Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Russula Species
2.1.1. Nutritional Composition of the Russula Species
2.1.2. Hydrophilic Compounds of the Russula Species
2.1.3. Lipophilic Compounds of the Russula Species
2.2. Antioxidant Activity of the Russula Species
2.3. Antibacterial Activity of the Russula Species
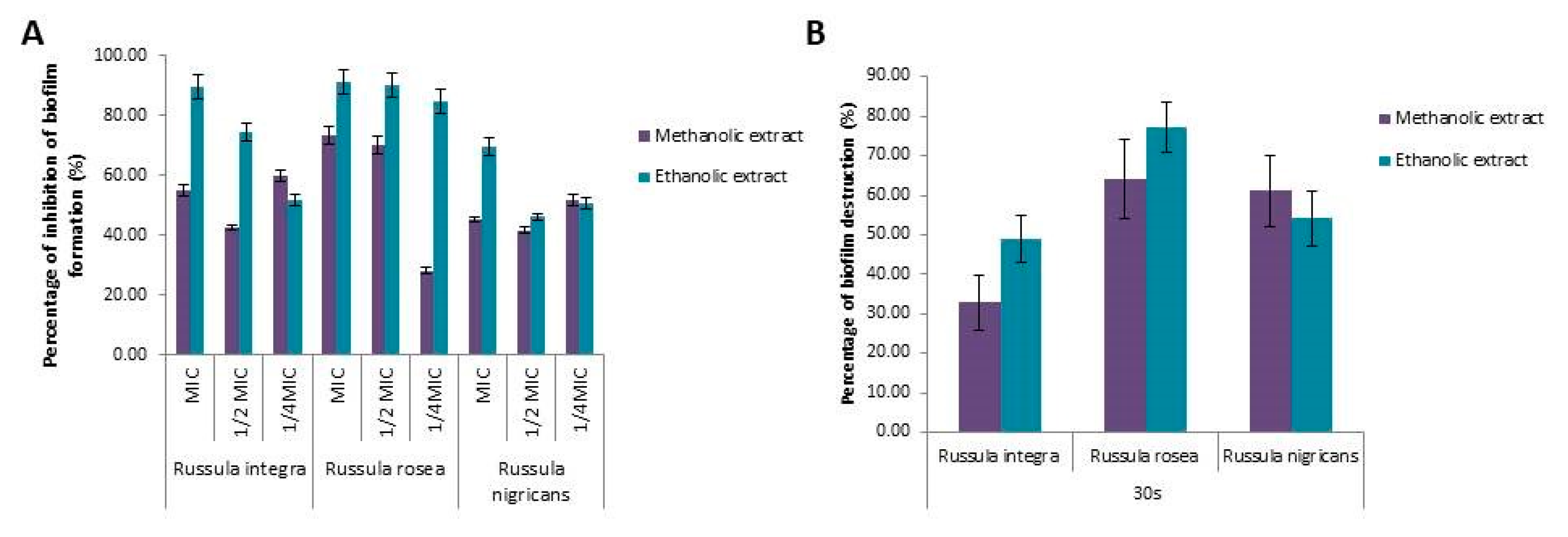
2.4. Antibiofilm Activity of the Russula Species
2.5. Cytotoxicity of the Russula Species
3. Materials and Methods
3.1. Mushroom Material
3.2. Extract Preparation of the Russula Species
3.3. Chemical Characterization of Russula Species
3.3.1. Nutritional Composition
3.3.2. Hydrophilic Compounds
3.3.3. Lipophilic Compounds
3.3.4. Phenolic Compounds Characterization
3.4. Antioxidant Activity of the Russula Species
3.4.1. Oxidative Hemolysis Inhibition Assay (OxHLIA)
3.4.2. Thiobarbituric Acid Reactive Substances (TBARS) Assay
3.5. Antibacterial and Antibiofilm Activity
3.5.1. Microorganisms: Culture Conditions and Identification
3.5.2. Microdilution Assay
3.5.3. Inhibition of Biofilm Formation
3.6. Evaluation of the Cytotoxicity in Tumor and Non-Tumor Cells
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stajic, M.; Vukojevic, J.; Knezevic, A.; Lausevic, S.; Milovanovic, I. Antioxidant Protective Effects of Mushroom Metabolites. Curr. Top. Med. Chem. 2013, 13, 2660–2676. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [Green Version]
- Stojković, D.; Živković, J.; Stanisavljević, D.; Smiljković, M.; Popović, J.; Stevanović, M.; Glamočlija, J.; Soković, M. Cytotoxicity through Molecular Targets Involved in Apoptosis. Where Should We Further Search for Mushrooms Functionalities in Future Cancer Treatment? In Frontiers in Natural Product Chemistry; Atta-ur-Rahman, Ed.; Bentham Science Publishers Pte. Ltd.: Singapore, 2019; Volume 5, pp. 146–191. [Google Scholar]
- Kaygusuz, R.; Ilhan, N.; Karlidag, T.; Keles, E.; Yalcin, S.; Cetineer., H. Free radicals and scavenging enzymes in chronic tonsillitis. J. Otolaryngol.-Head N. 2003, 129, 265–268. [Google Scholar] [CrossRef]
- Cvetkovic, T.; Vlahovic, P.; Todorovic, M.; Stankovic, M. Investigation of oxidative stress in patients with chronic tonsillitis. Auris Nasus Larynx 2009, 36, 340–344. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Stojković, D.; Smiljković, M.; Kostić, M.; Soković, M. Root vegetables as a source of biologically active agents-lesson from soil, In Phytochemicals in Vegetables: A valuable source of bioactive compounds; Petropoulos, S.A., Ferreira, I.C.F.R., Barros, L., Eds.; Bentham Science Publishers Ltd.: Singapore, 2018; Volume 1, pp. 1–39. [Google Scholar]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 496–505. [Google Scholar]
