Optimization of 5-CQA Extraction Conditions from Green Coffee By-Product (Coffea arabica) Using a Response-Surface Design and the Study of Its Extraction Kinetics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Raw Material
2.2. 5-CQA Quantification via Liquid Chromatography
2.3. Optimization of 5-CQA Extraction Parameters from Green-Coffee By-Product via RSM
2.3.1. Statistical Analysis of the Model
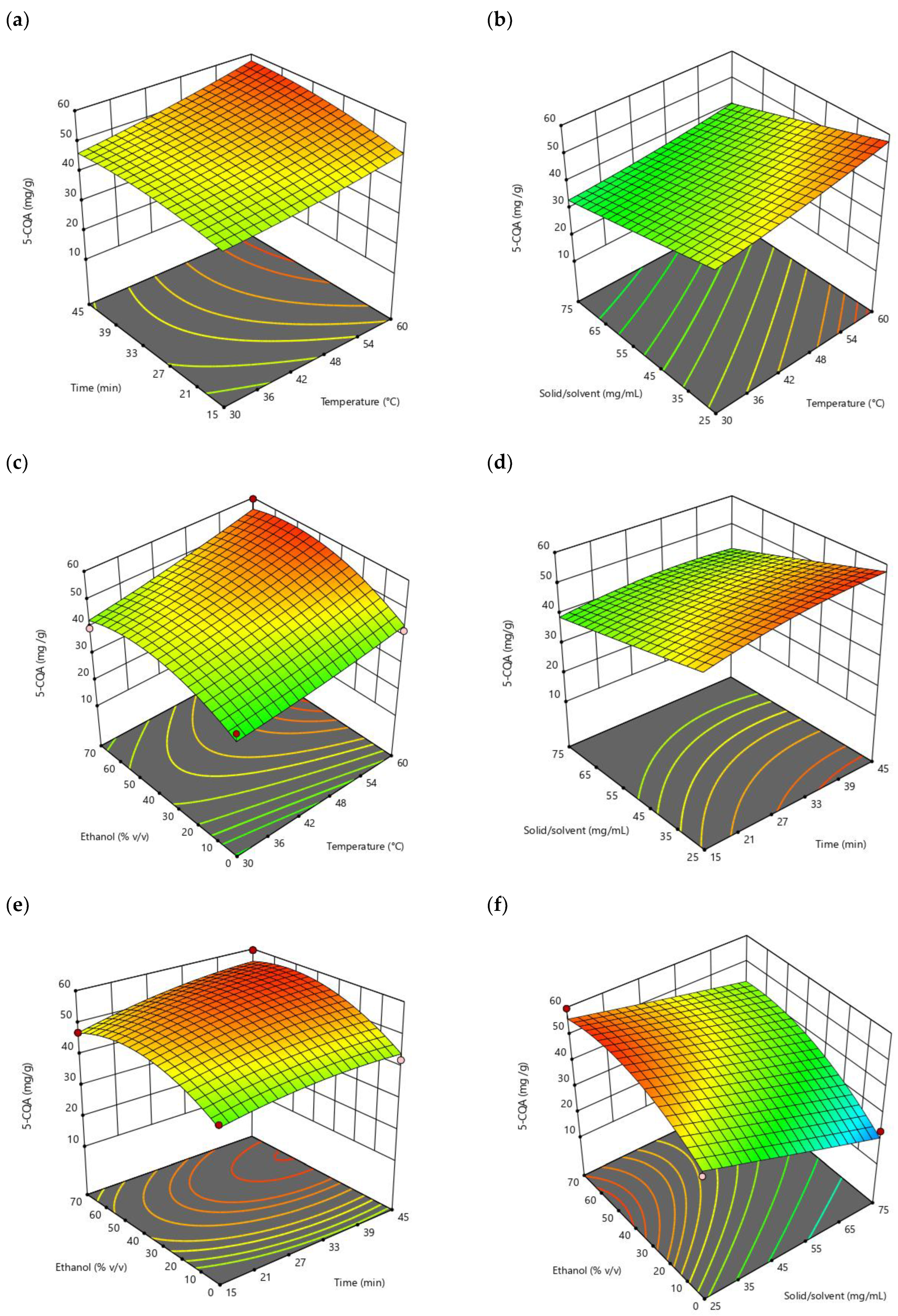
2.3.2. Analysis of Response Surface
2.3.3. Optimization of Extraction Parameters and Verification of Optimized Conditions
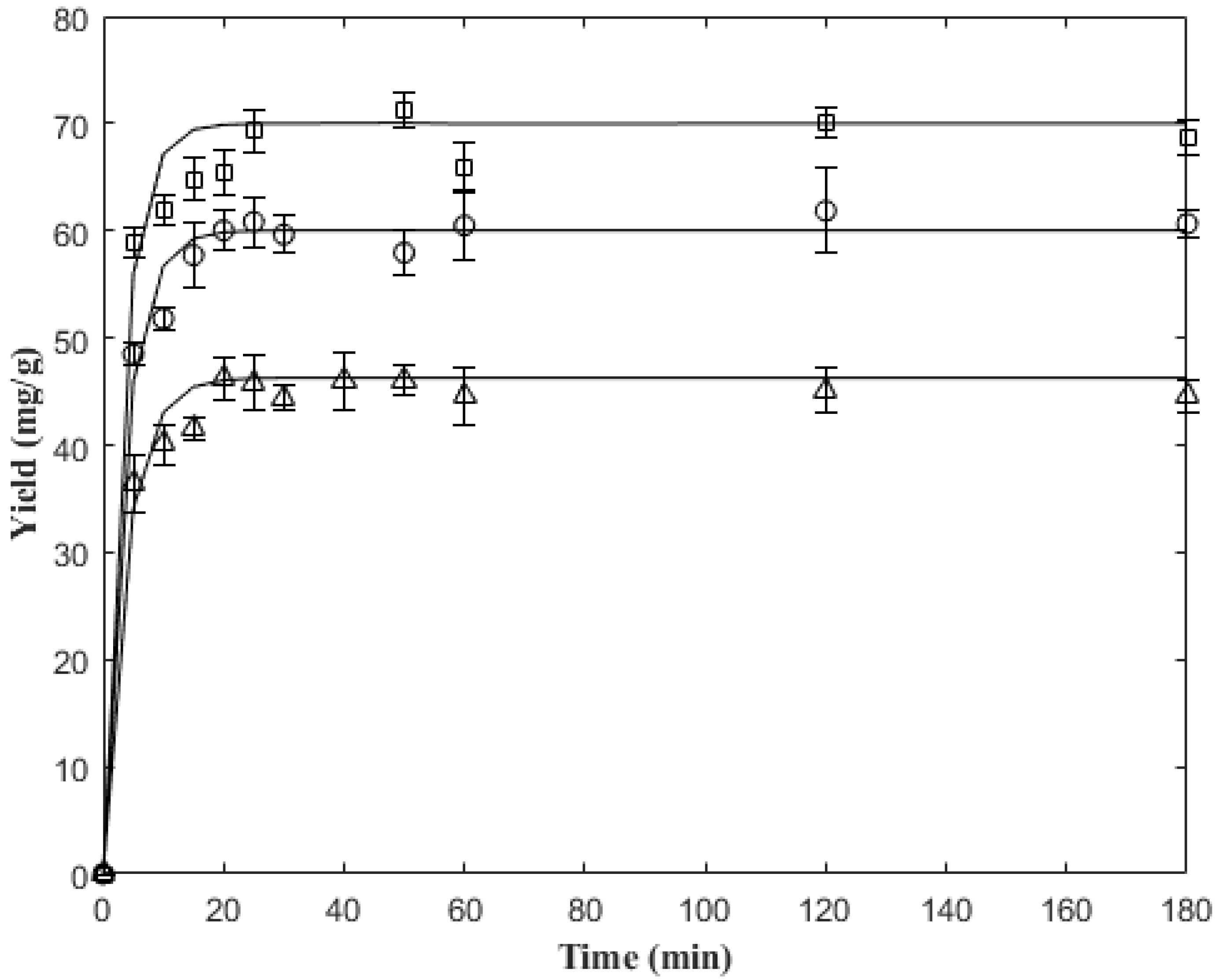
2.4. Analysis of 5-CQA Extraction Kinetics
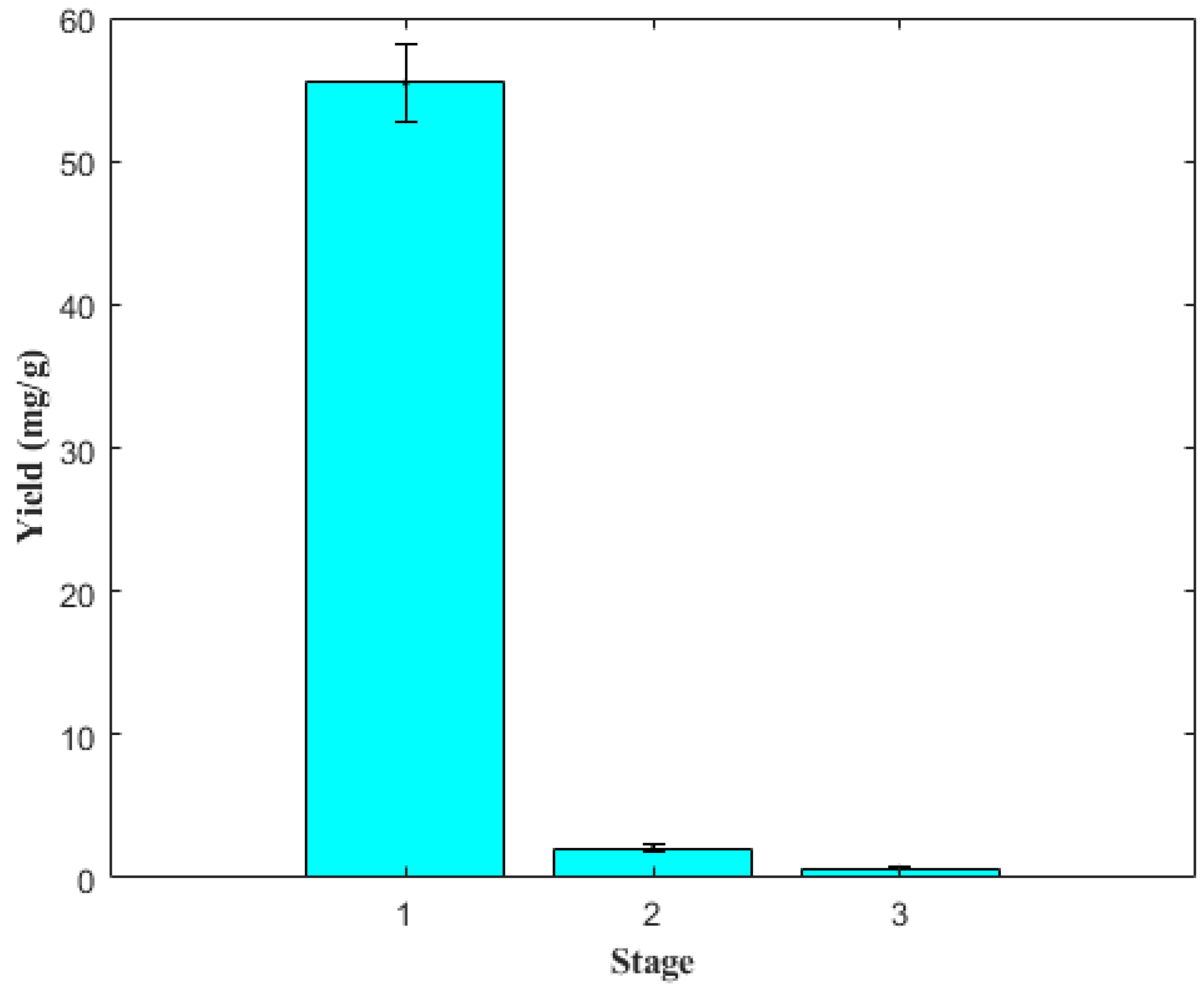
2.5. Extraction by Stages
3. Materials and Methods
3.1. Raw Material
