Vivo Imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef]
- Walkey, C.; Sykes, E.A.; Chan, W.C.W. Application of semiconductor and metal nanostructures in biology and medicine. Hematology 2009, 2009, 701–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Pan, C.; Wei, Z.; Wei, X.; Yang, F.; Mao, L. Bionanosensor based on N-doped graphene quantum dots coupled with CoOOH nanosheets and their application for in vivo analysis of ascorbic acid. Anal. Chim. Acta 2020, 1100, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.-G.; Zhu, S.; Feng, P.-J.; Chen, Y.-L.; Yu, J.-C.; Tang, X.; Liu, Y.; Shen, Q.-D. Conjugated Polymer Nanoparticles for Fluorescence Imaging and Sensing of Neurotransmitter Dopamine in Living Cells and the Brains of Zebrafish Larvae. ACS Appl. Mater. Interfaces 2015, 7, 18581–18589. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.B.; Schmitz, Y.; Mészáros, J.; Merchant, P.; Hu, G.; Li, S.; Henke, A.; Lizardi-Ortiz, J.E.; Karpowicz, R.J., Jr.; Morgenstern, T.J.; et al. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat. Neurosci. 2016, 19, 578–586. [Google Scholar] [CrossRef]
- Black, C.A.; Bucher, M.L.; Bradner, J.M.; Jonas, L.; Igarza, K.; Miller, G.W. Assessing Vesicular Monoamine Transport and Toxicity Using Fluorescent False Neurotransmitters. Chem. Res. Toxicol. 2020. [Google Scholar] [CrossRef]
- Sulzer, D.; Sames, D. Fluorescent False Neurotransmitters. Compendium of In Vivo Monitoring In Real-time Probing Brain Function, Disease and Injury With Enhanced Optical And Electrochemical Sensors. Mol. Neurosci. 2019, 3, 33. [Google Scholar]
- Zhen, X.; Zhang, C.; Xie, C.; Miao, Q.; Lim, K.L.; Pu, K. Intraparticle Energy Level Alignment of Semiconducting Polymer Nanoparticles to Amplify Chemiluminescence for Ultrasensitive In Vivo Imaging of Reactive Oxygen Species. ACS Nano 2016, 10, 6400–6409. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Lee, Y.D.; Na, J.; Oh, J.M.; Her, S.; Kim, K.; Choi, K.; Kim, S.; Kwon, I.C. Chemiluminescence-Generating Nanoreactor Formulation for Near-Infrared Imaging of Hydrogen Peroxide and Glucose Level in vivo. Adv. Funct. Mater. 2010, 20, 2644–2648. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, P.; Wang, G.; Feng, J.; Mesik, L.; Zeng, J.; Jiang, H.; Wang, S.; Looby, J.C.; Guagliardo, N.A.; et al. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat. Biotechnol. 2018, 36, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Marvin, J.S.; Borghuis, B.G.; Tian, L.; Cichon, J.; Harnett, M.T.; Akerboom, J.; Gordus, A.; Renninger, S.L.; Chen, T.-W.; Bargmann, C.I.; et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 2013, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
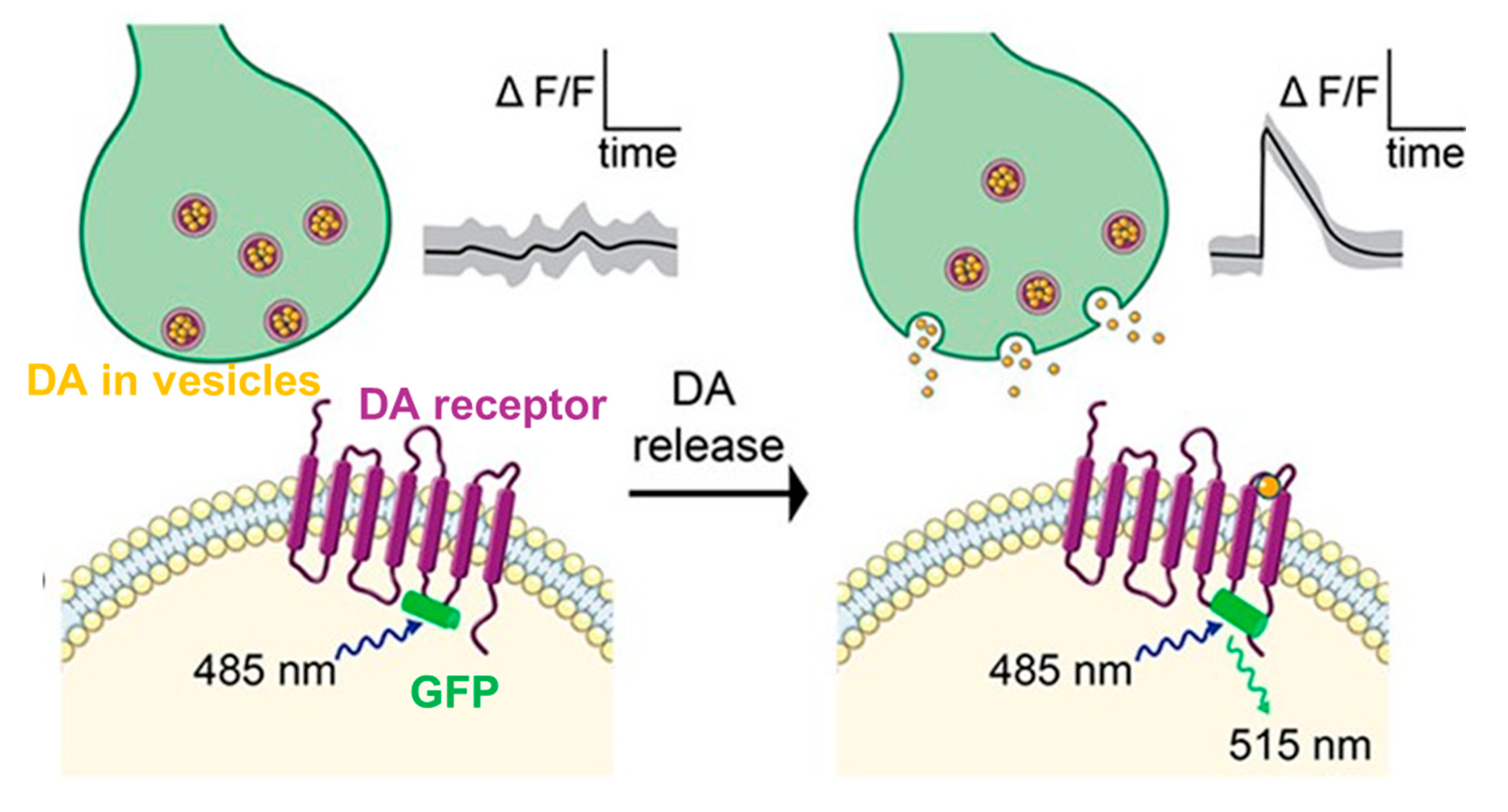
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T.; et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, 481–496.e19. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron 2019, 102, 745–761.e8. [Google Scholar] [CrossRef] [PubMed]
