Role of Basic Surface Groups of Activated Carbon in Chlordecone and β-Hexachlorocyclohexane Adsorption: A Molecular Modelling Study
Abstract
:1. Introduction
2. Materials and Methods
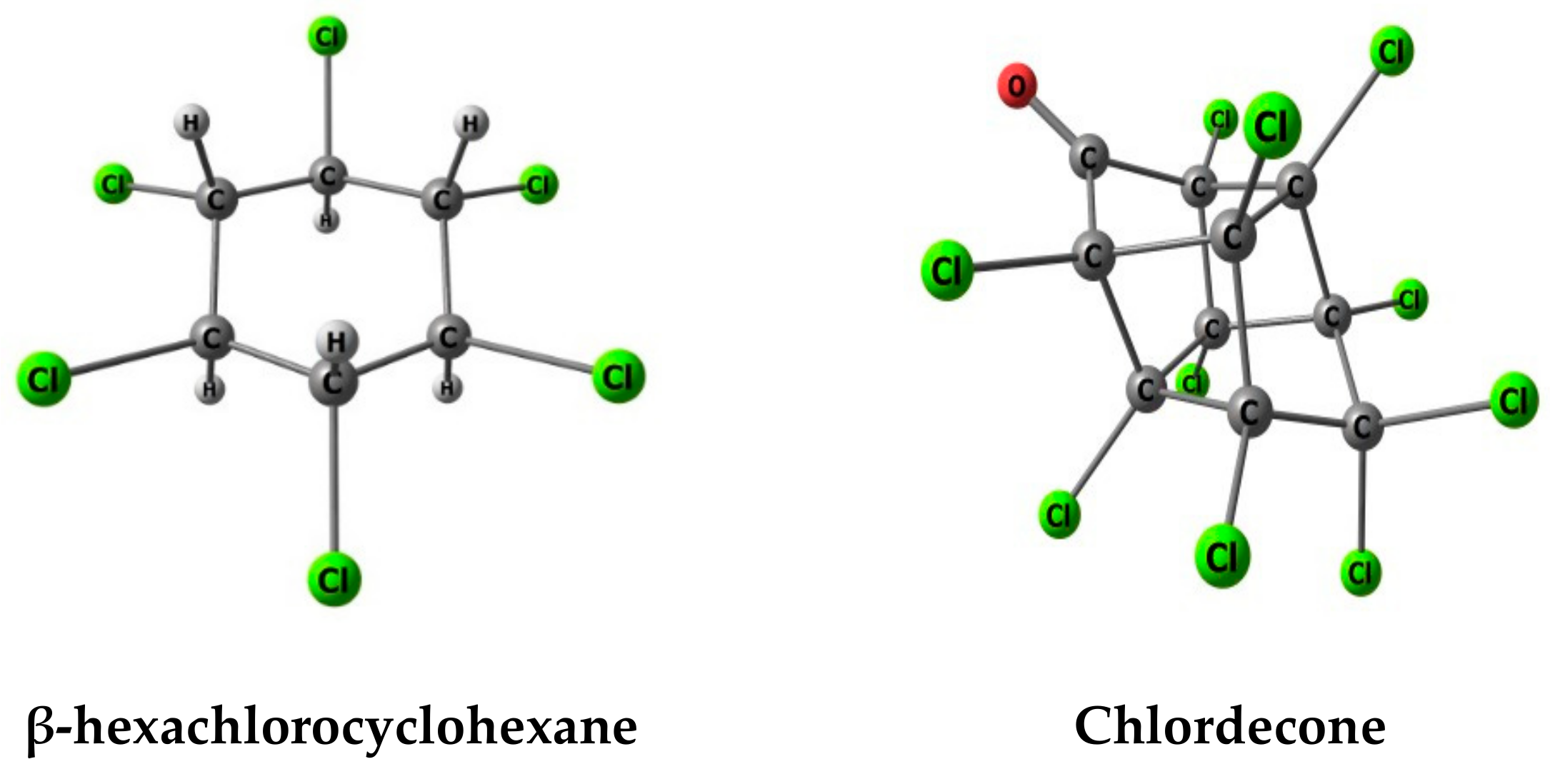
2.1. System under Study: Activated Carbon Model
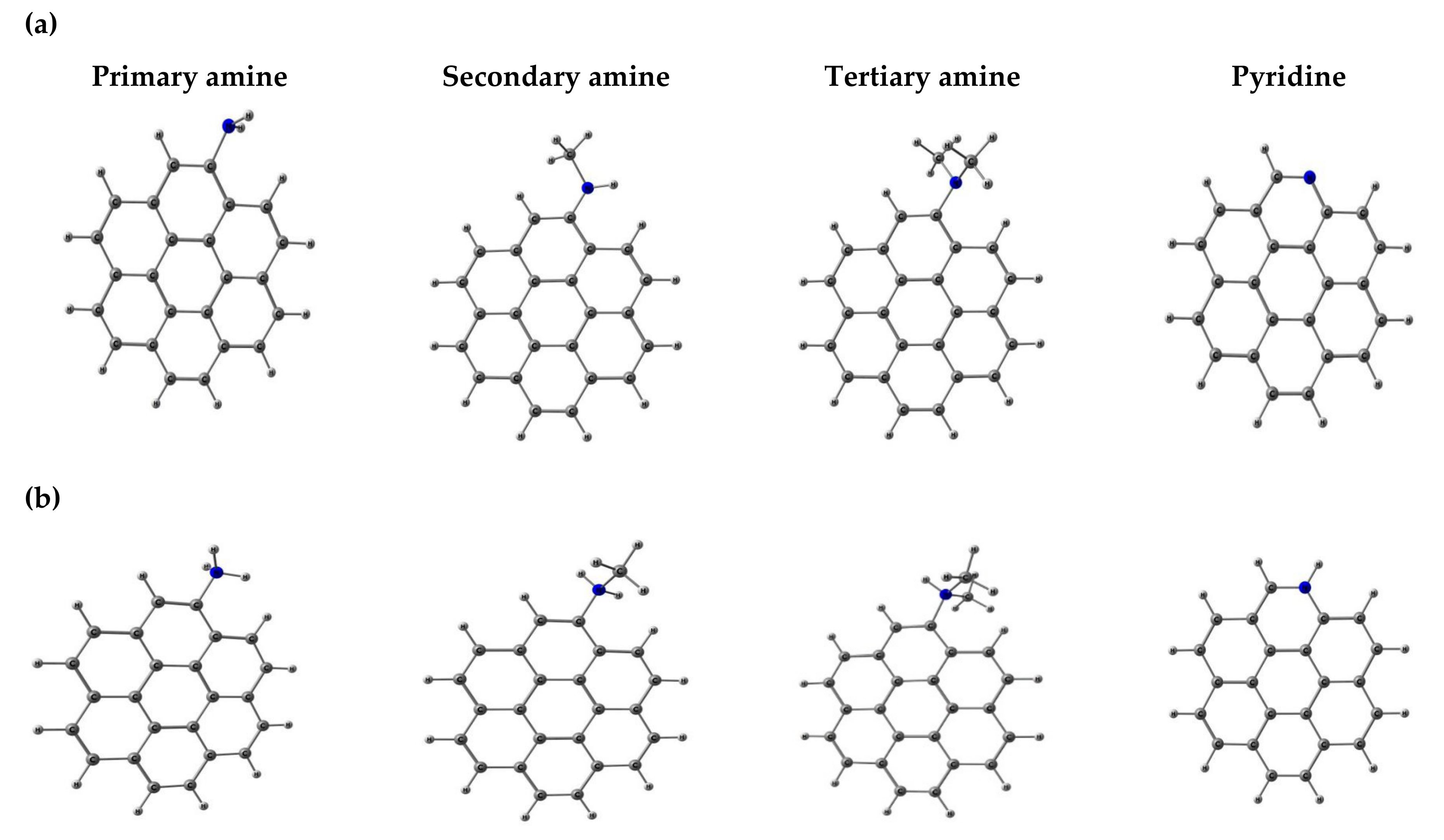
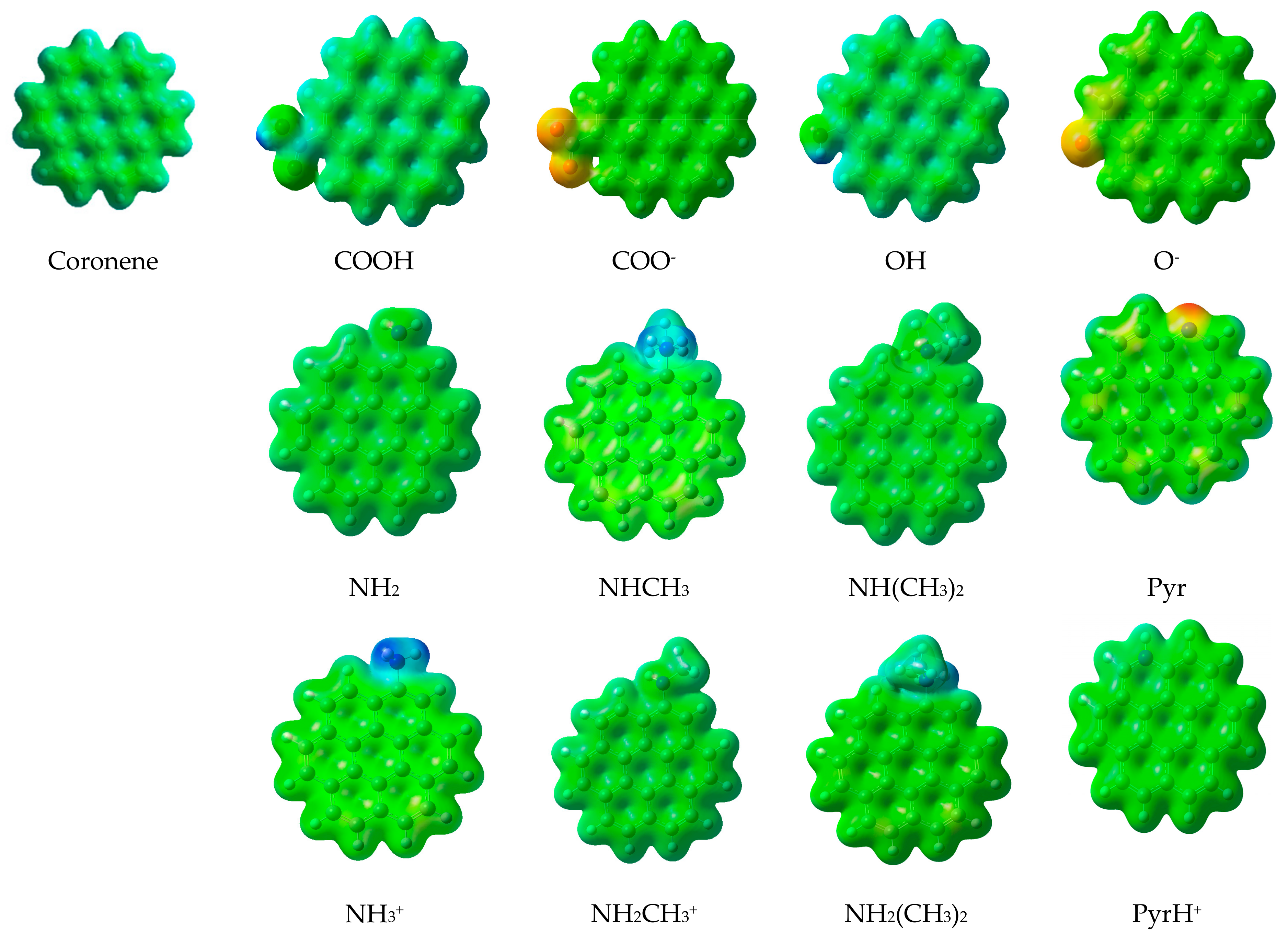
2.2. Basic Surface Groups
2.3. Computational Methodology
2.4. Multiple Minima Hypersurface Methodology
2.5. Reoptimization by Density Functional Theory
2.6. Topological Analysis of the Electron Density by Quantum Theory of Atoms in Molecules
3. Results and Discussion
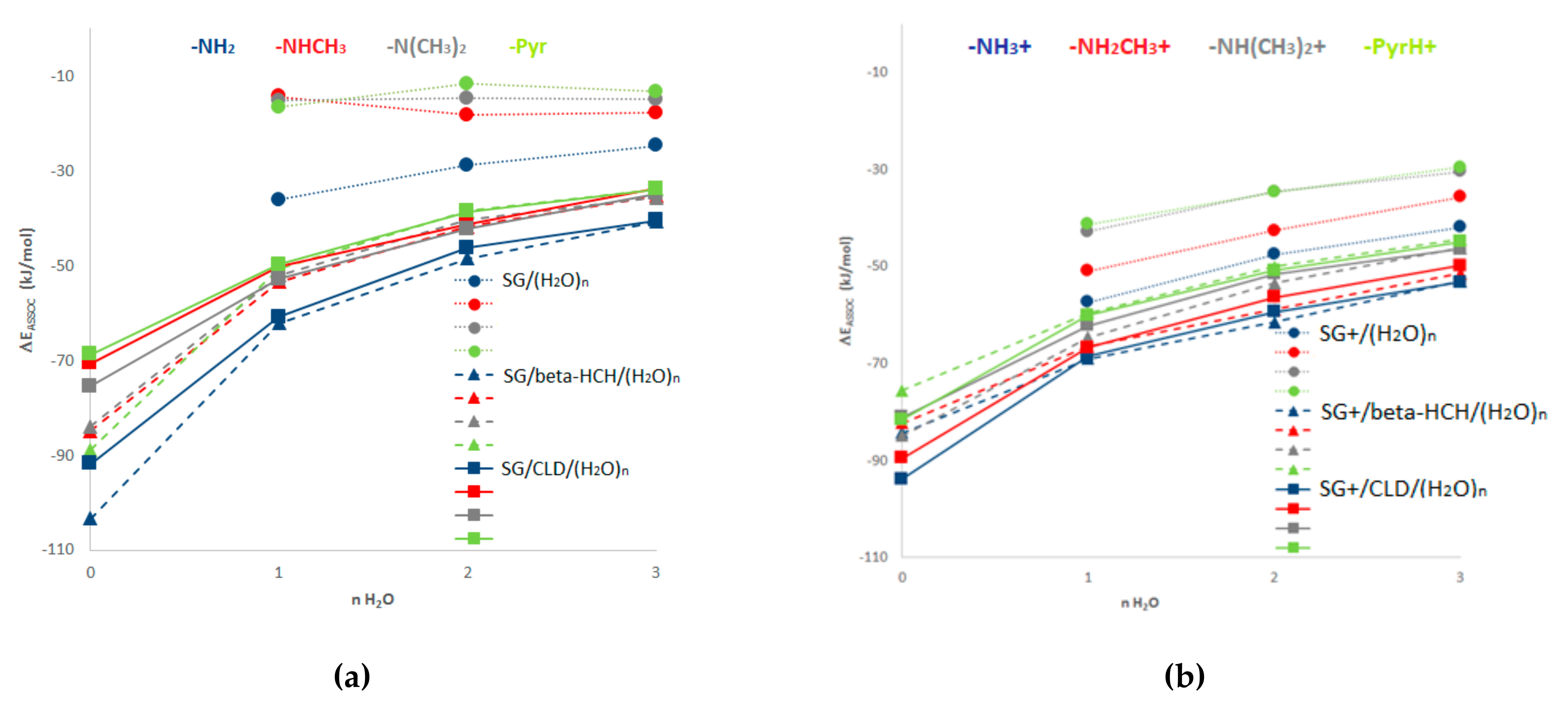
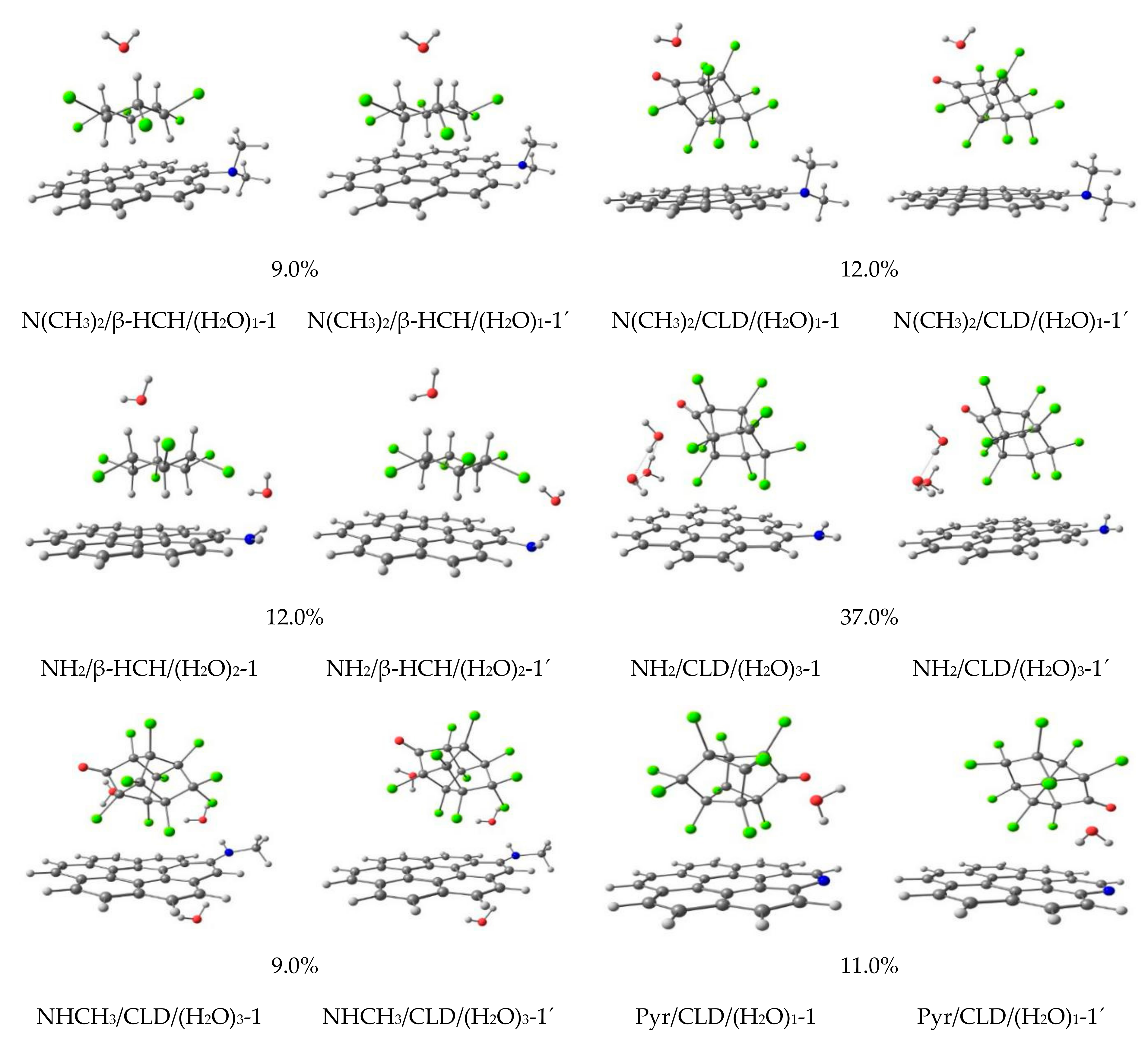
3.1. MMH Calculations
3.2. Influence of the Surface Group’s Nature
3.3. DFT Reoptimization by M06-2X/6-31+G(d,p)
3.4. QTAIM Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cabidoche, Y.M.; Achard, R.; Cattan, P.; Clermont-Dauphin, C.; Massat, F.; Sansoulet, J. Long-term pollution by chlordecone of tropical volcanic soils in the French West Indies: A simple leaching model accounts for current residue. Environ. Pollut. 2009, 157, 1697–1705. [Google Scholar] [CrossRef]
- Coat, S.; Monti, D.; Legendre, P.; Bouchon, C.; Massat, F.; Lepoint, G. Organochlorine pollution in tropical rivers (Guadeloupe): Role of ecological factors in food web bioaccumulation. Environ. Pollut. 2011, 159, 1692–1701. [Google Scholar] [CrossRef]
- Jannoyer, M.L. Crisis Management of Chronic Pollution Contaminated Soil and Human Health. Urbanization, Industrialization, and the Environment, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2017; pp. 3–10. [Google Scholar]
- Listing of POPs in the Stockholm Convention: Annex a (Elimination); UN: New York, NY, USA, 2011; Volume 2015.
- Fernández-Bayo, J.D.; Saison, C.; Voltz, M.; Disko, U.; Hofmann, D.; Berns, A.E. Chlordecone fate and mineralization in a tropical soil (andosol) microcosm under aerobic conditions. Sci. Total Environ. 2013, 463, 395–403. [Google Scholar] [CrossRef]
- Merlin, C.; Devers, M.; Crouzet, O.; Heraud, C.; Steinberg, C.; Mougin, C.; Martin-Laurent, F. Characterization of chlordecone-tolerant fungal populations isolated from long-term polluted tropical volcanic soil in the French West Indies. Environ. Sci. Pollut. Res. 2014, 21, 4914. [Google Scholar] [CrossRef]
