Flavonoids from Agrimonia pilosa Ledeb: Free Radical Scavenging and DNA Oxidative Damage Protection Activities and Analysis of Bioactivity-Structure Relationship Based on Molecular and Electronic Structures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Flavonoids from Agrimonia pilosa Ledeb
2.2. DPPH Radical Scavenging Activity of Flavonoids from Agrimonia pilosa Ledeb
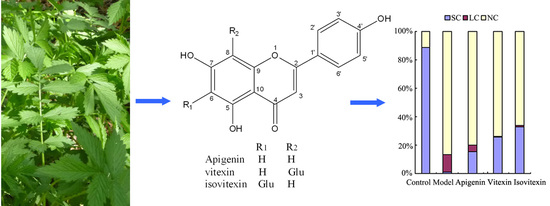
2.3. Protective Effect against DNA Oxidative Damage
2.4. Analysis of Bioactivity-Structure Relationship Based on the Electronic Structure
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Chemical Experiments
3.3.1. Extraction and Isolation
3.3.2. Structure Elucidation of the Isolated Compounds
3.4. DPPH Radical Scavenging Assay
3.5. DNA Nicking Assay
3.6. Quantum Theory Study of Bioactivity-Structure Relationship

3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sorg, O. Oxidative stress: A theoretical model or a biological reality? C. R. Biol. 2004, 327, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Hana, A.H. Lipid peroxidation end-products as a key of oxidative stress: Effect of Antioxidant on their production and transfer of free radicals. Biochem. Genet. Mol. Biol. 2012, 3, 63–68. [Google Scholar]
- Dean, R.; Fu, S.; Stocker, R.; Davies, M. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell Mol. Biol. Lett. 2003, 8, 391–413. [Google Scholar] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Mariani, E.; Polidori, M.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B 2005, 827, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Isabella, D.; Ranieri, R.; Roberto, C.; Daniela, G.; Aldo, M. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 603–621. [Google Scholar]
- Li, H.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Nithya, N.; Balakrishnan, K.P. Evaluation of some medicinal plants for their antioxidant properties. Int. J. Pharm. Tech. Res. 2010, 3, 381–385. [Google Scholar]
- Zeng, Y.W.; Deng, M.C.; Lv, Z.C.; Peng, Y.L. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus 2014, 3, 315. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Flatt, P.R. Actions of the traditional anti-diabetic plant, Agrimony eupatoria (agrimony): Effects on hyperglycaemia, cellular glucose metabolism and insulin secretion. Br. J. Nutr. 1998, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Zhu, L.C.; Tan, J.; Wang, B.C.; He, R.; Liu, Y.P.; Zheng, C. Antioxidant activities of aqueous extract from Agrimonia pilosa Ledeb and its fractions. Chem. Biodiver. 2009, 6, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Zhang, K.; Zhao, S.Q.; Zhang, J.H. Studies on the lowering blood sugar substances from agrimony (II). J. Chin. Med. Mater. 2010, 33, 724–726. [Google Scholar]
- Wang, X.; Zhang, K.; Chen, Y.S. Extraction and isolation of lowering blood sugar substances from the agrimony. Chin. J. Exp. Tradit. Med. Formul. 2010, 16, 85–87. [Google Scholar]
- Kato, H.; Li, W.; Koike, M.; Wang, Y.H.; Koike, K. Phenolic glycosides from Agrimonia pilosa. Phytochemistry 2010, 71, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, L.C.; Tan, J.; Zhou, X.M.; Xiao, L.; Yang, X.; Wang, B.C. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia Pilosa Ledeb. BMC Complem. Alter. Med. 2014, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. Carbon-13 NMR of flavonoids; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Markham, K.R.; Geiger, H. 1H-NMR spectroscopy of flavonoids and their glycosides in hexadeuterodimethylsulfoxide. In The Flavonoids, Advances in Research since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1994; pp. 441–497. [Google Scholar]
