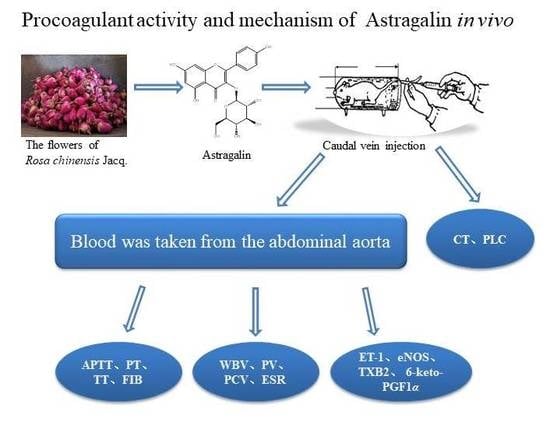
Evaluation Procoagulant Activity and Mechanism of Astragalin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coagulation Time Test In Vitro
2.1.1. Effects on Plasma Coagulation Parameters In Vitro
2.1.2. Effect of Different Concentration of Astragalin on Coagulation In Vitro
2.2. Effects on Plasma Coagulation Parameters In Vivo
2.2.1. Effects of Astragalin on Coagulation Time (CT) in Rats
2.2.2. Effects of Astragalin on Platelets (PLC)
2.2.3. Effect of Astragalin on Plasma Coagulation Parameters In Vivo
2.2.4. Effects of Astragalin on Thromboxane B2 (TXB2) and 6-Keto Prostaglandin F1α (6-Keto-PGF1α)
2.2.5. Effects of Astragalin on Endothelin-1 (ET-1) and Nitric Oxide Synthase (eNOS)
2.2.6. Effects of Astragalin on Whole Blood Viscosity (WBV) and Plasma Viscosity (PV)
2.2.7. Effects of Astragalin on Blood Erythrocyte Sedimentation Rate (ESR) and Packed Cell Volume (PCV)
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Extraction and Isolation
3.4. Coagulation Time Test In Vitro
3.4.1. APTT Assay
3.4.2. PT Assay
3.4.3. TT Assay
3.4.4. FIB Assay
3.5. Assays of the Procoagulant Effect of Astragalin In Vivo
3.5.1. Determination of Coagulation Time
3.5.2. Number of Platelets (PLC)
3.5.3. Blood Sample Collection
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APTT | activated partial thromboplastin time |
| PT | prothrombin time |
| TT | thrombin time |
| FIB | plasma fibrinogen |
| 6-keto-PGF1α | 6-keto prostaglandin F1α |
| eNOS | nitric oxide synthase |
| TXB2 | thromboxane B2 |
| ET-1 | endothelin-1 |
| ESR | erythrocyte sedimentation rate |
| PCV | packed cell volume |
| WBV | whole blood viscosity |
| PV | plasma viscosity |
| TXA2 | thromboxane A2 |
| PGI2 | prostaglandin I2 |
| NO | nitric oxide |
| SD | Sprague–Dawley |
| CT | coagulation time |
| PLC | platelet count |
| EI-MS | electronimpact mass spectrometry |
| HPLC | high performance liquid chromatography |
References
- Li, M.X. Study on the Hemostatic Active Part of Tibetan Medicine Single Herb; Lanzhou University: Lanzhou, China, 2008. [Google Scholar]
- Zhang, M.L. Study on the Substance Basis and Mechanism of Nepeta Charcoal Hemostasis; Beijing University of Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Liu, X.M. Discussion on “Charred Charcoal Hemostasis” and “Charred Ash Retention” from the Hemostatic Effect and Mechanism of Phellodendron; Beijing University of Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Jean, C.F.; Jay, E.; Thierry, B. Improving haemophilia therapy in developing countries: Virus-safe cryoprecipitate. Vox Sang. 2019, 114, 635–636. [Google Scholar]
- Ahnström, J. The potential of serpins for future treatment for haemophilia. J. Throm. Haemoph. 2019, 17, 1629–1631. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.D.; Wolberg, A.S. Mechanistic rationale for factor XIII cotreatment in haemophilia. Haemophilia 2019, 25, e377–e378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Clinical Effect of Gene Recombinant F VIII in Hemophilia Patients and Its Effect onThromboxane B2. World’s Latest Med. Inform. Abstr. 2018, 18, 3–4. [Google Scholar]