- Manickam, M.; Mau, M.; Schlömann, M. Characterization of the novel HCH-degrading strain, Microbacterium sp. ITRC1. Appl. Microbiol. Biotechnol. 2006, 69, 580. [Google Scholar] [CrossRef]
- Calvelo, R.; Monterroso, C.; Macias, F.; Camps-Arbestain, M. Distribution pathways of hexachlorocyclohexane isomers in a soil-plantair system. A case study with Cynara scolymus L. and Erica sp. Plants grown in a contaminated site. Environ. Pollut. 2008, 155, 350–358. [Google Scholar] [CrossRef]
- Cabidoche, Y.M.; Lesueur-Jannoyer, M. Pollution durable des sols par la chlordécone aux antilles: Comment la gérer? Innovat. Agron. 2011, 16, 117–133. [Google Scholar]
- Wu, W.Z.; Schramm, K.W.; Henkelmann, B.; Xu, Y.; Yediler, A.; Kettrup, A. PCDD/Fs, PCBs, HCHs and HCB in sediments and soils of Ya-Er Lake area in China: Results on residual levels and correlation to the organic carbon and the particle size. Chemosphere 1997, 34, 191–202. [Google Scholar] [CrossRef]
- De Proft, F.; Sablon, N.; Tozer, D.J.; Geerlings, P. Calculation of negative electron affinity and aqueous anion hardness using Kohn-Sham HOMO and LUMO energies. Faraday Discuss. 2007, 135, 151–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Tang, L.; Zhang, F.; Zeng, G.; Peng, X.; Luo, L.; Deng, Y.; Pang, Y.; Zhang, J. Insight into highly efficient co-removal of p-nitrophenol and lead by nitrogen functionalized magnetic ordered mesoporous carbon: Performance and modelling. J. Hazard. Mater. 2017, 33, 80–87. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Padhye, L.P. Influence of surface chemistry of carbon materials on their interactions with inorganic nitrogen contaminants in soil and water. Chemosphere 2017, 184, 532–547. [Google Scholar]
- Sellaoui, L.; Kehili, M.; Lima, E.C.; Thue, P.S.; Bonilla-Petriciolet, A.; Ben Lamine, A.; Dotto, G.L.; Erto, A. Adsorption of phenol on microwave-assisted activated carbons: Modelling and interpretation. J. Mol. Liq. 2019, 274, 309–314. [Google Scholar] [CrossRef]
- Francoeur, M.; Ferino-Pérez, A.; Yacou, C.; Jean-Marius, C.; Emmanuel, E.; Chérémond, Y.; Jáuregui-Haza, U.J.; Gaspard, S. Activated carbon synthetized from Sargassum (sp) for adsorption of caffeine: Understanding the adsorption mechanism using molecular modeling. J. Environ. Chem. Eng. 2021, 9, 104795. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Swiatkowski, A.; Biniak, S.; Walczyk, M. Effect of properties of chemically modified activated carbon and aromatic adsorbate molecule on adsorption from liquid phase. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 1–8. [Google Scholar] [CrossRef]
