Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone
Abstract
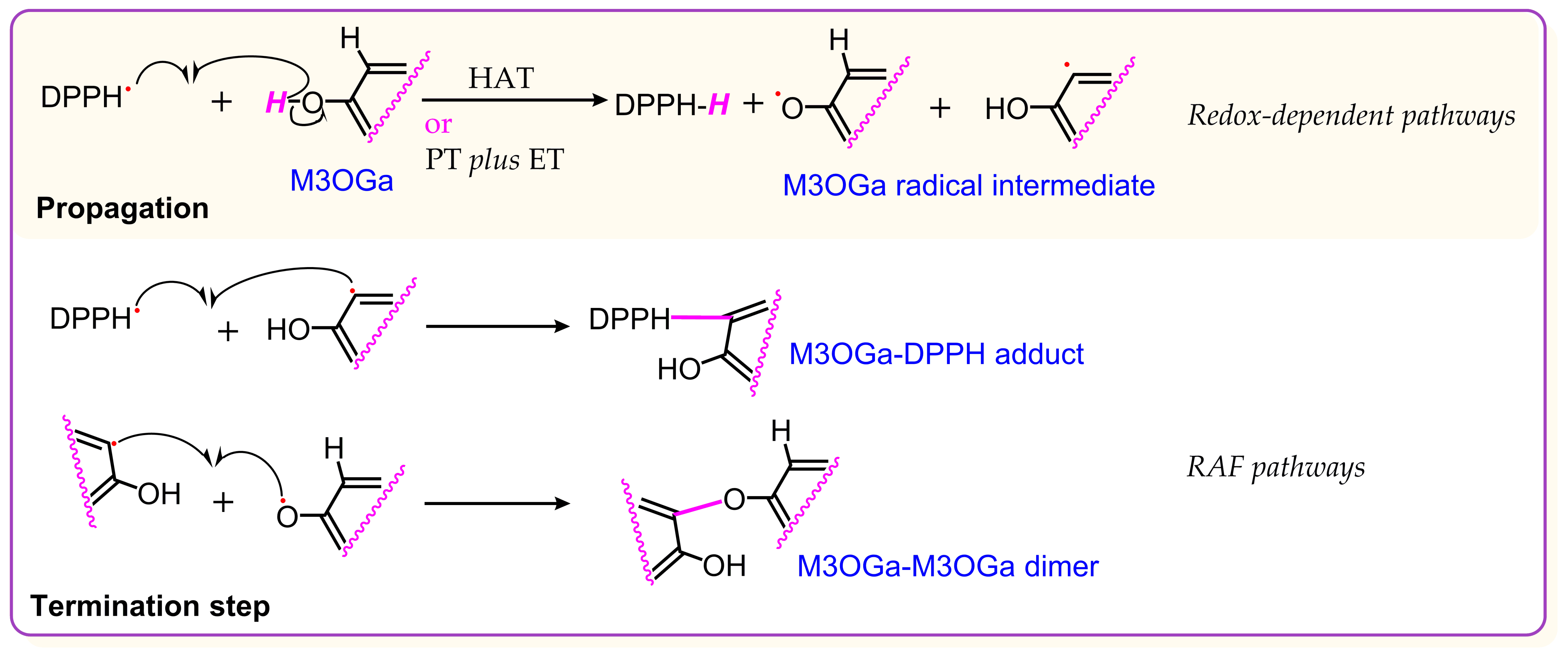
:1. Introduction
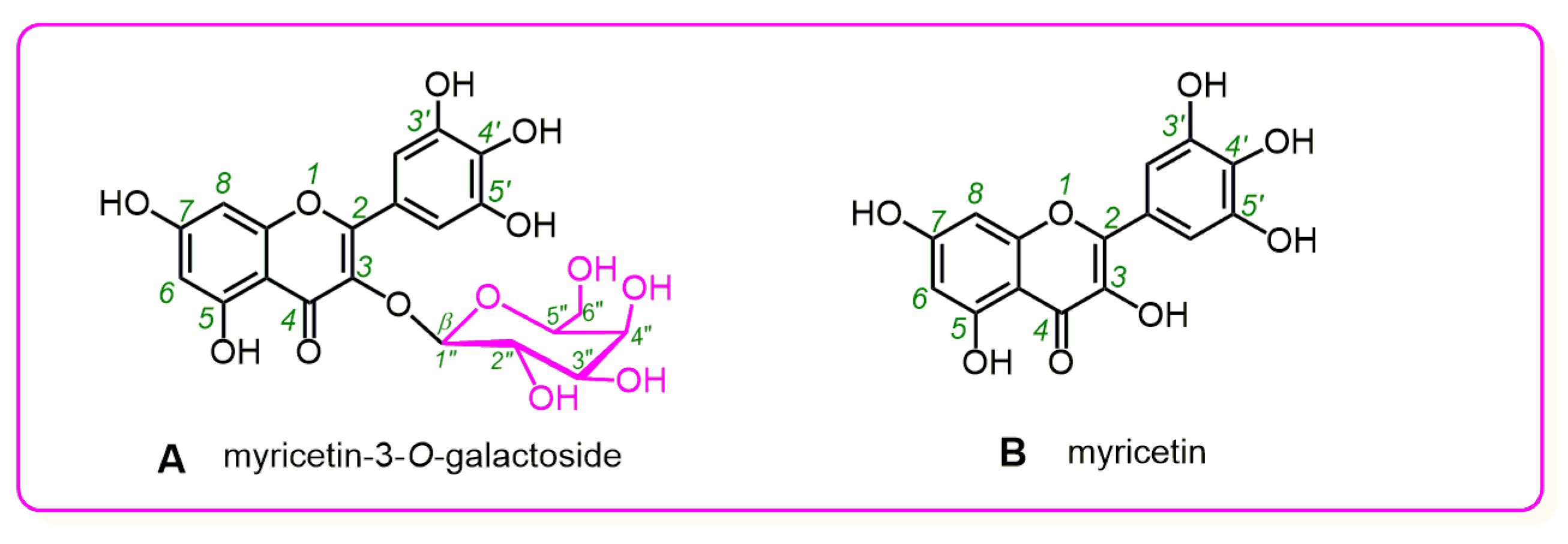
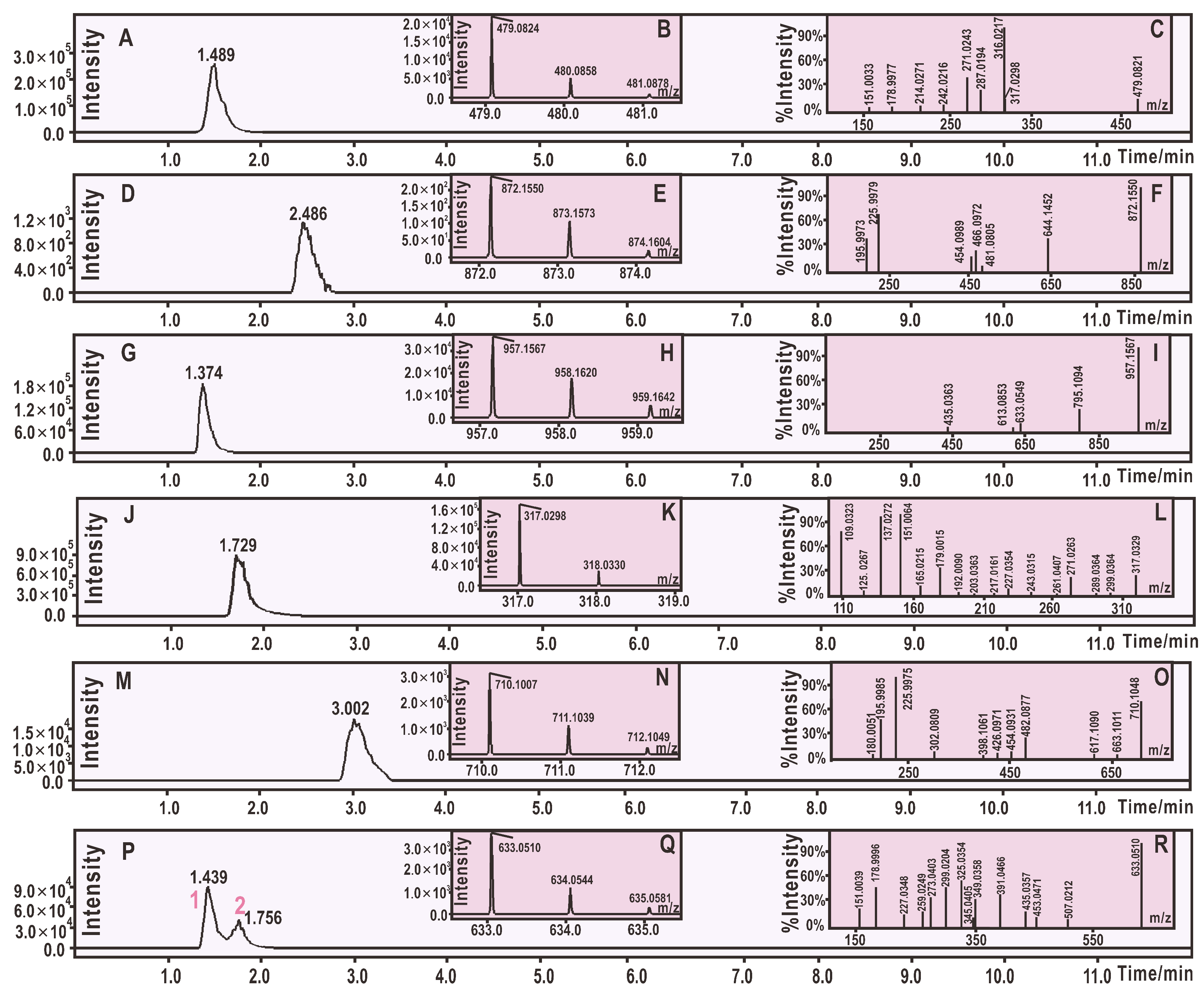
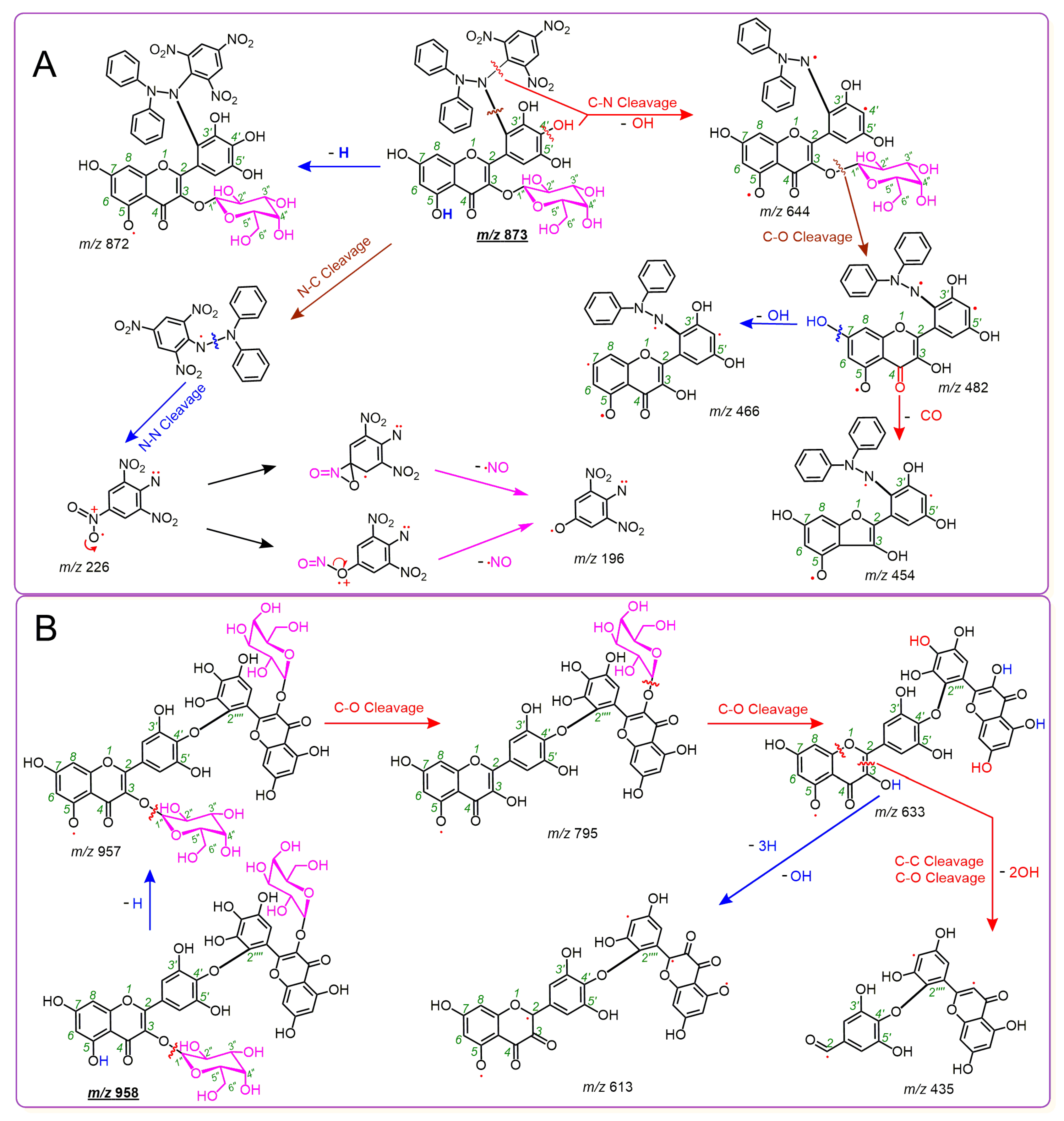
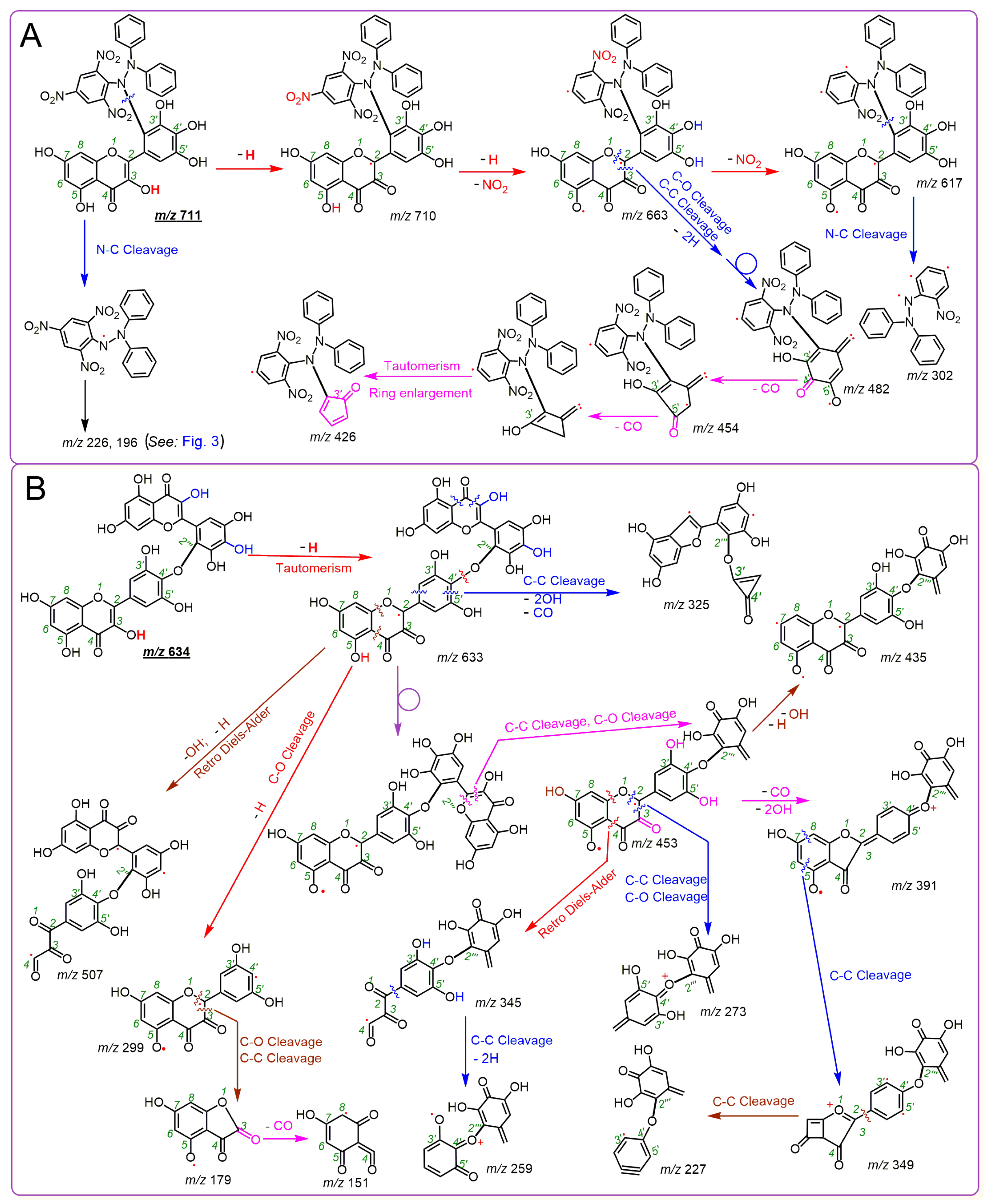
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. UPLC-ESI-Q-TOF-MS Analysis of DPPH• Reaction Products with M3OGa and Myricetin
3.3. DPPH• Radical-Trapping Analysis
3.4. PTIO•-Trapping Spectrophotometric Analysis
3.5. Superoxide Anion (•O2−)-Scavenging Spectrophotometric Analysis (Pyrogallol Autoxidation Method)
3.6. Preferential Conformation Analysis by Computational Chemistry and Molecular Weight Calculation
3.7. Statistical Analysis
4. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DPPH | α,α-diphenyl-β-picrylhydrazyl radical |
| EDTA | ethylenediaminetetraacetic acid |
| ET | electron transfer |
| PTIO | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| RAF | radical adduct formation |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SPSS | statistical product and service solutions |
| Tris | tris-hydroxymethyl amino methane |
| UPLC–ESI–Q–TOF–MS | ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry |
References
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.T.; Craven, F.L.; Voglmeir, J.; Sardzik, R.; Flitsch, S.L.; Webb, S.J. Accelerated enzymatic galactosylation of N-acetylglucosaminolipids in lipid microdomains. J. Am. Chem. Soc. 2012, 134, 13010–13017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Xu, Z.; Li, M.; Chen, K.; Zhang, Y.; Hu, Z.; Zhang, M.; Zhang, Z.; Qiao, X.; et