- Sokovic, M.; Glamoclija, J.; Ciric, A.; Petrovic, J.; Stojkovic, D. Mushrooms as Sources of Therapeutic Foods. In Therapeutic Foods Handbook of Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Elsevier: London, UK, 2018; Volume 8, pp. 141–178. [Google Scholar]
- Li, G.; Zhao, R.; Zhang, C.; Lin, F. A preliminary DNA barcode selection for the genus. Mycology 2019, 10, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćirić, A.; Kruljević, I.; Stojković, D.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; Glamočlija, J. Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: Functional foods with diverse biological activities. Food Funct. 2019, 10, 7678–7686. [Google Scholar]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef]
- Reis, F.S.; Heleno, S.A.; Barros, L.; Jo, M.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Toward the antioxidant and chemical characterization of mycorrhizal mushrooms from Northeast Portugal. J. Food Sci 2011, 76, C824–C830. [Google Scholar] [CrossRef]
- Reis, F.S.; Stojković, D.; Soković, M.; Glamočlija, J.; Ćirić, A.; Barros, L.; Ferreira, I.C.F.R. Chemical characterization of Agaricus bohusii, antioxidant potential and antifungal preserving properties when incorporated in cream cheese. Food Res. Int. 2012, 48, 620–626. [Google Scholar] [CrossRef]
- Stojković, D.; Reis, F.S.; Glamočlija, J.; Ćirić, A.; Barros, L.; JLD van Griensven, L.; Ferreira, I.C.F.R.; Soković, M. Cultivated strains of Agaricus bisporus and A. brasiliensis: Chemical characterization and evaluation of antioxidant and antimicrobial properties for final healthy product—Natural preservatives in yoghurt. Food Funct. 2014, 5, 1602–1612. [Google Scholar]
- Kouassi, K.A.; Kouadio, E.J.P.; Djè, K.M.; Dué, A.E.; Kouamé, L.P. Edible Ectomycorrhizal mushrooms Russula spp. of Cote d’Ivoire: Total phenolic content, HPLC-profiles of phenolic compounds and organic acids, antioxidant activities. J. Agric. Chem. Environ. 2016, 5, 73–84. [Google Scholar]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonçalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P. Comparative study of phytochemicals and antioxidant potential of wild edible mushroom caps and stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra-fast liquid chromatography and photodiode array detection. Food Anal. Method 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Expanding current knowledge on the chemical composition and antioxidant activity of the genus Lactarius. Molecules 2014, 19, 20650–20663. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom: Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Khatua, S.; Gupta, S.S.; Ghosh, M.; Tripathi, S.; Acharya, K. Exploration of nutritional, antioxidative, antibacterial and anticancer status of Russula alatoreticula: Towards valorization of a traditionally preferred unique mycofood. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Keleş, A.; Koca, İ.; Gençcelep, H. Antioxidant Properties of Wild Edible Mushrooms. J. Food Process. Tehnol. 2011, 2, 6. [Google Scholar]