- Lladó, J.; Gil, R.R.; Lao-Luque, C.; Solé-Sardans, M.; Fuente, E.; Ruiz, B. Highly microporous activated carbons derived from biocollagenic wastes of the leather industry as adsorbents of aromatic organic pollutants in water. J. Environ. Chem. Eng. 2017, 5, 2090–2100. [Google Scholar] [CrossRef]
- Khalil, K.M.S.; Khairy, M.; Allam, O.A.S.; Khalil, M.K. Formation of improved activated carbons from sugarcane bagasse as environmental materials for adsorption of phenolic pollutants. Int. J. Environ. Sci. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Montero, L.A.; Esteva, A.M.; Molina, J.; Zapardiel, A.; Hernandez, L.; Marquez, H.; Acosta, A. A theoretical approach to analytical properties of 2,4-diamino-5-phenylthiazole in water solution. Tautomerism and dependence on pH. J. Am. Chem. Soc. 1998, 120, 12023–12033. [Google Scholar] [CrossRef]
- Montero, L.A.; Llano, J.; Molina, J.; Fabian, J. Multiple minima hypersurfaces of water clusters for calculations of association energy. Int. J. Quantum Chem. 2000, 79, 8–16. [Google Scholar] [CrossRef]
- Durimel, A.; Altenor, S.; Miranda-Quintana, R.; Du Mesnil, P.C.; Jauregui-Haza, U.; Gadiou, R.; Gaspard, S. pH dependence of chlordecone adsorption on activated carbons and role of adsorbent physico-chemical properties. Chem. Eng. J. 2013, 229, 239–349. [Google Scholar] [CrossRef]
- Enriquez-Victorero, C.; Hernández-Valdés, D.; Montero-Alejo, A.L.; Durimel, A.; Gaspard, S.; Jáuregui-Haza, U.J. Theoretical study of γ-hexachlorocyclohexane and β-hexachlorocyclohexane isomers interaction with surface groups of activated carbon model. J. Mol. Graph. Model. 2014, 51, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Durimel, A.; Passé-Coutrin, N.; Jean-Marius, C.; Gadiou, R.; Enriquez-Victorero, C.; Hernández-Valdés, D.; Jauregui-Haza, U.; Gaspard, S. Role of acidic sites in beta-hexachlorocyclohexane (β-HCH) adsorption by activated carbons: Molecular modelling and adsorption-desorption studies. RSC Adv. 2015, 5, 85153–85164. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–898. [Google Scholar] [CrossRef]
- Bader, R.F.W. The density in density functional theory. J. Mol. Struct. 2010, 943, 2–18. [Google Scholar] [CrossRef]