- Germond, A.; Fujita, H.; Ichimura, T.; Watanabe, T.M. Design and development of genetically encoded fluorescent sen-sors to monitor intracellular chemical and physical parameters. Biophys. Rev. 2016, 8, 121–138. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Dai, B.; Qian, T.; Zeng, J.; Li, X.; Zhuo, Y.; Zhang, Y.; Wang, Y.; Qian, C.; et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 2020, 17, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Looger, L.L.; Micheva, K.D.; Reimer, R.J.; Smith, S.J.; Frommer, W.B. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 8740–8745. [Google Scholar] [CrossRef]
- Patriarchi, T.; Cho, J.R.; Merten, K.; Howe, M.W.; Marley, A.; Xiong, W.-H.; Folk, R.W.; Broussard, G.J.; Liang, R.; Jang, M.J.; et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360, eaat4422. [Google Scholar] [CrossRef] [PubMed]
- Hefendehl, J.K.; LeDue, J.; Ko, R.W.Y.; Mahler, J.; Murphy, T.H.; MacVicar, B.A. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat. Commun. 2016, 7, 13441. [Google Scholar] [CrossRef]
- Beyene, A.G.; Delevich, K.; Yang, S.J.; Landry, M.P. New Optical Probes Bring Dopamine to Light. Biochemestry 2018, 57, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Khaja, S.D.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; A Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, O.; Lee, I.; Lee, G.; Yoo, D.; Khang, G.; Kang, P.M.; Lee, D. Chemiluminescent and Antioxidant Micelles as Theranostic Agents for Hydrogen Peroxide Associated-Inflammatory Diseases. Adv. Funct. Mater. 2012, 22, 4038–4043. [Google Scholar] [CrossRef]
- Yao, D.; Vlessidis, A.G.; Evmiridis, N.P. Microdialysis sampling and monitoring of uric acid in vivo by a chemilumi-nescence reaction and an enzyme on immobilized chitosan support membrane. Anal. Chim. Acta 2003, 478, 23–30. [Google Scholar] [CrossRef]
- Wu, N.; Xie, H.; Lu, H.; Li, W.; Zhang, Q. Sensitive determination of norepinephrine, epinephrine, dopamine and 5-hydroxytryptamine by coupling HPLC with [Ag(HIO6)2]5−-luminol chemiluminescence detection. Biomed. Chromatogr. 2016, 30, 1458–1466. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Yu, Y.; Zou, G. A monochromatic electrochemiluminescence sensing strategy for dopamine with dual-stabilizers-capped CdSe quantum dots as emitters. Anal. Chem. 2014, 86, 2784–2788. [Google Scholar] [CrossRef]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex-vivo optical clearing methods for biological tissues: Review. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Kim, G.; Nagarajan, N.; Pastuzyn, E.; Jenks, K.; Capecchi, M.; Shepherd, J.; Menon, R. Deep-brain imaging via epi-fluorescence Computational Cannula Microscopy. Sci. Rep. 2017, 7, srep44791. [Google Scholar] [CrossRef]