- Tripathy, S.S.; Rajoriya, A.; Mahapatra, A.; Gupta, N. Biochemical and antioxidant properties of wild edible mushrooms used for food by tribal of eastern India. Int. J. Pharm. Pharm. Sci. 2016, 8, 194–199. [Google Scholar]
- Yaltirak, T.; Aslim, B.; Ozturk, S.; Alli, H. Antimicrobial and antioxidant activities of Russula delica Fr. Food Chem. Toxicol. 2009, 47, 2052–2056. [Google Scholar] [CrossRef]
- Khatua, S.; Dutta, A.K.; Chandra, S.; Paloi, S.; Das, K.; Acharya, K. Introducing a novel mushroom from mycophagy community with emphasis on biomedical potency. PLoS ONE 2017, 12, e0178050. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Ferreira, I.C.F.R.; Martins, A.; Pintado, M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Ivanov, M.; Babić, S.S.; Petrović, J.; Soković, M.; Ćirić, A. An up-to-date review on bio-resource therapeutics effective against bacterial species frequently associated with chronic sinusitis and tonsillitis. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, A.; Petrović, J.; Ivanov, M.; Kostić, M.; Soković, M. Recent Advances in Science of Quorum Sensing: An Overview of Natural Product Inhibitors. In Trends in Quorum Sensing and Quorum Quenching: New Perspectives and Applications; Ravishankar Rai, V., Jamuna, A.B., Eds.; Taylor & Francis Ltd.: London, UK, 2020; pp. 225–241. [Google Scholar]
- Zhao, Y.-Y.; Shen, X.; Chao, X.; Ho, C.C.; Cheng, X.L.; Zhang, Y.; Lin, R.-C.; Du, K.-J.; Luo, W.-J.; Chen, J.-Y.; et al. Ergosta-4, 6, 8 (14), 22-tetraen-3-one induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T.B. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 2010, 17, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Barros, L.; Antonio, A.L.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Using gamma irradiation to attenuate the effects caused by drying or freezing in Macrolepiota procera organic acids and phenolic compounds. Food Bioprocess Technol. 2014, 7, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W.; Latimer, G. (Eds.) AOAC Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Takebayashi, J.; Iwahashi, N.; Ishimi, Y.; Tai, A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem. 2012, 134, 606–610. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [CrossRef] [Green Version]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćircović, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm producton by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Smiljković, M.; Dias, M.I.; Stojković, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Soković, M. Characterization of phenolic compounds intincture of edible Nepeta nuda: Development of antimicrobial mouthwash. Food Funct. 2018, 9, 5417. [Google Scholar] [CrossRef] [Green Version]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Constituent | R. integra | R. rosea | R. nigricans |
|---|---|---|---|
| Nutritional Value (g/100 g dw) | |||
| Fat | 1.12 ± 0.04 b | 0.84 ± 0.01 c | 1.70 ± 0.06 a |
| Protein | 21.3 ± 0.5 a | 12.2 ± 0.01 c | 19.33 ± 0.5 b |
| Ash | 5.19 ± 0.04 a | 4.9 ± 0.01 b | 3.71 ± 0.08 c |
| Carbohydrates | 72.4 ± 0.3 c | 82.03 ± 0.1 a | 75.26 ± 0.4 b |
| Energy (Kcal/100 g dw) | 384.9 ± 0.3 b | 384.6 ± 0.3 b | 393.66 ± 0.4 a |
| Free Sugars (g/100 g dw) | |||
| Fructose | 0.347 ± 0.002 b | nd | 0.39 ± 0.01 a |
| Mannitol | 14.6 ± 0.3 c | 25.8 ± 0.3 b | 34 ± 1 a |
| Trehalose | 0.202 ± 0.004 b | nd | 7.2 ± 0.5 a |
| Total Sugars | 15.2 ± 0.3 c | 25.8 ± 0.3 b | 42 ± 1 a |
| Organic Acids (g/100 g dw) | |||
| Oxalic acid | 0.110 ± 0.003 b | 1.70 ± 0.01 a | tr |
| Quinic acid | 1.56 ± 0.03 b | nd | 11.26 ± 0.01 a |
| Malic acid | 1.29 ± 0.02 b | 2.06 ± 0.09 a | 0.60 ± 0.01 c |
| Citric acid | nd | nd | nd |
| Fumaric acid | 0.080 ± 0.001 a | tr | 0.020 ± 0.001 b |
| Total organic acids | 3.04 ± 0.05 c | 3.76 ± 0.1 b | 11.89 ± 0.02 a |
| Constituent | R. integra | R. rosea | R. nigricans |
|---|---|---|---|
| Fatty Acids (relative %) | |||
| C6:0 | nd | 0.39 ± 0.02 | nd |
| C8:0 | 0.12 ± 0.01 a | 0.085 ± 0.001 b | 0.042 ± 0.001 c |
| C10:0 | 0.093 ± 0.008 c | 0.197 ± 0.008 a | 0.159 ± 0.007 b |
| C12:0 | 2.20 ± 0.03 a | nd | 0.079 ± 0.05 b |
| C14:0 | 2.53 ± 0.04 a | 0.61 ± 0.02 b | 0.241 ± 0.006 c |
| C14:1 | 0.126 ± 0.006 | nd | nd |
| C15:0 | 1.1 ± 0.1 a | 0.33 ± 0.01 b | 0.35 ± 0.01 b |
| C16:0 | 16.6 ± 0.2 a | 12.0 ± 0.3 b | 8.8 ± 0.3 c |
| C16:1 | 1.55 ± 0.01 a | nd | 0.80 ± 0.01 b |
| C17:0 | 0.572 ± 0.006 b | 0.88 ± 0.03 a | 0.127 ± 0.001 c |
| C18:0 | 6.57 ± 0.02 c | 36.3 ± 0.5 a | 7.8 ± 0.3 b |
| C18:1 n9c | 21.85 ± 0.04 c | 35.74 ± 0.06 a | 27.1 ± 0.1 b |
| C18:2 n6c | 38.2 ± 0.2 b | 13.39 ± 0.06 c | 52.0 ± 0.7 a |
| C18:3n3 | 1.45 ± 0.03 a | nd | 0.061 ± 0.001 b |
| C18:3n6 | 3.27 ± 0.09 | nd | nd |
| C20:0 | 1.98 ± 0.01 a | nd | 0.210 ± 0.004 b |
| C20:1 | 0.082 ± 0.001 b | nd | 0.247 ± 0.002 a |
| C20:2 | 0.58 ± 0.03 a | nd | 0.11 ± 0.01 b |
| C20:3n3 | nd | nd | nd |
| C20:3n6 | 0.274 ± 0.001 | nd | nd |
| C20:5n3 | nd | nd | nd |
| C22:0 | nd | nd | 0.264 ± 0.003 |
| C22:1 | 0.21 ± 0.01 a | nd | 0.16 ± 0.01 b |
| C22:1n9 | nd | nd | nd |
| C22:2 | nd | nd | 0.207 ± 0.001 |