- Gamboa-Carballo, J.J.; Melchor-Rodríguez, K.; Hernández-Valdés, D.; Enriquez-Victorero, C.; Montero-Alejo, A.L.; Gaspard, S.; Jáuregui-Haza, U.J. Theoretical study of chlordecone and surface groups interaction in an activated carbon model under acidic and neutral conditions. J. Mol. Graph. Model. 2016, 65, 83–93. [Google Scholar] [CrossRef]
- Melchor-Rodríguez, K.; Gamboa-Carballo, J.J.; Ferino-Pérez, A.; Passe-Coutrin, N.; Gaspard, S.; Jáuregui-Haza, U.J. Theoretical study on the interactions between chlordecone hydrate and acidic surface groups of activated carbon under basic pH conditions. J. Mol. Graph. Model. 2018, 81, 146–154. [Google Scholar] [CrossRef]
- Melchor-Rodríguez, K.; Gaspard, S.; Jáuregui-Haza, U.J. Chlordecone adsorption on functionalized activated carbons: Computational chemistry as a tool for understanding the adsorption process. Quim. Nova 2021, 44, 172–179. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Atoms-in-molecules dual parameter analysis of weak to strong interactions: Behaviors of electronic energy densities versus Laplacian of electron densities at bond critical points. J. Phys. Chem. A 2008, 112, 13593–13599. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Polar coordinate representation of Hb (rc) versus (ℏ2/8m)∇2ρb(rc) at BCP in AIM analysis: Classification and evaluation of weak to strong interactions. J. Phys. Chem. A 2009, 113, 10050–10057. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Carballo, J.J.; Ferino-Pérez, A.; Rana, V.K.; Levalois-Grützmacher, J.; Gaspard, S.; Montero-Cabrera, L.A.; Jáuregui-Haza, U.J. Theoretical evaluation of the molecular inclusion process between chlordecone and cyclodextrins: A new method for mitigating the basis set superposition error in the case of an implicit solvation model. J. Chem. Inf. Model. 2020, 60, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Jáuregui-Haza, U.J.; Ferino-Pérez, A.; Gamboa-Carballo, J.J.; Gaspard, S. Guest-host complexes of 1-iodochlordecone and β-1-iodo-pentachlorocyclohexane with cyclodextrins as radiotracers of organochlorine pesticides in polluted water. Environ. Sci. Pollut. Res. 2020, 27, 41105–41116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A prototype for graphene material simulation: Structures and interaction potentials of coronene dimers. J. Phys. Chem. C 2008, 112, 4061–4067. [Google Scholar] [CrossRef]