3.2. Reagents
3.3. Extraction of 5-CQA from Green-Coffee By-Product
3.4. HPLC Analysis of Extracts
3.5. Extraction Optimization via RSM
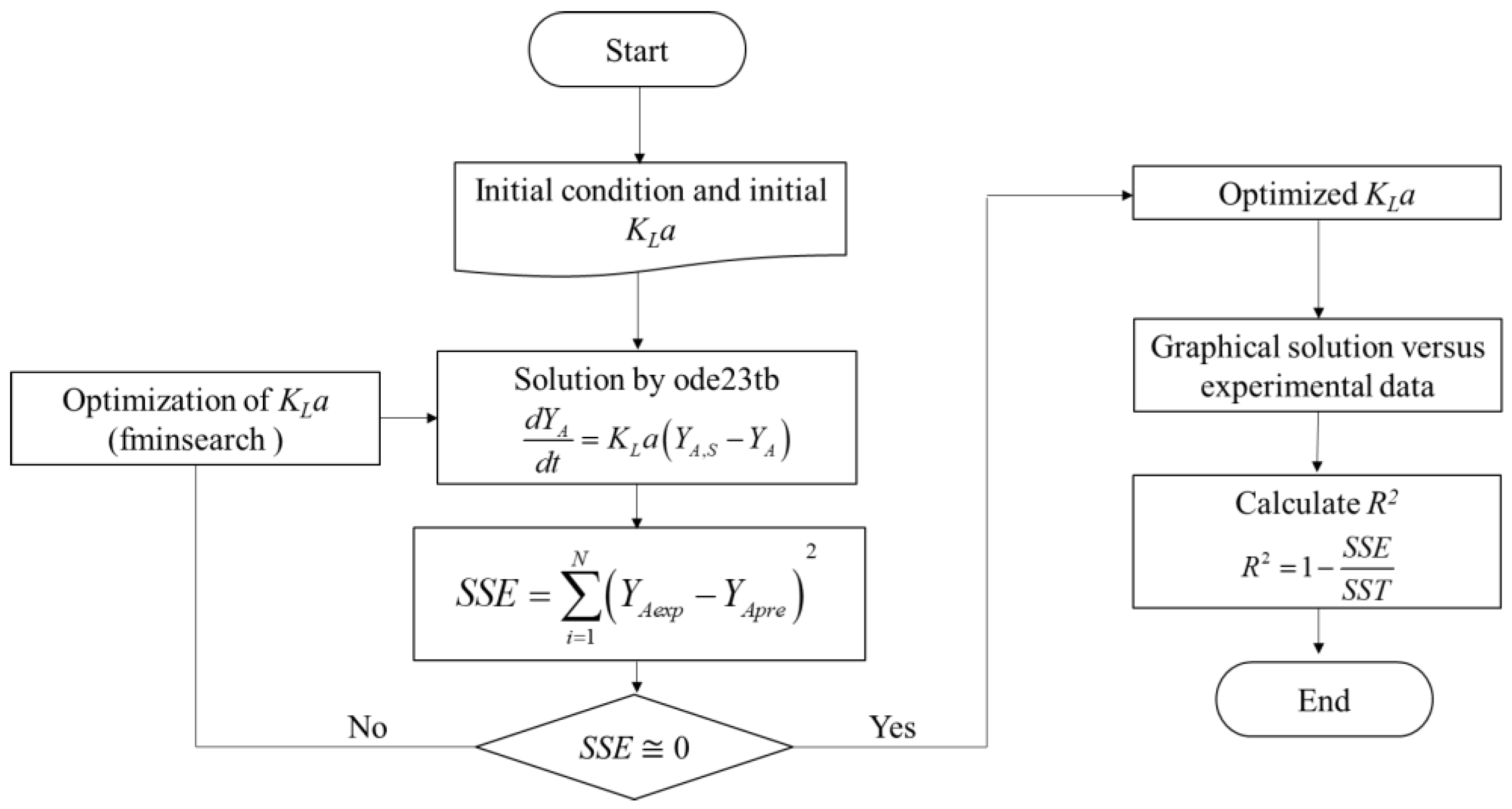
3.6. Kinetic Model
3.7. Effect of Extraction in Stages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, J.H.; Im, S.S.; Song, D.K. Coffee and health. Integr. Med. Res. 2014, 129, 189–191. [Google Scholar] [CrossRef] [PubMed]
- USDA Foreign Agricultural Service “Coffee: World Markets and Trade” 2022. Available online: https://www.fas.usda.gov/data/coffee-world-markets-and-trade (accessed on 5 August 2022).
- Wan, C.W.; Wong, C.N.Y.; Pin, W.K.; Wong, M.H.Y.; Kwok, C.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phyther. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, L.; Stuknytė, M.; Ciaramelli, C.; Magoni, C.; Bruni, I.; De Noni, I.; Airoldi, C.; Regonesi, M.E.; Palmioli, A. Green coffee extract enhances oxidative stress resistance and delays aging in Caenorhabditis elegans. J. Funct. Foods 2017, 33, 297–306. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Kojoori, S.M.H.; Moazeni-Pourasil, R.S. Optimization of chlorogenic acid extraction from Elm tree, Ulmus minor Mill., fruits, using response surface methodology. Sep. Purif. Technol. 2021, 256, 117773. [Google Scholar] [CrossRef]
- Market and Research, “Chlorogenic Acid Market”, United States, 2022. Available online: https://www.marketandresearch.biz/report/235796/global-chlorogenic-acid-market-growth-2022-2028?fbclid=IwAR0Lt5or7XbIWnxH3RA2NiK5KzpX36uuPQ--yz3Q-SvrjDbnC2yoOg2R4IM (accessed on 4 August 2022).
- Madhava, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod. Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Castro, A.C.; Oda, F.B.; Almeida-Cincotto, M.G.J.; Davanço, M.G.; Chiari-Andréo, B.G.; Cicarelli, R.M.B.; Peccinini, R.G.; Zocolo, G.J.; Ribeiro, P.R.V.; Corrêa, M.A.; et al. Green coffee seed residue: A sustainable source of antioxidant compounds. Food Chem. 2018, 246, 48–57. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Optimization of autohydrolysis conditions to extract antioxidant phenolic compounds from spent coffee grounds. J. Food Eng. 2017, 199, 1–8. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liu, T.A.; Chen, J.H.; Chang, C.M.J.; Shieh, C.J. Response surface methodology and artificial neural network optimized synthesis of enzymatic 2-phenylethyl acetate in a solvent-free system. Biocatal. Agric. Biotechnol. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Pavlović, M.D.; Buntić, A.V.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Pettinato, M.; Casazza, A.A.; Ferrari, P.F.; Palombo, D.; Perego, P. Eco-sustainable recovery of antioxidants from spent coffee grounds by microwave-assisted extraction: Process optimization, kinetic modeling and biological validation. Food Bioprod. Process. 2019, 114, 31–42. [Google Scholar] [CrossRef]
- Norma Mexicana NMX-F-177-SCFI-2009; Café Verde de Especialidad—Especificaciones, Clasificación y Evaluación Sensorial. Diario Oficial de la Federación: CDMX, Mexico, 2009; pp. 1–23.