- Wang, Z.; Zhang, Y.; Qiu, X.S.; Zhang, Z.T.; Chen, Y.X. Perioperative characteristics and treatment of hemophilia B patients with fracture. J. Pract. Orthop. 2017, 23, 554–556. [Google Scholar]
- Luo, H.S.; Ren, J. Therapeutic effect of coagulation factor on hemophilia complicated with joint bleeding. Chin. Urban Rural Enterp. Health. 2017, 32, 81–83. [Google Scholar]
- Zhang, Y. Perioperative Nursing of Severe Obstetric Hemorrhagic Diseases with Interventional Therapy. Wisdom Health. 2019, 5, 185–186. [Google Scholar]
- He, Y.F. Effect of Catnip Charcoal on the Pharmacokinetics of the Main Component of RhizomaCoptidis and Pulsatilla Decoction; Anhui Agricultural University: Hefei, China, 2016. [Google Scholar]
- Ou, L.; Cheng, H.Y.; Zhao, P. Research progress on the effects of nepeta charcoal on blood system. China Pharm. Ind. 2010, 19, 19–20. [Google Scholar]
- Zhao, Y.; Guo, J.; Liu, T.; Li, C.; Cao, C.; Yi, Y.; Li, R. Pharmacology experimental study of new hematischesis compounds after FlosSophorae carbonized. Chin. J. Trad. Chin. Med. 2010, 35, 2346–2349. [Google Scholar]
- Yin, J.; Guo, L.G. Modern Research and Clinical Application of Traditional Chinese Medicine I; XueYuan Press: Beijing, China, 1994. [Google Scholar]
- Cai, H.F.; Luo, P.; Wang, K.; Xu, Z.P.; Wang, D.K.; Gao, J.B.; Deng, X.Y. Effects of Rhubarb Injection on Fibrinolytic System of Dogs after Vascular Bypass Surgery. Chin. J. Ani. Veter. Sci. 2010, 41, 897–901. [Google Scholar]
- Xu, X. Research progress of rhubarb. Shanghai J. Trad. Chin. Med. 2003, 37, 319. [Google Scholar]
- Li, M.; Jia, Z.; Hu, Z.; Zhang, R.; Shen, T. Experiental study on the Hemostatic Activity of Tibet medicinal herb Lamiophlomisrotate. Phys. Res. 2008, 22, 759–765. [Google Scholar]
- Shen, T.; Jia, Z.P.; Li, M.X.; Zhang, R.X.; Zhang, H.X. Preliminary study on hemostatic effect and mechanism of water extract of monogonum solanum. New Chin. Med. Clin. Pharm. 2006, 17, 93–96. [Google Scholar]
- Kagawa, K.; Tokura, K.; Uchida, K.; Kakushi, H.; Shike, T.; Nakai, H. Platelet Aggregation Inhibitors and Inotropic Constituents in PyrolaeHerba. Chem. Pharm. Bull. 1992, 40, 2083–2087. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, P.-F.; Zheng, R.-L.; Liu, Z.-M.; Jia, Z.-J. Protection of phenylpropanoid glycosides from Pedicularis against oxidative hemolysis In Vitro. Planta Med. 1993, 59, 315–317. [Google Scholar]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Toda, S.; Shirataki, Y.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Zhao, Q. Chemical constituents of Chinese rose (I). In Proceedings of the 9th National Symposium Traditional Chinese Medicine, Nature Medicine, Jiangxi, China, 4 December 2007; pp. 408–411. [Google Scholar]