- Jenness, G.R.; Jordan, K.D. DF-DFT-SAPT Investigation of the Interaction of a water molecule to coronene and dodecabenzocoronene: Implications for the water graphite interaction. J. Phys. Chem. 2009, 123, 10242–10248. [Google Scholar] [CrossRef]
- Ishimoto, T.; Koyama, M. Theoretical study on interaction energy between water and graphene model compound. J. Comput. Chem. Jpn. 2014, 13, 171–172. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Valdés, D.; Enriquez-Victorero, C.; Pizarro-Lou, L.; Turiño-Pérez, D.; Ducat-Pagés, L.; Arias, M.; Jáuregui-Haza, U.J. Interaction of paracetamol and 125I-paracetamol with surface groups of activated carbon: Theoretical and experimental study. J. Radioanal. Nucl. Chem. 2015, 305, 609–622. [Google Scholar] [CrossRef]
- Ferino-Perez, A.; Gamboa-Carballo, J.J.; Li, Z.; Campos, L.C.; Jáuregui-Haza, U.J. Explaining the interactions between metaldehyde and acidic surface groups of activated carbon under different pH conditions. J. Mol. Graph. Model. 2019, 90, 94–103. [Google Scholar] [CrossRef]
- Di Biase, E.; Sarkisov, L. Systematic development of predictive molecular models of high surface area activated carbons for adsorption applications. Carbon 2013, 64, 262–280. [Google Scholar] [CrossRef]
- Bahamon, D.; Carro, L.; Guri, S.; Vega, L. Computational study of ibuprofen removal from water by adsorption in realistic activated carbons. J. Colloid Interface Sci. 2017, 498, 323–334. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Suárez, D.; Menéndez, J.A.; Fuente, E. On the nature of basic sites on carbon surfaces: An overview. Carbon 2004, 42, 1219–1225. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Kumar, K.V.; Müller, E.A.; Rodríguez-Reinoso, F. Effect of pore morphology on the adsorption of methane/hydrogen mixtures on carbon micropores. J. Phys. Chem. C 2012, 116, 11820–11829. [Google Scholar] [CrossRef]
- Supong, A.; Bhomick, P.C.; Baruah, M.; Pongener, C.; Sinha, U.B.; Sinha, D. Adsorptive removal of Bisphenol A by biomass activated carbon and insights into the adsorption mechanism through density functional theory calculations. Sustain. Chem. Pharm. 2019, 13, 100159. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.J.P. Stewart Computational Chemistry; Mercury Consortium: Colorado Springs, CO, USA, 2016. [Google Scholar]
- Zhurko, G.; Zhurko, D. Chemcraft, Version 1.8. User Guide. 2015. Available online: https://www.chemcraftprog.com/news.html (accessed on 20 September 2021).