- Storniolo, C.E.; Quifer-Rada, P.; Lamuela-Raventos, R.M.; Moreno, J.J. Piceid presents antiproliferative effects in intestinal epithelial Caco-2 cells, effects unrelated to resveratrol release. Food Funct. 2014, 5, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Rebai, B.A.; Wissem, B.; Mohamed, B.S.; Jihed, B.; Ines, S.; Aicha, N.; Ines, B.; Soumaya, K.; Anne-Marie, M.; Leila, C.G.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnusalaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar]
- Zhu, L.C.; Liu, X.; Tan, J.; Wang, B.C. Influence of harvest season on antioxidant activity and constituents of rabbiteye blueberry (Vaccinium ashei) Leaves. J. Agric. Food Chem. 2013, 61, 11477–11483. [Google Scholar] [CrossRef] [PubMed]
- Takuya, K.; Hiromasa, T.; Yukari, O.; Yukikazu, Y.; Erdembileg, A.; Kuninori, S.; Yosuke, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging Assay, and Folin−Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar]
- Miliauskasa, G.; Venskutonisa, P.R.; Beekb, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Falcioni, G.; Fedeli, D.; Tiano, L.; Calzuola, I.; Mancinelli, L.; Marsili, V.; Gianfranceschi, G. Antioxidant activity of wheat sprouts extract in vitro: Inhibition of DNA oxidative damage. J. Food Sci. 2002, 67, 2918–2922. [Google Scholar] [CrossRef]
- Russo, A.; Piovano, M.; Lombardo, L.; Garbarino, J.; Cardile, V. Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci. 2008, 83, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; Korth, H.G.; Pratt, D.A.; Labio, G.A.; Valgimigh, L.; Pedulli, G.F.; Ingold, K.U. Critical re-evaluation of the O-H bond dissociation enthalpy in phenol. J Phys. Chem. A 2005, 109, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y. Theoretical methods used in elucidating activity differences of phenolic antioxidants. J. Am. Oil Chem. Soc. 1999, 76, 745–748. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Sun, Y.M.; Wang, X.L. Substituent effects on O-H bond dissociation enthalpies and ionization potentials of catechols: A DFT study and its implications in rational design of phenolic antioxidants and elucidation of structure-activity relationships for flavonoid antioxidants. Chem.-Eur. J. 2003, 9, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Carpenter, D.J.; McKay, D.J.; Ingold, K.U. Theoretical calculation of substituent effects on the O-H bond strength of phenolic antioxidants related to vitamin E. J. Am. Chem. Soc. 1997, 119, 4245–4252. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision A.1, Gaussian, Inc.: Pittsburgh, PA, USA, 2003.
- Sample Availability: Samples of the compounds flavonoids from Agrimonia pilosa Ledeb are available from the authors.



| Number | Compound | IC50 (μM) |
|---|---|---|
| 1 | catechin | 5.06 ± 0.08 |
| 2 | taxifolin | 11.98 ± 0.12 |
| 3 | kaempferol | 16.09 ± 0.10 |
| 4 | apigenin | >200 |
| 5 | luteolin | 7.29 ± 0.09 |
| 6 | quercetin | 4.36 ± 0.10 |
| 7 | quercitrin | 7.12 ± 0.11 |
| 8 | hyperoside | 6.34 ± 0.10 |
| 9 | rutin | 6.36 ± 0.12 |
| 10 | tiliroside | >200 |
| 11 | kaempferol-3-O-glucoside | >200 |
| 12 | luteolin-7-O-β-glucoside | 8.12 ± 0.14 |
| 13 | vitexin | >200 |
| 14 | isovitexin | 122.83 ± 0.20 |
| 15 | vitamin C | 14.62 ± 0.15 |
| 16 | BHT | 17.67 ± 0.11 |