- Ou, L.; Miao, Y.X.; Gao, F.; Li, M. Study on the Hemostatic Effect and Mechanism of Total Flavonoids of Limonium Bicolor in the Treatment of Anovulatory Dysfunctional Uterine Bleeding. Jilin J. Trad. Chin. Med. 2019, 39, 638–641. [Google Scholar]
- Wu, L.; Sang, Z.J.; Tang, T.; Xia, Y.T.; Wu, J.G.; Zhang, N.; Ma, J.M.; Kang, W.; Yang, Y.X.; Zhang, G.L.; et al. Regulation of clotting factor and microcirculation in RVO rabbit model by cold blood hemostasis and activating blood circulation. Chin. J. Opht. 2013, 23, 2–6. [Google Scholar]
- Ren, D.Y.; Wang, Y.H. Study on the value of coagulation function and platelet parameters in the auxiliary diagnosis of preeclampsia and its severity. Chin. J. Gen. Med. 2019, 22, 2698–2704. [Google Scholar]
- Facciorusso, A.; Takahashi, M.S.; Postula, C.E.; Buccino, V.R.; Muscatiello, N. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zheng, Z.; Wang, S.; Chen, S.; Ma, J.; Liu, G.; Li, J. Polydopamine-coated chitosan/calcium pyrophosphate hybrid microflowers as an effective hemostatic agent. Carbohydr. Polym. 2019, 224, 115175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, H.J.; Hou, R.W.; Tong, J.F.; Liu, X.H. Clinical application of coagulation four. Lab. Med. Clin. 2013, 10, 450–452. [Google Scholar]
- Yang, S.A.; Im, N.K.; Lee, I.S. Effects of methanolic extract from Salvia miltiorrhiza Bunge on In Vitro antithrombotic and antioxidative activities. Korean J. Food Sci. Technol. 2007, 39, 83–87. [Google Scholar]
- Yoo, J.H.; Han, S.H.; Kil, G.J. Active effect of anticoagulant effects in Chaenomelis fructus water extract. Korean J. Herbol. 2009, 24, 7–11. [Google Scholar]
- Wang, J. Correlation between PT APTT FIB and hematocrit. Inn. Mong. J. Med. 2009, 41, 1467–1468. [Google Scholar]
- Zhang, Y.L.; Xi, M.Z.; Choi, Y.B.; Lee, B.H. Antithrombotic Effect of Fermented, Ophiopogon japonicus, in Thrombosis-Induced Rat Models. J. Med. Food. 2017, 20, 637–645. [Google Scholar] [CrossRef]
- Li, X.Q.; Li, X.W.; Chen, Z.Q.; Shi, L.J.; Li, J.B.; Hu, S.Y.; Chen, C.H.; Zhang, H.N.; Zhang, X.; Yin, Q.R.; et al. Studies on Plasma TXB2 and PGI2 in Patients with Different Gan-Zheng. J. Hunan Univ. Trad. Chin. Med. 2001, 21, 9–11. [Google Scholar]
- He, X.R. Preparation, Hemostatic Activity Screening and Mechanism Study of Monoedoid Glycoside Monomers; Lanzhou University: Lanzhou, China, 2011. [Google Scholar]
- Nong, X.X. Hematischetic Effect of Dracaena cochinensis (Lour.) S. C. Chen. Chin. J. Trad. Chin. Med. 1997, 22, 240. [Google Scholar]
- Kim, S.G.; Choi, J.W.; Park, H.J.; Lee, S.M.; Jung, H.J. Anti-hyperlipidemic effects of the flavonoid-rich fraction from the methanol extract of Orostachy japonicus in rats. Korean J. Pharm. 2009, 40, 51–58. [Google Scholar]
- Stricker-Krongrad, A.H.; Alikhassy, Z.; Matsangos, N.; Sebastian, R.; Marti, G.; Lay, F.; Harmon, J.W. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. Eplasty 2018, 18, e14. [Google Scholar]
- Jia, X.R.; Wang, J. Discussion on hemostatic mechanism of water extract alone. J. Gansu Univ. Trad. Chin. Med. 1994, 11, 44–46. [Google Scholar]