- Valdés, D.H.; Victorero, C.E.; Haza, U.J.; Valdés, P.H.; Santana, S.G. Granada modificado con restricción geométrica. Granada modified with geometric restriction. RIC 2013, 7, 9–15. [Google Scholar]
- Collignon, B.; Hoang, P.; Picaud, S.; Rayez, J. Ab initio study of the water adsorption on hydroxylated graphite surfaces. Chem. Phys. Lett. 2005, 406, 430–435. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states and transitions elements: Two new functionals and systematic testing of four M06-class functionals and 12 others functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Johnson, E.R.; Mackie, I.D.; DiLabio, G.A. Dispersion interactions in density-functional theory. J. Phys. Org. Chem. 2009, 22, 1127–1135. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision d. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Graham-Solomons, T.W. Organic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-55659-7. [Google Scholar]
- Villacañas, F.; Pereira, M.F.; Órfão, J.; Figueiredo, J. Adsorption of simple aromatic compounds on activated carbons. J. Colloid Interface Sci. 2006, 293, 128–136. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Hameed, B.H. Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol. Chem. Eng. J. 2011, 169, 180–185. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, R.; Zhang, R.; Chen, J. Efficient adsorption of benzoic acid from aqueous solution by nitrogen-containing activated carbon. Water Sci. Technol. 2017, 3, 686–694. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wave function analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Lefevre, S.; Guy, L.; Robert, V.; Dutasta, J.P.; Chevallier, M.L.; Della-Negra, O.; Saaidi, P.; Martinez, A. Recognition of the persistent organic pollutan chlordecone by hemicryptophane cage. New J. Chem. 2019, 43, 10222–10226. [Google Scholar] [CrossRef] [Green Version]
- Shang, T.X.; Zhang, J.; Jin, X.J.; Gao, J.M. Study of Cr(VI) adsorption onto nitrogen-containing activated carbon preparation from bamboo processing residues. J. Wood Sci. 2014, 60, 215–224. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.L.; Qin, H.D.; Feng, Y.J. Amination of activated carbon for enhancing phenol adsorption: Effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. [Google Scholar] [CrossRef]