- Vasquez-Lopez, S.A.; Turcotte, R.; Koren, V.; Plöschner, M.; Padamsey, Z.; Booth, M.J.; Čižmár, T.; Emptage, N.J. Subcellular spatial resolution achieved for deep-brain imaging in vivo using a minimally invasive multimode fiber. Light Sci. Appl. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Wang, Z.; Liang, B.; Barbera, G.; Moffitt, C.; Li, Y.; Lin, D.-T. A Two-Step GRIN Lens Coating for In Vivo Brain Imaging. Neurosci. Bull. 2019, 35, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Quantitative liquid chromatography/mass spectrometry for the analysis of microdialysates. Talanta 2008, 74, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.T. Emerging trends in in vivo neurochemical monitoring by microdialysis. Curr. Opin. Chem. Biol. 2013, 17, 860–867. [Google Scholar] [CrossRef]
- Weltin, A.; Kieninger, J.; Urban, G.A. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal. Bioanal. Chem. 2016, 408, 4503–4521. [Google Scholar] [CrossRef]
- Zestos, A.G.; Luna-Munguia, H.; Stacey, W.C.; Kennedy, R.T. Use and Future Prospects of in Vivo Microdialysis for Epilepsy Studies. ACS Chem. Neurosci. 2018, 10, 1875–1883. [Google Scholar] [CrossRef]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of Brain Microdialysis. Curr. Protoc. Neurosci. 2009, 47, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Venton, B.J.; Kennedy, R.T. In Vivo Measurements of Neurotransmitters by Microdialysis Sampling. Anal. Chem. 2006, 78, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Gowers, S.A.N.; Hamaoui, K.; Vallant, N.; Hanna, G.B.; Darzi, A.; Casanova, D.; Papalois, V.; Boutelle, M.G. An improved rapid sampling microdialysis system for human and porcine organ monitoring in a hospital setting. Anal. Methods 2018, 10, 5273–5281. [Google Scholar] [CrossRef] [PubMed]
- Ngernsutivorakul, T.; Steyer, D.J.; Valenta, A.C.; Kennedy, R.T. In Vivo Chemical Monitoring at High Spatiotemporal Resolution Using Microfabricated Sampling Probes and Droplet-Based Microfluidics Coupled to Mass Spectrometry. Anal. Chem. 2018, 90, 10943–10950. [Google Scholar] [CrossRef]
- Yang, H.; Thompson, A.B.; McIntosh, B.J.; Altieri, S.C.; Andrews, A.M. Physiologically Relevant Changes in Serotonin Resolved by Fast Microdialysis. ACS Chem. Neurosci. 2013, 4, 790–798. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Xu, X.; Zhao, M.K.; Andrews, A.M.; Weber, S.G. Capillary ultrahigh performance liquid chromatography with elevated temperature for sub-one minute separations of basal serotonin in submicroliter brain microdialysate samples. Anal. Chem. 2010, 82, 9611–9616. [Google Scholar] [CrossRef]
- Ngo, K.T.; Varner, E.L.; Michael, A.C.; Weber, S.G. Monitoring Dopamine Responses to Potassium Ion and Nomifensine by in Vivo Microdialysis with Online Liquid Chromatography at One-Minute Resolution. ACS Chem. Neurosci. 2017, 8, 329–338. [Google Scholar] [CrossRef]
- Zhang, J.; Jaquins-Gerstl, A.; Nesbitt, K.M.; Rutan, S.C.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Serotonin in the Striatum of Freely Moving Rats with One Minute Temporal Resolution by Online Microdialysis-Capillary High-Performance Liquid Chromatography at Elevated Temperature and Pressure. Anal. Chem. 2013, 85, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- Hascup, E.R.; Hascup, K.N.; Talauliker, P.M.; Price, D.A.; Pomerleau, F.; Quintero, J.E.; Huettl, P.; Gratton, A.; Strömberg, I.; Gerhardt, G.A. Sub-Second Measurements of Glutamate and Other Neurotransmitter Signaling Using Enzyme-Based Ceramic Microelectrode Arrays. In Microelectrode Biosensors; Humana Press: Totowa, NJ, USA, 2013; pp. 179–199. [Google Scholar]