al. Highly Promiscuous Flavonoid 3-O-Glycosyltransferase from Scutellaria baicalensis. Org. Lett. 2019, 21, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xu, Z.S.; Liu, J.X.; Li, J.W.; Wang, F.; Xiong, A.S. Isolation, purification, and characterization of AgUCGalT1, a galactosyltransferase involved in anthocyanin galactosylation in purple celery (Apium graveolens L.). Planta 2018, 247, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.E.; David, A.J.; Polo, M.A.S.; Marina, A. Structural Snapshots of the Reaction Center of Family GT6 α-1,3-Galactosyltransferase with Native Substrates. Insights into the Catalytic Mechanism of Retaining Glycosyltransferases. Angew. Chem. Int. Ed. 2017. [Google Scholar] [CrossRef]
- Lee, C.W.; Seo, J.Y.; Lee, J.; Choi, J.W.; Cho, S.; Bae, J.Y.; Sohng, J.K.; Kim, S.O.; Kim, J.; Park, Y.I. 3-O-Glucosylation of quercetin enhances inhibitory effects on the adipocyte differentiation and lipogenesis. Biomed. Pharmacoter. 2017, 95, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, B.N.; Valcic, S.; Liu, Y.L.; Montenegro, G. Flavonols from Cryptocarya alba. Z. Naturforsch C 1995, 50, 898–899. [Google Scholar] [CrossRef]
- Choi, S.J.; Tai, B.H.; Cuong, N.M.; Kim, Y.H.; Jang, H.D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Diaz, J.G.; Carmona, A.J.; Torres, F.; Quintana, J.; Estevez, F.; Herz, W. Cytotoxic activities of flavonoid glycoside acetates from Consolida oliveriana. Planta Med. 2008, 74, 171–174. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Serreli, G.; Jerkovic, I.; Gil, K.A.; Marijanovic, Z.; Pacini, V.; Tuberoso, C.I.G. Phenolic Compounds, Volatiles and Antioxidant Capacity of White Myrtle Berry Liqueurs. Plant Food Hum. Nutr. 2017, 72, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, L.C.; Xi, H.F.; Guan, L.; Fang, J.B.; Liu, Y.L.; Wu, B.H.; Li, S.H. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Li, X.; Lu, W.; Zhao, X.; Chen, D. A Null B-Ring Improves the Antioxidant Levels of Flavonol: A Comparative Study between Galangin and 3,5,7-Trihydroxychromone. Molecules 2018, 23, 3083. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, C.Y. Comprehensive Study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Various Polyphenolics in Scavenging a Free Radical and its Structural Relationship. Crit. Rev. Food Sci. 2004, 44, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Djeradi, H.; Rahmouni, A.; Cheriti, A. Antioxidant activity of flavonoids: A QSAR modeling using Fukui indices descriptors. J. Mol. Model. 2014, 20, 2476. [Google Scholar] [CrossRef] [PubMed]
- Amic, D.; Lucic, B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorgan. Med. Chem. 2010, 18, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.-L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 2010, 39, 2106–2119. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Maeda-Yamamoto, M.; Nesumi, A.; Murakami, A. Delphinidin-3-O-galactoside protects mouse hepatocytes from (-)-epigallocatechin-3-gallate-induced cytotoxicity via up-regulation of heme oxygenase-1 and heat shock protein 70. Nutr. Res. 2012, 32, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Reina, M.; Martinez, A. A new free radical scavenging cascade involving melatonin and three of its metabolites (3OHM, AFMK and AMK). Comput. Theor. Chem. 2018, 1123, 111–118. [Google Scholar] [CrossRef]