| C23:0 | 0.149 ± 0.006 a | nd | 1.17 ± 0.06 a |
| C24:0 | 0.425 ± 0.006 a | nd | 0.16 ± 0.01 b |
| C24:1 | nd | nd | nd |
| Total SFA (% of total FA) | 32.4 ± 0.3 b | 50.9 ± 0.1 a | 19.4 ± 0.6 c |
| Total MUFA (% of total FA) | 23.81 ± 0.04 c | 35.74 ± 0.06 a | 28.3 ± 0.1 b |
| Total PUFA (% of total FA) | 43.8 ± 0.3 b | 13.39 ± 0.06 c | 52.3 ± 0.7 a |
| Tocopherols (µg/100 g dw) | |||
| α-Tocopherol | 13.1 ± 0.4 c | 19.5 ± 0.3 b | 100 ± 3 a |
| β-Tocopherol | 15.8 ± 0.8 | nd | nd |
| γ-Tocopherol | 766 ± 2 | nd | nd |
| δ-Tocopherol | 15.7 ± 0.4 b | nd | 42 ± 4 a |
| Total Tocopherols | 810± 3 a | 19.5± 0.3 c | 142± 1 b |
| P-Hydroxybenzoic Acid | Cinnamic Acid | |
|---|---|---|
| RIM | nd | nd |
| RIE | nd | nd |
| RRM | nd | 20.0 ± 0.1 |
| RRE | nd | 89 ± 2 |
| t-test | - | <0.001 |
| RNM | nd | nd |
| RNE | 101 ± 5 | nd |
| Extract | OxHLIA (IC50, µg/mL) | TBARS (IC50, µg/mL) | ||
|---|---|---|---|---|
| Δt 10 min | Δt 30 min | Δt 60 min | ||
| RIM | 111 ± 4 a | na | na | 1720 ± 5 a |
| RIE | 23 ± 1 b | 69 ± 2 | 139 ± 3 | 960 ± 22 b |
| RRM | na | na | na | 92 ± 4 e |
| RRE | na | na | na | 116 ± 4 d |
| RNM | 9.1 ± 0.7 c | na | na | 120 ± 22 c |
| RNE | 1.02 ± 0.07 c | na | na | 17 ± 2 f |
| Trolox | 3.1 ± 0.3 | 8.1 ± 0.2 | 20.6 ± 0.7 | 19.6 ± 0.1 |
| Bacteria | RIM | RIE | RRM | RRE | RNM | RNE | Amoxicillin + Clavulanic Acid | Cefixime | |
|---|---|---|---|---|---|---|---|---|---|
| Micrococcus luteus | MIC | 0.78 | 3.12 | 0.20 | 0.39 | 0.78 | 3.12 | 0.0002 | 0.002 |
| MBC | 1.56 | 6.25 | 0.39 | 0.78 | 1.56 | 6.25 | 0.0004 | 0.003 | |
| Rothia mucilagenosa | MIC | 3.12 | 0.78 | 3.12 | 0.39 | 1.56 | 1.56 | 0.007 | 0.002 |
| MBC | 6.25 | 1.56 | 6.25 | 0.78 | 3.12 | 3.12 | 0.014 | 0.003 | |
| Streptococcus agalactiae | MIC | 3.12 | 1.56 | 3.12 | 0.78 | 1.56 | 1.56 | 0.007 | 0.002 |
| MBC | 6.25 | 3.12 | 6.25 | 1.56 | 3.12 | 3.12 | 0.014 | 0.004 | |
| Streptococcus angiosus | MIC | 1.56 | 3.12 | 6.25 | 0.78 | 1.56 | 3.12 | 0.028 | 0.0002 |
| MBC | 3.12 | 6.25 | 12.50 | 1.56 | 3.12 | 6.25 | 0.056 | 0.0004 | |
| Streptococcus conselatus | MIC | 3.12 | 6.25 | 3.12 | 6.25 | 0.78 | 6.25 | 0.0002 | 0.0002 |
| MBC | 6.25 | 12.50 | 6.25 | 12.50 | 1.56 | 12.50 | 0.0004 | 0.0004 | |
| Streptococcus dysgalactiae | MIC | 3.12 | 6.25 | 0.39 | 0.20 | 3.12 | 3.12 | 0.007 | 0.0002 |
| MBC | 6.25 | 12.50 | 0.78 | 0.39 | 6.25 | 6.25 | 0.014 | 0.0004 | |
| Streptococcus oralis | MIC | 0.39 | 0.78 | 6.25 | 3.12 | 3.12 | 3.12 | 0.0004 | 0.002 |
| MBC | 0.78 | 1.56 | 12.50 | 6.25 | 6.25 | 6.25 | 0.001 | 0.003 | |
| Streptococcus parasanquinis | MIC | 3.12 | 6.25 | 0.78 | 0.39 | 1.56 | 1.56 | 0.004 | 0.003 |
| MBC | 6.25 | 12.50 | 1.56 | 0.78 | 3.12 | 3.12 | 0.01 | 0.006 | |