- Kozai, T.D.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials 2016, 87, 157–169. [Google Scholar] [CrossRef]
- Stenken, J.A.; Church, M.K.; Gill, C.A.; Clough, G.F. How Minimally Invasive is Microdialysis Sampling? A Cautionary Note for Cytokine Collection in Human Skin and other Clinical Studies. AAPS J. 2009, 12, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Borland, L.M.; Shi, G.; Yang, H.; Michael, A.C. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J. Neurosci. Methods 2005, 146, 149–158. [Google Scholar] [CrossRef]
- Jaquins-Gerstl, A.; Shu, Z.; Zhang, J.; Liu, Y.; Weber, S.G.; Michael, A.C. Effect of Dexamethasone on Gliosis, Ischemia, and Dopamine Extraction during Microdialysis Sampling in Brain Tissue. Anal. Chem. 2011, 83, 7662–7667. [Google Scholar] [CrossRef]
- Nesbitt, K.M.; Varner, E.L.; Jaquins-Gerstl, A.; Michael, A.C. Microdialysis in the rat striatum: Effects of 24 h dexamethasone retrodialysis on evoked dopamine release and penetration injury. ACS Chem. Neurosci. 2015, 6, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Varner, E.L.; Leong, C.L.; Jaquins-Gerstl, A.; Nesbitt, K.M.; Boutelle, M.G.; Michael, A.C. Enhancing continuous online microdialysis using dexamethasone: Measurement of dynamic neurometabolic changes during spreading depolarization. ACS Chem. Neurosci. 2017, 8, 1779–1788. [Google Scholar] [CrossRef]
- Robbins, E.M.; Jaquins-Gerstl, A.; Fine, D.F.; Leong, C.L.; Dixon, C.E.; Wagner, A.K.; Boutelle, M.G.; Michael, A.C. Extended (10-Day) Real-Time Monitoring by Dexamethasone-Enhanced Microdialysis in the Injured Rat Cortex. ACS Chem. Neurosci. 2019, 10, 3521–3531. [Google Scholar] [CrossRef]
- Daghighian, F.; Sumida, R.; Phelps, M.E. PET imaging: An overview and instrumentation. J. Nucl. Med. Technol. 1990, 18, 5–13. [Google Scholar]
- Thanos, P.K.; Wang, G.-J.; Volkow, N.D. Positron Emission Tomography as a Tool for Studying Alcohol Abuse. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2008, 31, 233–237. [Google Scholar]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J. Neurosci. 2007, 27, 12700–12706. [Google Scholar] [CrossRef]
- Steeves, T.; Miyasaki, J.; Zurowski, M.; Lang, A.; Pellecchia, G.; Van Eimeren, T.; Rusjan, P.; Houle, S.; Strafella, A. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: A [11C] raclopride PET study. Brain 2009, 132, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Finnema, S.J.; Scheinin, M.; Shahid, M.; Lehto, J.; Borroni, E.; Bang-Andersen, B.; Sallinen, J.; Wong, E.; Farde, L.; Halldin, C.; et al. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology 2015, 232, 4129–4157. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Kolarcik, C.L.; Catt, K.; Rost, E.; Albrecht, I.N.; Bourbeau, D.; Du, Z.; Kozai, T.D.; Luo, X.; Weber, D.J.; Cui, X.T. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J. Neural Eng. 2015, 12, 016008. [Google Scholar] [CrossRef]