- Qin, H.D.; Chen, H.L. Pretreatment of concentrated leachate by the combination of coagulation and catalytic ozonation with Ce/AC catalyst. Water Sci. Technol. 2016, 73, 511–519. [Google Scholar] [CrossRef] [PubMed]


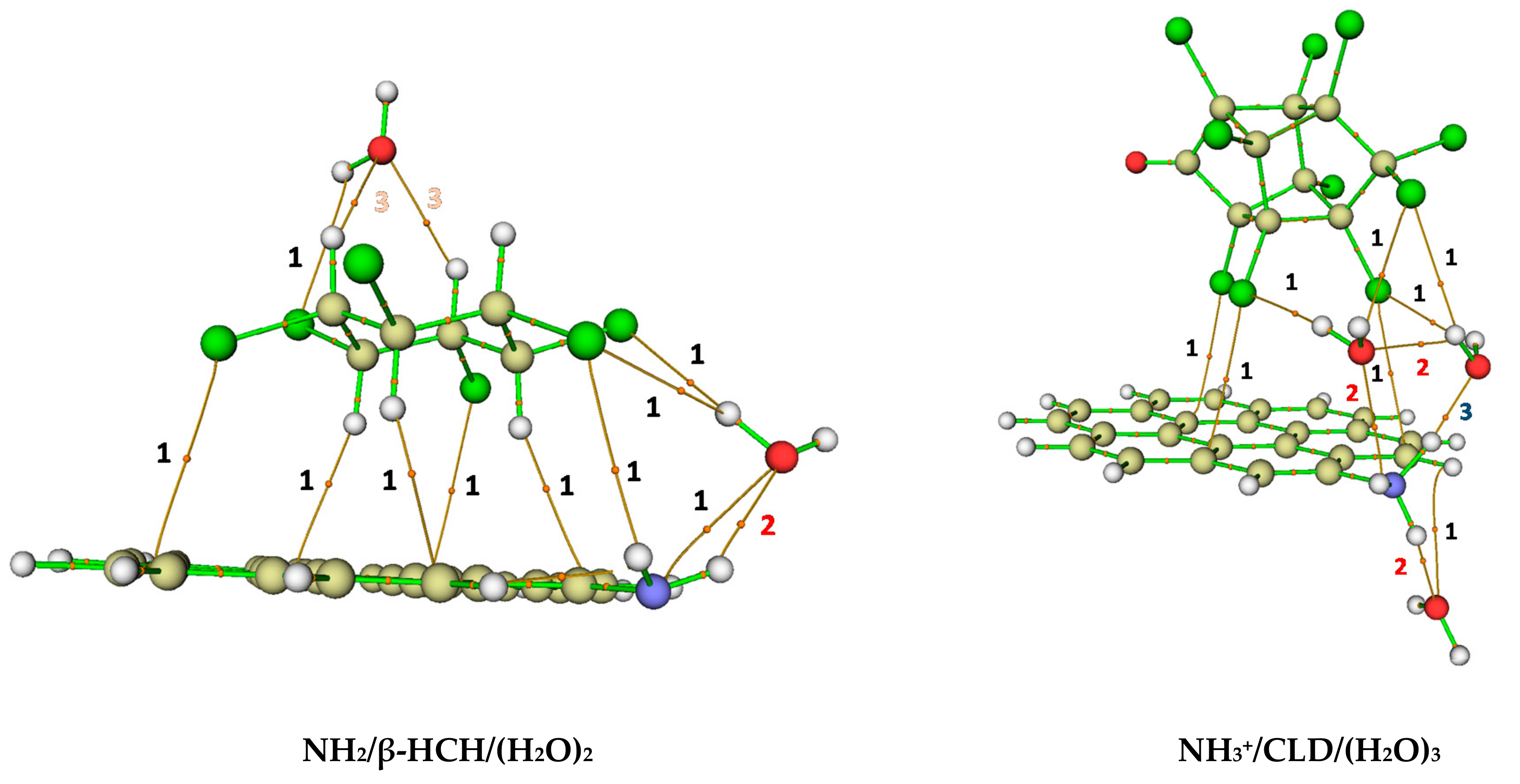
| Interaction Number | Atoms 1 | d (Ȧ) | ε | Interaction Type 2,3 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | O42…H19 (H2O…AC) | 2.77 | 0.0069 | 0.023 | 0.0007 | −0.85 | 0.17 | vdW |
| 2 | O42…H37 (H2O…AC) | 2.06 | 0.0189 | 0.078 | 0.0026 | −0.85 | 0.02 | HB |
| 1 | Cl62…C14 (P…AC) | 3.47 | 0.0067 | 0.022 | 0.0011 | −0.75 | 3.01 | vdW |
| 1 | H44...Cl60 (H2O…P) | 2.61 | 0.0100 | 0.034 | 0.0015 | −0.78 | 0.13 | vdW |
| 1 | H44...Cl59 (H2O…P) | 2.72 | 0.0080 | 0.029 | 0.0014 | −0.75 | 0.18 | vdW |
| 1 | Cl59…N36 (P…AC) | 3.47 | 0.0072 | 0.023 | 0.0009 | −0.80 | 0.73 | vdW |
| 1 | H51…C22 (P…AC) | 2.70 | 0.0082 | 0.027 | 0.0011 | -0.80 | 8.21 | vdW |
| 1 | Cl58…C27 (P…AC) | 3.51 | 0.0068 | 0.021 | 0.0010 | −0.76 | 2.63 | vdW |
| 1 | H55…C9 (P…AC) | 2.69 | 0.0079 | 0.026 | 0.0012 | −0.78 | 4.95 | vdW |
| 1 | H53…C6 (P…AC) | 2.66 | 0.0091 | 0.030 | 0.0012 | −0.81 | 3.79 | vdW |
| 3 | O39…H54 (H2O…P) | 2.28 | 0.0134 | 0.050 | 0.0017 | −0.85 | 0.10 | HB-w |
| 3 | O39…H56 (H2O…P) | 2.29 | 0.0133 | 0.049 | 0.0016 | −0.85 | 0.09 | HB-w |
| 1 | H40…Cl61 (H2O…P) | 2.66 | 0.0099 | 0.036 | 0.0015 | −0.79 | 0.12 | vdW |
| Interaction Number | Atoms 1 | d (Ȧ) | ε | Interaction Type 2,3 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | O64…H25 (H2O…AC) | 2.81 | 0.0065 | 0.023 | 0.0009 | −0.83 | 0.44 | vdW |
| 2 | O64…H38 (H2O…AC) | 1.81 | 0.0333 | 0.111 | −0.0011 | −1.04 | 0.06 | HB |
| 1 | Cl46…C12 (P…AC) | 3.20 | 0.0090 | 0.032 | 0.0015 | −0.76 | 1.62 | vdW |
| 1 | Cl60…C1 (P…AC) | 3.42 | 0.0069 | 0.022 | 0.0011 | −0.76 | 1.73 | vdW |
| 1 | Cl60…H69 (P…H2O) | 2.76 | 0.0076 | 0.026 | 0.0012 | −0.76 | 0.16 | vdW |
| 3 | O67…H39 (H2O…AC) | 1.63 | 0.0574 | 0.100 | −0.0176 | −1.41 | 0.03 | CT |
| 1 | H68…Cl59 (H2O…P) | 2.71 | 0.0087 | 0.030 | 0.0013 | −0.79 | 0.25 | vdW |
| 2 | H68…O61 (H2O…H2O) | 2.08 | 0.0191 | 0.076 | 0.0021 | −0.87 | 0.20 | HB |
| 1 | O61…Cl59 (H2O…P) | 3.36 | 0.0070 | 0.024 | 0.0009 | −0.81 | 0.51 | vdW |
| 2 | O61…H37 (H2O…AC) | 2.36 | 0.0128 | 0.050 | 0.0018 | −0.83 | 0.51 | HB |
| 1 | H62…Cl47 (H2O…P) | 2.53 | 0.0110 | 0.038 | 0.0016 | −0.80 | 0.04 | vdW |
| 1 | Cl47…C3 (P…AC) | 3.29 | 0.0081 | 0.027 | 0.0013 | −0.76 | 1.08 | vdW |