| Streptococcus pseudopneumoniae | MIC | 0.78 | 0.78 | 1.56 | 0.78 | 3.12 | 1.56 | 0.001 | 0.013 |
| MBC | 1.56 | 1.56 | 3.12 | 1.56 | 6.25 | 3.12 | 0.002 | 0.027 | |
| Streptococcus pyogenes | MIC | 0.78 | 3.12 | 0.78 | 3.12 | 0.39 | 3.12 | 0.0004 | 0.0008 |
| MBC | 1.56 | 6.25 | 1.56 | 6.25 | 0.78 | 6.25 | 0.001 | 0.004 | |
| Streptococcus salivarius | MIC | 6.25 | 6.25 | 3.12 | 3.12 | 6.25 | 6.25 | 0.01 | 0.013 |
| MBC | 12.50 | 12.50 | 6.25 | 6.25 | 12.50 | 12.50 | 0.014 | 0.027 | |
| Staphylococcus aureus | MIC | 12.50 | 12.50 | 12.50 | 12.50 | 12.50 | 6.25 | 0.001 | 0.003 |
| MBC | >12.50 | >12.50 | >12.50 | >12.50 | >12.50 | 12.50 | 0.002 | 0.006 | |
| Staphylococcus hominis | MIC | >12.50 | 7.50 | >12.50 | >12.50 | >12.50 | 7.50 | 0.004 | 0.002 |
| MBC | >12.50 | >12.50 | >12.50 | >12.50 | >12.50 | >12.50 | 0.007 | 0.003 | |
| Staphylococcus warnerii | MIC | 6.25 | 6.25 | 3.12 | 6.25 | 6.25 | 3.12 | 0.001 | 0.003 |
| MBC | 12.50 | 12.50 | 6.25 | 12.50 | 12.50 | 6.25 | 0.002 | 0.006 | |
| Enterobacter cloacae | MIC | >12.50 | 3.12 | 6.25 | 6.25 | 3.12 | 1.56 | 0.028 | 0.003 |
| MBC | >12.50 | 6.25 | 12.50 | 12.50 | 6.25 | 3.12 | 0.056 | 0.007 | |
| Stenotrophomonas maltophilia | MIC | >12.50 | 12.50 | >12.50 | 12.50 | 12.50 | 6.25 | 0.003 | 0.003 |
| MBC | >12.50 | >12.50 | >12.50 | >12.50 | >12.50 | 12.5 | 0.007 | 0.006 |
| Cytotoxicity to Non-Tumor Cell Line | Cytotoxicity to Tumor Cell Lines | ||||
|---|---|---|---|---|---|
| PLP2 (porcine liver primary culture) | HeLa (cervical carcinoma) | HepG2 (hepatocellular carcinoma) | MCF-7 (breast carcinoma) | NCI-H460 (non-small cell lung cancer) | |
| RIM | >400 | 338 ± 13 | >400 | 253 ± 1 | 236 ± 3 |
| RIE | >400 | >400 | >400 | 305 ± 12 | 281 ± 9 |
| RRM | >400 | 333 ± 7 | 303 ± 8 | 381 ± 16 | 323 ± 7 |
| RRE | >400 | >400 | >400 | >400 | >400 |
| RNM | >400 | >400 | 372 ± 23 | >400 | >400 |
| RNE | >400 | >400 | >400 | >400 | >400 |
| Ellipticine | 2.3 ± 0.1 | 0.91 ± 0.1 | 1.10 ± 0.09 | 1.21 ± 0.02 | 1.03 ± 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, M.; Ivanov, M.; Fernandes, Â.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. https://doi.org/10.3390/molecules25184336
Kostić M, Ivanov M, Fernandes Â, Pinela J, Calhelha RC, Glamočlija J, Barros L, Ferreira ICFR, Soković M, Ćirić A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules. 2020; 25(18):4336. https://doi.org/10.3390/molecules25184336
Chicago/Turabian StyleKostić, Marina, Marija Ivanov, Ângela Fernandes, José Pinela, Ricardo C. Calhelha, Jasmina Glamočlija, Lillian Barros, Isabel C. F. R. Ferreira, Marina Soković, and Ana Ćirić. 2020. "Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity" Molecules 25, no. 18: 4336. https://doi.org/10.3390/molecules25184336