- Bayat, A.; Fattahi, A. A quantum chemical study on the OH radical quenching by natural antioxidant fisetin. J. Phys. Org. Chem. 2017, 30, 8. [Google Scholar] [CrossRef]
- Liang, M.S.; Li, X.C.; Ouyang, X.J.; Xie, H.; Chen, D.F. Antioxidant Mechanisms of Echinatin and Licochalcone, A. Molecules 2019, 24, 14. [Google Scholar] [CrossRef]
- Hassan, I.; Pinto, S.; Weisbecker, C.; Attygalle, A.B. Competitive Deprotonation and Superoxide O-2(-center dot) Radical-Anion Adduct Formation Reactions of Carboxamides under Negative-Ion Atmospheric-Pressure Helium-Plasma Ionization (HePI) Conditions. J. Am. Soc. Mass Spectr. 2016, 27, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Li, W.G.; Chen, L.F.; Xiao, B.K.; Yang, J.Y.; Yang, L.; Zhang, C.G.; Huang, R.Q.; Dong, J.X. ABTS+• scavenging potency of selected flavonols from Hypericum perforatum L. by HPLC-ESI/MS QQQ: Reaction observation, adduct characterization and scavenging activity determination. Food Res. Int. 2014, 58, 47–58. [Google Scholar] [CrossRef]
- Gross, J.H. Mass Spectrometry, 2nd ed.; Science Press: Beijing, China, 2013; pp. 718–719. [Google Scholar]
- Li, X. Antioxidant Change in Biosynthesis from Naringenin Chalcone to Flavonoid Apingenin. ChemistrySelect 2019, 4, 5155–5159. [Google Scholar]