- Golabchi, A.; Wu, B.; Li, X.; Carlisle, D.L.; Kozai, T.D.; Friedlander, R.M.; Cui, X.T. Melatonin improves quality and longevity of chronic neural recording. Biomaterials 2018, 180, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.S.; Snyder, N.R.; Woeppel, K.; Barengo, J.H.; Li, X.; Eles, J.; Kolarcik, C.L.; Cui, X.T.; Hanner, J. A superoxide scavenging coating for improving tissue response to neural implants. Acta Biomater. 2019, 99, 72–83. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid. Redox Signal 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical Nitric Oxide Sensors: Principles of Design and Characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar] [CrossRef]
- Dugan, E.A.; Bennett, C.; Tamames, I.; Dietrich, W.D.; King, C.S.; Prasad, A.; Rajguru, S.M. Therapeutic hypothermia reduces cortical inflammation associated with utah array implants. J. Neural Eng. 2020, 17, 026035. [Google Scholar] [CrossRef]
- Bedell, H.W.; Capadona, J.R. Anti-inflammatory approaches to mitigate the Neuroinflammatory response to brain-dwelling intracortical microelectrodes. J. Immunol. Sci. 2018, 2, 15. [Google Scholar]
- Chatard, C.; Sabac, A.; Moreno-Velasquez, L.; Meiller, A.; Marinesco, S. Minimally Invasive Microelectrode Biosensors Based on Platinized Carbon Fibers for in Vivo Brain Monitoring. ACS Central Sci. 2018, 4, 1751–1760. [Google Scholar] [CrossRef]
- Obien, M.E.J.; Deligkaris, K.; Bullmann, T.; Bakkum, D.J.; Frey, U. Revealing neuronal function through microelectrode array recordings. Front. Neurosci. 2015, 8, 423. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Song, S.; Kang, J.; Tsao, Y.; Chen, S.; Mottini, V.; McConnell, K.; Xu, W.; Zheng, Y.-Q.; et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020, 1–6. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Woeppel, K.; Yang, Q.; Cui, X.T. Recent advances in neural electrode–tissue interfaces. Curr. Opin. Biomed. Eng. 2017, 4, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Popov, P.; Caldwell, C.M.; Welle, E.J.; Egert, D.; Pettibone, J.R.; Roossien, D.H.; Becker, J.B.; Berke, J.D.; A Chestek, C.; et al. High density carbon fiber arrays for chronic electrophysiology, fast scan cyclic voltammetry, and correlative anatomy. J. Neural Eng. 2020, 17, 056029. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, H.N.; Kim, M.; Karasan, E.; Amemori, S.; Homma, D.; Shimazu, H.; Yoshida, T.; Langer, R.; Graybiel, A.M.; Cima, M.J. Subcellular electrode arrays for multisite recording of dopamine in vivo. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017. [Google Scholar]
- Miller, E.M.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Glaser, P.E. Simultaneous glutamate recordings in the frontal cortex network with multisite biomorphic microelectrodes: New tools for ADHD research. J. Neurosci. Methods 2015, 252, 75–79. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. Neurometabolic and electrophysiological changes during cortical spreading depolarization: Multimodal approach based on a lactate-glucose dual microbiosensor arrays. Sci. Rep. 2017, 7, 6764. [Google Scholar] [CrossRef]