| No. | Complex | Interaction Types | |||
|---|---|---|---|---|---|
| vdW | HB | HBw | CT | ||
| 1 | NH3+/β-HCH/(H2O)3 | 10 (71.4%) | 3 (21.4%) | 1 (7.1%) | - |
| 2 | NH2CH3+/β-HCH/(H2O)2 | 11 (73.3%) | 2 (13.3%) | 2 (13.3%) | - |
| 3 | NH2CH3+/β-HCH/(H2O)3 | 13 (64.4%) | 4 (21.0%) | 2 (10.5%) | - |
| 4 | PyrH+/β-HCH/(H2O)3 | 9 (64.3%) | 3 (21.4%) | 1 (7.1%) | 1 (7.1%) |
| 5 | NH3+/CLD | 5 (83.3%) | 1 (16.6%) | - | - |
| 6 | NH3+/CLD/(H2O)1 | 6 (75.0%) | 2 (25.0%) | - | - |
| 7 | NH3+/CLD/(H2O)2 | 8 (72.7%) | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) |
| 8 | NH3+/CLD/(H2O)3 | 8 (66.6%) | 3 (25.0%) | - | 1 (8.3%) |
| 9 | NH2/β-HCH/(H2O)2 | 10 (76.9%) | 1 (7.7%) | 2 (15.4%) | - |
| 10 | N(CH3)2/β-HCH/(H2O)1 | 9 (81.8%) | 2 (18.2%) | - | - |
| 11 | NH2/CLD/(H2O)3 | 10 (71.4%) | 4 (28.6%) | - | - |
| 12 | NHCH3/CLD/(H2O)3 | 12 (85.7%) | 1 (7.1%) | 1 (7.1%) | - |
| 13 | N(CH3)2/CLD/(H2O)1 | 9 (100.0%) | - | - | - |
| 14 | Pyr/CLD/(H2O)1 | 7 (100.0%) | - | - | - |
| Total | 127 (76.0%) | 27 (16.2%) | 10 (6.0%) | 3 (1.8%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Rodríguez, K.; Carmenate-Rodríguez, C.; Ferino-Pérez, A.; Gaspard, S.; Jáuregui-Haza, U.J. Role of Basic Surface Groups of Activated Carbon in Chlordecone and β-Hexachlorocyclohexane Adsorption: A Molecular Modelling Study. Molecules 2021, 26, 6969. https://doi.org/10.3390/molecules26226969
Melchor-Rodríguez K, Carmenate-Rodríguez C, Ferino-Pérez A, Gaspard S, Jáuregui-Haza UJ. Role of Basic Surface Groups of Activated Carbon in Chlordecone and β-Hexachlorocyclohexane Adsorption: A Molecular Modelling Study. Molecules. 2021; 26(22):6969. https://doi.org/10.3390/molecules26226969
Chicago/Turabian StyleMelchor-Rodríguez, Kenia, Chayan Carmenate-Rodríguez, Anthuan Ferino-Pérez, Sarra Gaspard, and Ulises J. Jáuregui-Haza. 2021. "Role of Basic Surface Groups of Activated Carbon in Chlordecone and β-Hexachlorocyclohexane Adsorption: A Molecular Modelling Study" Molecules 26, no. 22: 6969. https://doi.org/10.3390/molecules26226969
APA StyleMelchor-Rodríguez, K., Carmenate-Rodríguez, C., Ferino-Pérez, A., Gaspard, S., & Jáuregui-Haza, U. J. (2021). Role of Basic Surface Groups of Activated Carbon in Chlordecone and β-Hexachlorocyclohexane Adsorption: A Molecular Modelling Study. Molecules, 26(22), 6969. https://doi.org/10.3390/molecules26226969