- Liu, Q.; Li, X.; Ouyang, X.; Chen, D. Dual Effect of Glucuronidation of a Pyrogallol-Type Phytophenol Antioxidant: A Comparison between Scutellarein and Scutellarin. Molecules 2018, 23, 3225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure-Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, H.; Zhan, R.; Chen, D. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules 2018, 23, 754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, X.; Cai, R.; Chen, D. 3′,8″-Dimerization Enhances the Antioxidant Capacity of Flavonoids: Evidence from Acacetin and Isoginkgetin. Molecules 2019, 24, 2039. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.C.; Zeng, H.P. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH•) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Markovic, S.; Tosovic, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef]
- Liu, Z.Q. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Daquino, C.; Mackie, I.D.; DiLabio, G.A.; Ingold, K.U. Reaction of phenols with the 2,2-diphenyl-1-picrylhydrazyl radical. Kinetics and DFT calculations applied to determine ArO-H bond dissociation enthalpies and reaction mechanism. J. Org. Chem. 2008, 73, 9270–9282. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, J.N.; Cattaneo, M.; Day, T.C.; Tenderholt, A.L.; Mayer, J.M. Multiple-site concerted proton-electron transfer reactions of hydrogen-bonded phenols are nonadiabatic and well described by semiclassical Marcus theory. J. Am. Chem. Soc. 2012, 134, 16635–16645. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Nauser, T.; Koppenol, W.H.; Kettle, A.J.; Winterbourn, C.C.; Nagy, P. Rapid reaction of superoxide with insulin-tyrosyl radicals to generate a hydroperoxide with subsequent glutathione addition. Free