| Compounds | BDE (Kcal/mol) | ||||
|---|---|---|---|---|---|
| 3-OH | 5-OH | 7-OH | 3′-OH | 4′-OH | |
| Quercetin | 78.95 | 102.70 | 83.37 | 71.86 | 69.02 |
| Taxifolin | 96.05 | 100.78 | 84.62 | 80.17 | 72.64 |
| Catechin | 78.44 | 77.86 | 80.20 | 77.13 | 69.47 |
| Luteolin | - | 102.77 | 83.97 | 80.10 | 72.44 |
| Kaempferol | 78.33 | 102.72 | 83.37 | - | 74.73 |
| Apigenin | - | 97.78 | 83.98 | - | 79.77 |
| Isovitexin | - | 80.68 | 79.70 | - | 78.55 |
| Vitexin | - | 101.61 | 101.52 | - | 99.20 |
| Kaempferol-3-O-glu | - | 89.93 | 81.22 | - | 78.69 |
| Luteolin-7-O-glu | - | 87.05 | - | 80.45 | 73.30 |
| Tiliroside | - | 77.96 | 79.49 | - | 76.32 |
| Rutin | - | 89.25 | 85.38 | 84.13 | 73.04 |
| Quercitrin | - | 91.22 | 82.36 | 80.10 | 69.89 |
| Hyperoside | - | 92.18 | 84.71 | 75.99 | 71.11 |
| Compound | 4′-O | 6′-C | 5′-C | 4′-C | 3′-C | 2′-C | 1′-C |
|---|---|---|---|---|---|---|---|
| Apigenin | 0.380083 | −0.169869 | 0.303191 | −0.113358 | 0.274517 | −0.163938 | 0.382626 |
| vitexin | 0.373175 | −0.168035 | 0.300523 | −0.108348 | 0.267208 | −0.160770 | 0.382844 |
| isovitexin | 0.396971 | −0.166433 | 0.301826 | −0.112689 | 0.290139 | −0.164036 | 0.383058 |
| Compound | 4′-O | 6′-C | 5′-C | 4′-C | 3′-C | 2′-C | 1′-C | 3′-O |
|---|---|---|---|---|---|---|---|---|
| Luteolin | 0.338555 | −0.076213 | 0.184145 | −0.015767 | 0.236716 | −0.135202 | 0.316232 | 0.083901 |
| luteolin-7-O-glu | 0.351547 | −0.131706 | 0.247106 | −0.080224 | 0.247929 | −0.146586 | 0.345674 | 0.069776 |
| Compound | 4′-O | 6′-C | 5′-C | 4′-C | 3′-C | 2′-C | 1′-C |
|---|---|---|---|---|---|---|---|
| Kaempferol | 0.362436 | −0.151734 | 0.263024 | −0.104162 | 0.278057 | −0.152102 | 0.351079 |
| kaempferol-3-O-Glu | 0.387743 | −0.161792 | 0.289969 | −0.108207 | 0.290449 | −0.162656 | 0.380708 |
| Tiliroside | 0.366781 | −0.159347 | 0.281211 | −0.103501 | 0.274765 | −0.158234 | 0.373993 |
| Compound | 4′-O | 6′-C | 5′-C | 4′-C | 3′-C | 2′-C | 1′-C | 3′-O |
|---|---|---|---|---|---|---|---|---|
| Quercetin | 0.316394 | −0.063693 | 0.165670 | −0.012205 | 0.246853 | −0.131286 | 0.299326 | 0.088008 |
| Quercitrin | 0.334737 | −0.097927 | 0.206668 | −0.093886 | 0.245379 | −0.142259 | 0.308832 | 0.097475 |
| Hyperoside | 0.323551 | −0.060549 | 0.164803 | −0.010714 | 0.254078 | −0.129996 | 0.297435 | 0.090597 |
| Rutin | 0.325680 | −0.070428 | 0.164597 | −0.016466 | 0.251577 | −0.129888 | 0.302433 | 0.090028 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Chen, J.; Tan, J.; Liu, X.; Wang, B. Flavonoids from Agrimonia pilosa Ledeb: Free Radical Scavenging and DNA Oxidative Damage Protection Activities and Analysis of Bioactivity-Structure Relationship Based on Molecular and Electronic Structures. Molecules 2017, 22, 195. https://doi.org/10.3390/molecules22030195
Zhu L, Chen J, Tan J, Liu X, Wang B. Flavonoids from Agrimonia pilosa Ledeb: Free Radical Scavenging and DNA Oxidative Damage Protection Activities and Analysis of Bioactivity-Structure Relationship Based on Molecular and Electronic Structures. Molecules. 2017; 22(3):195. https://doi.org/10.3390/molecules22030195
Chicago/Turabian StyleZhu, Liancai, Jinqiu Chen, Jun Tan, Xi Liu, and Bochu Wang. 2017. "Flavonoids from Agrimonia pilosa Ledeb: Free Radical Scavenging and DNA Oxidative Damage Protection Activities and Analysis of Bioactivity-Structure Relationship Based on Molecular and Electronic Structures" Molecules 22, no. 3: 195. https://doi.org/10.3390/molecules22030195