- Rogers, M.L.; Leong, C.L.; Gowers, S.A.; Samper, I.C.; Jewell, S.L.; Khan, A.; McCarthy, L.; Pahl, C.; Tolias, C.M.; Walsh, D.C.; et al. Simultaneous monitoring of potassium, glucose and lactate during spreading depolarization in the injured human brain—Proof of principle of a novel real-time neurochemical analysis system, continuous online microdialysis. Br. J. Pharmacol. 2016, 37, 1883–1895. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Y.; Wang, M.; Zhang, Z.; Fan, X.; Song, X.; Zhuang, P.; Yue, F.; Chan, P.; Cai, X. A silicon based implant-able microelectrode array for electrophysiological and dopamine recording from cortex to striatum in the non-human primate brain. Biosens. Bioelectron. 2016, 85, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yoon, H.; Choi, S.H.; Zhao, F.; Kim, J.; Song, K.D.; Lee, U. Miniaturized and wireless optical neurotransmitter sensor for real-time monitoring of dopamine in the brain. Sensors 2016, 16, 1894. [Google Scholar] [CrossRef]
- Zhang, H.; Gutruf, P.; Meacham, K.; Montana, M.C.; Zhao, X.; Chiarelli, A.M.; Vázquez-Guardado, A.; Norris, A.; Lu, L.; Guo, Q.; et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 2019, 5, eaaw0873. [Google Scholar] [CrossRef]
- Kasasbeh, A.; Lee, K.; Bieber, A.; Bennet, K.; Chang, S.-Y. Wireless neurochemical monitoring in humans. Ster. Funct. Neurosurg. 2013, 91, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Kim, I.; Marsh, M.P.; Jang, D.P.; Hwang, S.-C.; Van Gompel, J.J.; Goerss, S.J.; Kimble, C.J.; Bennet, K.E.; Garris, P.A.; et al. Wireless Fast-Scan Cyclic Voltammetry to Monitor Adenosine in Patients With Essential Tremor During Deep Brain Stimulation. Mayo Clin. Proc. 2012, 87, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Tageldeen, M.K.; Gowers, S.A.N.; Leong, C.L.; Boutelle, M.G.; Drakakis, E.M. Traumatic brain injury neuroelectrochemical monitoring: Behind-the-ear micro-instrument and cloud application. J. Neuroeng. Rehabil. 2020, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.D.; Doeven, E.H.; Tye, S.J.; Bennet, K.E.; Berk, M.; Kouzani, A.Z. TinyFSCV: FSCV for the Masses. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 28, 133–142. [Google Scholar] [CrossRef]
- Van Gompel, J.J.; Chang, S.-Y.; Goerss, S.J.; Kim, I.Y.; Kimble, C.; Bennet, K.E.; Lee, K.H. Development of intraoperative electrochemical detection: Wireless instantaneous neurochemical concentration sensor for deep brain stimulation feedback. Neurosurg. Focus 2010, 29, E6. [Google Scholar] [CrossRef]
- Mirza, K.B.; Golden, C.T.; Nikolic, K.; Toumazou, C. Closed-Loop Implantable Therapeutic Neuromodulation Systems Based on Neurochemical Monitoring. Front. Neurosci. 2019, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lujan, J.L.; Trevathan, J.K.; Ross, E.K.; Bartoletta, J.J.; Park, H.O.; Paek, S.B.; Nicolai, E.N.; Lee, J.H.; Min, H.-K.; et al. WINCS Harmoni: Closed-loop dynamic neurochemical control of therapeutic interventions. Sci. Rep. 2017, 7, srep46675. [Google Scholar] [CrossRef]