Radic. Biol. Med. 2014, 70, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Hara, Y.; Steenken, S.; Simic, M.G. Antioxidant Potential of Gallocatechins. A Pulse Radiolysis and Laser Photolysis Study. J. Am. Chem. Soc. 1995, 117, 9881–9888. [Google Scholar] [CrossRef]
- Quintero-Saumeth, J.; Rincon, D.A.; Doerr, M.; Daza, M.C. Concerted double proton-transfer electron-transfer between catechol and superoxide radical anion. Phys. Chem. Chem. Phys. 2017, 19, 26179–26190. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Uno, B. Importance of Proton-Coupled Electron Transfer from Natural Phenolic Compounds in Superoxide Scavenging. Chem. Pharm. Bull. 2015, 63, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Holtomo, O.; Nsangou, M.; Fifen, J.J.; Motapon, O. DFT study of the effect of solvent on the H-atom transfer involved in the scavenging of the free radicals •HO2 and •O2− by caffeic acid phenethyl ester and some of its derivatives. J. Mol. Model. 2014, 20, 13. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Chen, B.; Wei, G.; Chen, D. E-Configuration Improves Antioxidant and Cytoprotective Capacities of Resveratrols. Molecules 2018, 23, 1790. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Comparative Study of 1,1-Diphenyl-2-picryl-hydrazyl Radical (DPPH•) Scavenging Capacity of the Antioxidant Xanthones Family. Chemistryselect 2018, 3, 13081–13086. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Zhao, X.; Chen, D. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide Radical (PTIO•) Trapping Activity and Mechanisms of 16 Phenolic Xanthones. Molecules 2018, 23, 1692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the p-Coumaroyl Moiety in the Antioxidant and Cytoprotective Effects of Flavonoid Glycosides: Comparison of Astragalin and Tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jiang, Q.; Chen, B.; Luo, X.L.; Chen, D.F. Structure–Activity Relationship and Prediction of the Electron-Transfer Potential of the Xanthones Series. Chem. Open 2018, 7, 730–736. [Google Scholar] [CrossRef] [PubMed]
- MacAleese, L.; Hermelin, S.; Hage, K.E.; Chouzenoux, P.; Kulesza, A.; Antoine, R.; Bonacina, L.; Meuwly, M.; Wolf, J.P.; Dugourd, P. Sequential Proton Coupled Electron Transfer (PCET): Dynamics Observed over 8 Orders of Magnitude in Time. J. Am. Chem. Soc. 2016, 138, 4401–4407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Hernandez-Marin, E.; Galano, A. Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct. 2012, 3, 442–450. [Google Scholar] [CrossRef]
- Li, X.C.; Han, L.; Li, Y.R.; Zhang, J.; Chen, J.M.; Lu, W.B.; Zhao, X.J.; Lai, Y.Y.; Chen, D.F.; Wei, G. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Li, X.; Chen, J.; Deng, Y.; Lu, W.; Chen, D. pH Effect and Chemical Mechanisms of Antioxidant Higenamine. Molecules 2018, 23, 2176. [Google Scholar] [CrossRef]
- Li, X.C.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
- Li, X.C. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Barigye, S.J.; Shahamat, M.; Labute, P.; Moitessier, N. Atom Types Independent Molecular Mechanics Method for Predicting the Conformational Energy of Small Molecules. J. Chem. Inf. Model. 