- Núñez-Navarro, N.E.; Santana, F.M.; Parra, L.P.; Zacconi, F.C. Surfing the Blood Coagulation Cascade: Insight into the Vital Factor Xa. Curr. Med. Chem. 2019, 26, 3175–3200. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, P.; Niu, Y.; Chen, J.; Li, C.; Kang, W.Y. Evaluation antithrombotic activity and action mechanism of myricitrin. Ind. Crop. Prod. 2019, 129, 536–541. [Google Scholar] [CrossRef]
- Liu, Z.G.; Wang, X.Y.; Mao, B.P.; Xie, X.L. Study on the hemostatic mechanism of Toddaliaasiatica extracts. Chin. J. Pharm. 2016, 31, 157–159. [Google Scholar]
- Du, J.; Xu, Q.T. A study on hemostatic effect of stigma maydis polysaccharide. Henan Med. Res. 2011, 20, 398–400. [Google Scholar]
- Huang, A.Y. Screening of Hemostatic and Anti-Inflammatory Activities of Ethyl Acetate Extract from Yangxin Herb; Fujian University of Traditional Chinese Medicine: Fuzhou, China, 2014. [Google Scholar]
- Qu, X.J.; Zhang, J.L.; Zhou, X.H.; Xia, W.S. Selection of effective components of lotus root node promoting coagulation and study on coagulation effect. Chin. J. Food Biotechnol. 2009, 28, 1673–1689. [Google Scholar]
- Zhu, J. Study on the Hemostatic Substance Basis and Quality Control of ChrysanthemumNotoginseng; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2007. [Google Scholar]
- Tian, Y.; Tan, L.; Yu, J.T. Correlation between plasma thrombotic B2 level and vascular dementia. J. Med. Coll. Qingdao Univ. 2010, 46, 65–66. [Google Scholar]
- Liang, C.; Sha, Y.Y.; Zhu, X.M.; Wang, X.D.; Zhu, Y.H.; Zhao, Z.Y. Early changes in hemorheological parameters and TXA2 and PGI2 levels among patients with severe acute pancreatitis. J. Clin. Hepatobiliary Dis. 2014, 30, 549–551. [Google Scholar]
- Geerdink, L.M.; Bertram, H.; Hansmann, G. EXPRESS: First-in-Child Use of the Oral Selective Prostacyclin IP Receptor Agonist Selexipag in Pulmonary Arterial Hypertension. Pulm. Circ. 2017, 7, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Wu, R. Effective Fractions Selection of Folium Diospyris kaki and its Mechanism Research on Ischemic Stroke; Guangzhou University of Chinese Medicine: Guangzhou, China, 2012. [Google Scholar]
- Bruno, R.M.; Sudano, I.; Ghiadoni, L.; Masi, L.; Taddei, S. Interactions between Sympathetic Nervous System and Endogenous Endothelin in Patients with Essential Hypertension. Hypertension 2011, 57, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Zhu, L.; Qing, M.A.; Biao, A.I.; Tian, T. The relationship of HCT between age and ESR. Int. J. Lab. Med. 2017, 38, 209–210. [Google Scholar]
- Li, W.X.; Huang, M.Y.; Tang, Y.P.; Guo, J.M.; Shang, E.X.; Liu, X.; Duan, J.A. Establishment and optimization of acute blood stasis rat model. Chin. Pharmacol. Bull. 2011, 27, 1761–1765. [Google Scholar]