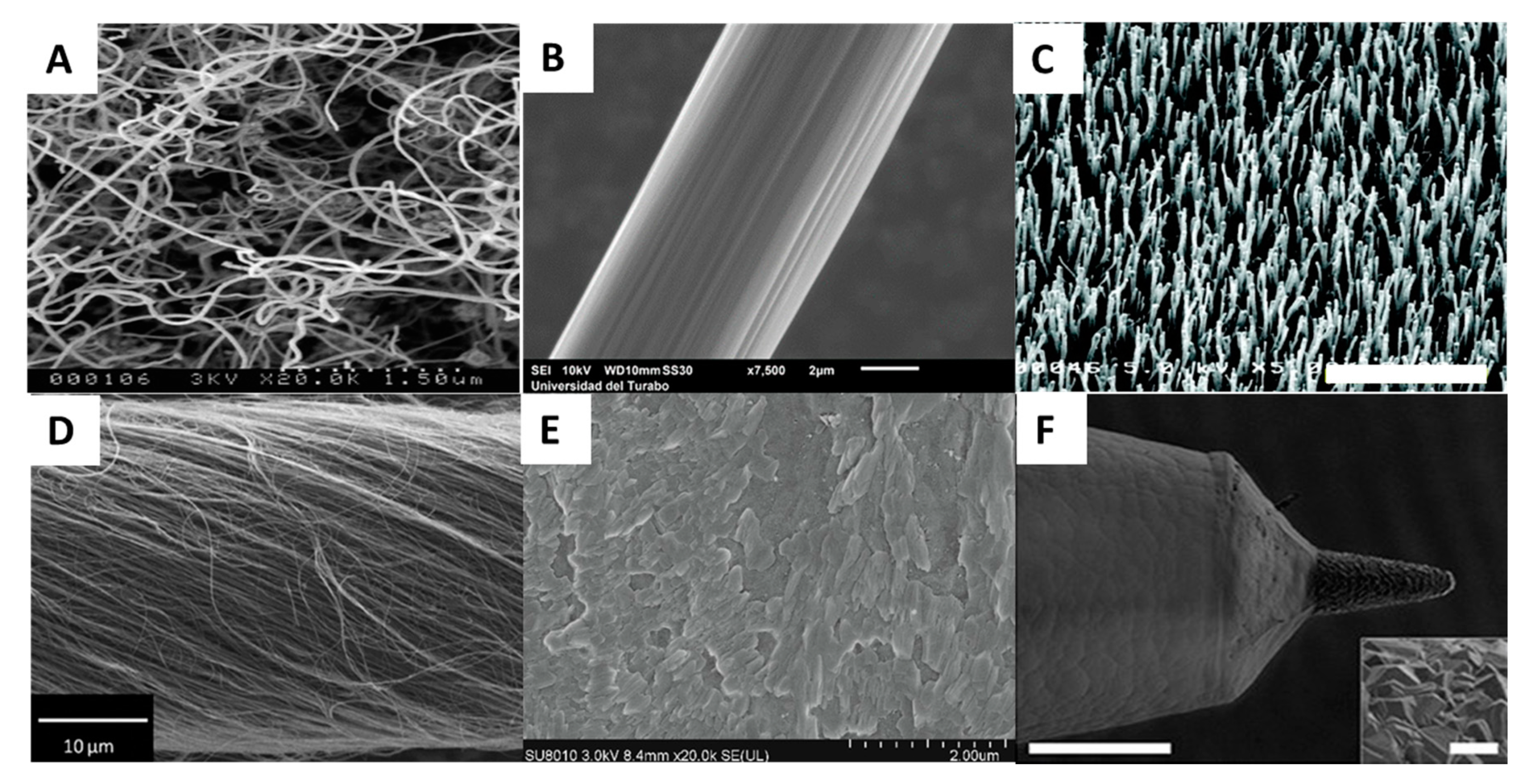
| Electrode Material | Method | Analyte | LOD | Selectivity Strategy | Stability | In Vivo Study | References |
|---|---|---|---|---|---|---|---|
| GC/Co-SAC/Nafion | CA | Glucose | N/A | Exclusive layer + low potential | Acute use | Microdialysate from insulin injected rat striatum | [124] |
| PtIr/oPD/Chit-GluOx/AAOx/BSA | CA | Glu | 44 nM | Exclusive layer + AAOx | 7 days, 95% in fridge | Electrically evoked Glu in subthalamic nucleus of anesthetized rat | [116] |
| Au/Poly-DA/AuNP/MPA | CA | DA | 50 nM | Exclusive layer | 1 h, 100% in vitro | K+ evoked DA in striatum of rat | [38] |
| CFM/PTA-PANI | CA | DA | N/A | N/A | Acute use | Electrically evoked DA in medial forebrain bundle of rat | [166] |
| CFM/Nafion/oPD | CA | NO | 6 nM | Distinct peaks | Acute use | NO release following NMDA stimulus in hippocampus of rat | [176] |
| CFM/CNT/Nafion | CA | AA | 0.7 µM | Exclusive layer + low potential | Acute use | Basal AA in hippocampus of rat | [117] |
| Pt/GluOx-(GABase)/mPD | CA | GABA, Glu | 0.2, 0.05 µM | Exclusive layer + self-referencing | Acute use | K+ and GABA transaminase inhibitor evoked GABA, Glu release in frontal cortex of rat | [143] |
| Pt/mPD/ChOx/(ACHE) | CA | Ach, Ch | 0.18 µM | Exclusive layer + self-referencing | Acute use | K+ evoked Ch, Ach in striatum of rat | [120] |
| BDD | FSCV | Adenosine, DA | N/A | Distinct peaks | 3 days, 93.3% in vitro | Mechanically evoked adenosine release in thalamus | [54] |
| CNTYM | FSCV | DA | 13 ± 2 nM | Distinct peaks | 4 h, 100% | Electrically evoked DA in caudate putamen of rat | [177] |
| CFM-PEDOT-PC | FSCV | DA | N/A | Distinct peaks | Acute use | Electrically evoked DA in medial forebrain bundle of rat | [161] |
| µIP-CFM | FSCV | DA | 5.7 nM | Distinct peaks | >1 yr | Electrically evoked DA in medial forebrain bundle of rat | [170] |
| CFM/Nafion | FSCV | DA | N/A | Distinct peaks | 2 h | Electrically evoked DA in striatum of rat | [24] |
| CFM | FSCV | H2O2, DA | N/A | Distinct peaks | Acute use | DA dynamics under modulation in striatum of rat | [53] |
| CFM/Chit | DPV | 5-HT | 1.6 nM | Exclusive layer + Distinct peaks | Acute use | Basal 5-HT in intestine of zebrafish | [178] |
| CFM/GR-FeTSPc | DPV | 5-HT, DA | 50, 20 nM | Distinct peaks | Acute use | Basal DA and 5-HT change in striatum of mouse | [45] |
| Tungsten/BDD | DPV | DA | 50 nM | Distinct peaks | Acute use | Nomifensine induced DA in medial forebrain bundle of rat | [40] |
| CFM/PEDOT-CNT | SWV | DA | 2.03 ± 0.09 nM | Exclusive layer + Distinct peaks | Acute use | Tonic and nomifensine induced DA in striatum of rat | [25] |
| Pt/rGO-AuNCs/adenosine aptamer/MB | SWV | Cocaine | NA | Aptamer sequence | 3 h | Infused and IV injected cocaine in striatum of rat | [95] |
| Sensor Material | Method | Analyte | LOD | Selectivity Strategy | Stability | In Vivo Study | References |
|---|---|---|---|---|---|---|---|