2018, 58, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ciric-Marjanovic, G.; Trchova, M.; Konyushenko, E.N.; Holler, P.; Stejskal, J. Chemical oxidative polymerization of aminodiphenylamines. J. Phys. Chem. B 2008, 112, 6976–6987. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, J.; Jensen, F. Conformational Interconversions of Amino Acid Derivatives. J. Chem. Theory Comput. 2016, 12, 694–705. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound myricetin-3-O-galactoside is available from the authors. |

| Antioxidant Analyses | M3OGa | Myricetin | Trolox |
|---|---|---|---|
| DPPH•-trapping | 12.9 ± 0.3 b | 10.7 ± 0.3 a | 26.4 ± 2.5 |
| PTIO•-trapping (pH 4.5) | 263.7 ± 3.5 b | 132.9 ± 5.1 a | 220.1 ± 4.6 |
| PTIO•-trapping (pH 7.4) | 131.2 ± 5.1 b | 81.5 ± 2.4 a | 142.9 ± 5.0 |
| •O2−-trapping | 88.9 ± 7.2 b | 73.3 ± 1.9 a | 2777.5 ± 35.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ouyang, X.; Liang, M.; Chen, D. Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone. Molecules 2019, 24, 2769. https://doi.org/10.3390/molecules24152769
Li X, Ouyang X, Liang M, Chen D. Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone. Molecules. 2019; 24(15):2769. https://doi.org/10.3390/molecules24152769
Chicago/Turabian StyleLi, Xican, Xiaojian Ouyang, Minshi Liang, and Dongfeng Chen. 2019. "Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone" Molecules 24, no. 15: 2769. https://doi.org/10.3390/molecules24152769
APA StyleLi, X., Ouyang, X., Liang, M., & Chen, D. (2019). Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone. Molecules, 24(15), 2769. https://doi.org/10.3390/molecules24152769