- Chen, J.; Ai, J.; Chen, S.; Xu, Z.; Lin, J.; Liu, H.; Chen, Q. Synergistic enhancement of hemostatic performance of mesoporous silica by hydrocaffeic acid and chitosan. Int. J. Biol. Macromol. 2019, 139, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, W.W.; Zhou, Y.H.; Jiang, X.K.; Jing, L.L.; Jia, L. Study on Chemical Constituents of Rosa chinensis Jacq. Flower. Chin. J. Pharm. 2012, 47, 500–503. [Google Scholar]
- Shen, T. Study on the Hemostatic Effect and Mechanism of Monopsis Solanum and Extraction and Separation of Its Active Parts; Lanzhou University: Lanzhou, China, 2006. [Google Scholar]
- Zhang, Y.; Sheng, Y.M.; Meng, X.L.; Long, Y. Effect of caffeic acid seopoletin and scutellarin on rat retinal neurons in vitro. Chin. J. Trad. Chin. Med. 2005, 12, 909–912. [Google Scholar]
Sample Availability: Sample of the compound astragalin is available from the authors. |










| Group | APTT (s) | PT (s) | TT (s) | FIB (mg/dL) |
|---|---|---|---|---|
| Control group | 55.33 ± 1.79 | 10.16 ± 0.03 | 31.33 ± 0.76 | 131.22 ± 4.18 |
| Breviscapine group (13.30 mg/mL) | 79.40 ± 0.46 *** | 11.30 ± 0.25 *** | 25.53 ± 0.18 *** | 157.61 ± 3.18 |
| Yunnan Baiyao group (20.00 mg/mL) | 34.83 ± 0.28 *** | 10.13 ± 0.06 | 28.73 ± 0.49 ** | 140.24 ± 1.06 |
| Astragalin group (2.000 mg/mL) | 41.46 ± 0.41 *** | 10.30 ± 0.10 | 19.66 ± 0.34 ***,&& | 160.91 ± 2.11 ***,&& |
| Group | APTT (s) | PT (s) | TT (s) | FIB (mg/dL) |
|---|---|---|---|---|
| Control group | 23.10 ± 0.10 | 32.43 ± 0.75 | 64.13 ± 0.81 | 83.07 ± 5.55 |
| Yunnan Baiyao group (20.00 mg/mL) | 17.30 ± 0.60 *** | 30.63 ± 0.65 *** | 55.46 ± 1.26 *** | 91.53 ± 3.34 * |
| Astragalin (5.000 mg/mL) | 17.50 ± 0.10 *** | 28.53 ± 0.32 ***,&&& | 52.66 ± 1.01 ***,&& | 102.93 ± 5.83 ***,& |
| Astragalin (2.500 mg/mL) | 20.77 ± 0.60 *** | 29.96 ± 0.66 *** | 54.20 ± 1.05 *** | 98.68 ± 6.70 ** |
| Astragalin (1.2500 mg/mL) | 21.76 ± 0.57 ** | 30.76 ± 0.15 ** | 56.23 ± 0.35 *** | 96.08 ± 2.71 ** |
| Astragalin (0.6250 mg/mL) | 22.60 ± 0.10 | 31.27 ± 0.31 * | 58.40 ± 0.60 *** | 94.41 ± 1.55 ** |
| Astragalin (0.3125 mg/mL) | 22.80 ± 0.26 | 31.83 ± 0.21 | 61.30 ± 1.25 ** | 88.71 ± 1.50 |
| Group | CT (Sec) |
|---|---|
| Control group | 145.50 ± 14.01 |
| Model group | 266.25 ± 19.96 *** |
| Positive group | 150.00 ± 21.38 ### |
| Astragalin (10 mg/kg) group | 152.25 ± 18.57 ### |
| Astragalin (5 mg/kg) group | 192.87 ± 15.15 ### |
| Astragalin (2.5 mg/kg) group | 199.87 ± 9.99 ### |
| Group | PLC (109/L) |
|---|---|
| Control group | 133 ± 11 |
| Model group | 89 ± 11 *** |
| Positive group | 131 ± 23 ### |
| Astragalin (10 mg/kg) group | 111 ± 10 ##,&& |
| Astragalin (5 mg/kg) group | 105 ± 9 &&& |
| Astragalin (2.5 mg/kg) group | 102 ± 13 &&& |
| Group | APTT (s) | PT (s) | TT (s) | FIB (mg/dL) |
|---|---|---|---|---|
| Control group | 16.60 ± 1.26 | 18.96 ± 1.21 | 44.10 ± 9.55 | 230.88 ± 41.44 |
| Model group | 120.72 ± 17.27 *** | 29.94 ± 4.24 *** | 51.25 ± 13.79 | 192.98 ± 18.16 * |
| Positive group | 42.95 ± 8.72 ### | 23.98 ± 1.84 ### | 46.16 ± 5.21 | 193.57 ± 33.37 |
| Astragalin (10 mg/kg) group | 73.10 ± 17.68 ### | 25.24 ± 2.02 ### | 43.18 ± 6.09 & | 239.39 ± 29.19 ##,& |