| Peroxalate TCPO+ PFPV + dye | CL | H2O2 | 5 nM | Affinity binding (chemical reaction) | Acute use | Intracerebral LPS induced neuroinflammation | [191] |
| Peroxyoxalate CPPO + dye | CL | H2O2 | 1 nM | Affinity binding (chemical reaction) | Acute use | LPS induced inflammation in ankle of tumor mice | [192] |
| GFP + Ach receptor | FL | Ach | 100 nM | Affinity binding (conformational change) | 4 h | Odor stim to Drosophila antenna lobe; Visual stim to mice cortex | [193] |
| NGQDs + CoOOH | FL | AA | 1.85 µM | Affinity binding (redox reaction) | Acute use | Microdialysate from rat brain | [186] |
| E. coli GltI + GFP | FL | Glu | NA | Affinity binding (conformational change) | Acute use | Neuronal process in C. elegans. Behavior related transient Glu in mice | [194] |
| PFBT+PBA | FL | DA | 38.8 nM | Affinity binding (boronic acid-diol recognition) | Acute use | Injected DA into zebrafish larvae brain ventricle | [187] |
| EGFP + DA receptor | FL | DA | NA | Affinity binding (conformational change) | Acute use | Odor and electrical stim to drosophila. Visual stim to zebrafish. Optogenetic, reward stim and sexual behavior triggered DA release in mice | [195] |
| EGFP + α-adrenergic receptors | FL | NE | NA | Affinity binding (conformational change) | Acute use | Looming-evoked NE release in the midbrain of live zebrafish. Optogenetically and behaviorally triggered NE release in the locus coeruleus and hypothalamus of mice | [196] |
| Methods | LOD | Temporal Resolution | Spatial Resolution | Tissue Damage | Cost | Limitations |
|---|---|---|---|---|---|---|
| Electrochemical sensors | <1 µM | <1 s | <100 µm | Invasive | Inexpensive | Limited number of analytes. Performances decay with time |
| Optical Methods | <1 µM | <1 s | <1 µm | Less invasive | Inexpensive | Rely on engineering of biomarkers. Optical access needed |
| Microdialysis | <1 nM | <10 min | <1 mm | Invasive | Cheap | Flow rate affects accuracy. Fluidic setup needed |
| Positron Emission Tomography | <1 nM | <1 min | <1 cm | Non-invasive | Expensive | Rely on engineering of radiotracers. Short half-life time of tracers. Large equipment |
| Nuclear Magnetic Resonance | <1 mM | <1 min | <1 cm | Non-invasive | Expensive | Structurally similar compounds may be difficult to separate. Complex data analysis. Large equipment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Robbins, E.M.; Wu, B.; Cui, X.T. Recent Advances in In Vivo Neurochemical Monitoring. Micromachines 2021, 12, 208. https://doi.org/10.3390/mi12020208
Tan C, Robbins EM, Wu B, Cui XT. Recent Advances in In Vivo Neurochemical Monitoring. Micromachines. 2021; 12(2):208. https://doi.org/10.3390/mi12020208
Chicago/Turabian StyleTan, Chao, Elaine M. Robbins, Bingchen Wu, and Xinyan Tracy Cui. 2021. "Recent Advances in In Vivo Neurochemical Monitoring" Micromachines 12, no. 2: 208. https://doi.org/10.3390/mi12020208
APA StyleTan, C., Robbins, E. M., Wu, B., & Cui, X. T. (2021). Recent Advances in In Vivo Neurochemical Monitoring. Micromachines, 12(2), 208. https://doi.org/10.3390/mi12020208