| Astragalin (5 mg/kg) group | 113.18 ± 26.09 | 26.34 ± 1.42 ### | 47.30 ± 3.75 | 211.95 ± 33.36 |
| Astragalin (2.5 mg/kg) group | 117.45 ± 11.77 | 29.61 ± 2.75 | 49.75 ± 8.43 | 204.83 ± 30.31 |
| Group | TXB2(ng/L) | Fla(6-keto-PGF1α)(ng/L) | TXB2/Fla(6-keto-PGF1α) |
|---|---|---|---|
| Control group | 150.64 ± 10.89 | 86.02 ± 7.29 | 1.74 ± 0.21 |
| Model group | 123.14 ± 6.73 *** | 106.85 ± 3.95 *** | 1.28 ± 0.18 *** |
| Positive group | 140.75 ± 11.79 # | 103.12 ± 4.69 | 1.31 ± 0.12 |
| Astragalin (10 mg/kg) group | 124.93 ± 8.72 | 89.8 ± 9.2 ###,&&& | 1.22 ± 0.12 |
| Astragalin (5 mg/kg) group | 133.04 ± 4.94 | 94.42 ± 6.7 ##,& | 1.50 ± 0.13 #,& |
| Astragalin (2.5 mg/kg) group | 141.11 ± 12.65 # | 92.27 ± 2.76 ###,&& | 1.51 ± 0.16 #,& |
| Group | ET-1 (ng/L) | eNOS (U/mL) |
|---|---|---|
| Control group | 120.49 ± 9.93 | 2.16 ± 0.16 |
| Model group | 63.91 ± 7.79 *** | 2.97 ± 0.08 *** |
| Positive group | 109.75 ± 16.80 ### | 2.16 ± 0.19 ### |
| Astragalin (10 mg/kg) group | 97.07 ± 13.23 ### | 2.22 ± 0.45 ### |
| Astragalin (5 mg/kg) group | 78.16 ± 12.54 # | 2.03 ± 0.20 ###,& |
| Astragalin (2.5 mg/kg) group | 60.95 ± 5.24 | 2.06 ± 0.16 ###,& |
| Group | WBV (mPa·s) | PV (mPa·s) | ||
|---|---|---|---|---|
| 200/s | 20/s | 3/s | ||
| Control group | 12.45 ± 1.03 | 15.32 ± 1.37 | 28.70 ± 0.98 | 3.60 ± 0.09 |
| Model group | 9.71 ± 0.62 *** | 13.21 ± 0.54 *** | 24.79 ± 0.91 *** | 3.31 ± 0.08 *** |
| Positive group | 11.33 ± 0.31 ### | 14.89 ± 1.07 ### | 26.86 ± 0.75 ### | 3.55 ± 0.05 ### |
| Astragalin (10 mg/kg) group | 11.69 ± 0.32 ### | 14.24 ± 0.77 # | 26.48 ± 1.15 ## | 3.48 ± 0.04 ### |
| Astragalin (5 mg/kg) group | 11.26 ± 0.59 ### | 14.06 ± 0.73 | 27.08 ± 1.06 ### | 3.43 ± 0.07 ## |
| Astragalin (2.5 mg/kg) group | 10.72 ± 0.17 ## | 13.67 ± 0.34 | 25.23 ± 0.57 | 3.44 ± 0.05 ## |
| Group | PCV (%) | ESR (mm/h) |
|---|---|---|
| Control group | 35.95 ± 0.89 | 3.93 ± 1.48 |
| Model group | 33.16 ± 1.11 * | 1.50 ± 0.55 *** |
| Positive group | 37.50 ± 2.58 ### | 2.50 ± 0.53 # |
| Astragalin (10 mg/kg) group | 35.24 ± 1.78 | 2.42 ± 0.53 # |
| Astragalin (5 mg/kg) group | 38.09 ± 2.02 ### | 2.85 ± 0.90 ##,& |
| Astragalin (2.5 mg/kg) group | 34.05 ± 2.33 | 2.64 ± 0.63 ## |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Hu, M.; Jiang, S.; Liang, Z.; Wang, J.; Liu, Z.; Wang, H.-M.D.; Kang, W. Evaluation Procoagulant Activity and Mechanism of Astragalin. Molecules 2020, 25, 177. https://doi.org/10.3390/molecules25010177
Li C, Hu M, Jiang S, Liang Z, Wang J, Liu Z, Wang H-MD, Kang W. Evaluation Procoagulant Activity and Mechanism of Astragalin. Molecules. 2020; 25(1):177. https://doi.org/10.3390/molecules25010177
Chicago/Turabian StyleLi, Changqin, Miyun Hu, Shengjun Jiang, Zhenhua Liang, Jinmei Wang, Zhenhua Liu, Hui-Min David Wang, and Wenyi Kang. 2020. "Evaluation Procoagulant Activity and Mechanism of Astragalin" Molecules 25, no. 1: 177. https://doi.org/10.3390/molecules25010177