- Sharapin, N.; Machado, L.; Souza, E.; Rocha, E.M.; Macedo, E.V.; Lopes, J.M. Fundamentos de Tecnología de Productos Fitoterapeuticos, 1st ed.; Quebecor impreandes: Santafé de Bogotá, Colombia, 2000; pp. 28–40. [Google Scholar]
- Oliveira, A.L.; Melo, V.L.S.; Guimarães, A.R.; Cabral, F.A. Modelling of high-pressure phase equilibrium in systems of interest in the food engineering field using the peng-robinson equation of state with two different mixing rules. J. Food Process. Eng. 2010, 33, 101–116. [Google Scholar] [CrossRef]
- Jokić, S.; Nagy, B.; Zeković, Z.; Vidović, S.; Bilić, M.; Velić, D.; Simándi, B. Effects of supercritical CO2 extraction parameters on soybean oil yield. Food Bioprod. Process. 2012, 90, 693–699. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Cornelio-Santiago, H.P.; Fukumasu, H.; Oliveira, A.L. Green coffee extracts rich in diterpenes—Process optimization of pressurized liquid extraction using ethanol as solvent. J. Food Process. Eng. 2018, 224, 148–155. [Google Scholar] [CrossRef]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.V.; Gavala, H.N. Integration of chlorogenic acid recovery and bioethanol production from spent coffee grounds. Biochem. Eng. J. 2016, 116, 54–64. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- De Assis Dias Alves, G.; De Souza, R.O.; Rogez, H.L.G.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit anti aging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar]
- Zhang, Y.; Prawang, P.; Li, C.; Meng, X.; Zhao, Y.; Wang, H.; Zhang, S. Ultrasonic assisted extraction of artemisinin from: Artemisia Annua L. using monoether-based solvents. Green Chem. 2018, 20, 713–723. [Google Scholar] [CrossRef]
- Gutiérrez Pulido, H.; De la vara Salazar, R. Análisis y Diseño de Experimentos, 2nd ed.; McGraw-Hill companies/Interamericana Editores S.A. de C.V.: Mexico City, Mexico, 2008; pp. 384–420. [Google Scholar]
- Karazhiyan, H.; Razavi, S.M.A.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Myers, R.; Montgomery, D.; Anderson, C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–12. [Google Scholar]
- Coelho, J.P.; Robalo, M.P.; Boyadzhieva, S.; Stateva, R.P. Microwave-assisted extraction of phenolic compounds from spent coffee grounds. Process optimization applying design of experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef]
- Soto-García, M.; Rosales-Castro, M. Effect of solvent and solvent-to-solid ratio on the phenolic extraction and the antioxidant capacity of extracts from Pinus durangensis and Quercus sideroxyla bark. Maderas Cienc. y Tecnol. 2016, 18, 701–714. [Google Scholar]
- Manohar, M.; Joseph, J.; Selvaraj, T.; Sivakumar, D. Application of desirability-function and RSM to optimise the multi-objectives while turning Inconel 718 using coated carbide tools. Int. J. Manuf. Technol. Manag. 2013, 27, 218–237. [Google Scholar] [CrossRef]
- Castillo-Santos, K.; Ruiz-López, I.I.; Rodríguez-Jimenes, G.C.; Carrillo-Ahumada, J.; García-Alvarado, M.A. Analysis of mass transfer equations during solid-liquid extraction and its application for vanilla extraction kinetics modeling. J. Food Eng. 2017, 192, 36–44. [Google Scholar] [CrossRef]
- Handayani, A.D.; Sutrisno; Indraswati, N.; Ismadji, S. Extraction of astaxanthin from giant tiger (Panaeus monodon) shrimp waste using palm oil: Studies of extraction kinetics and thermodynamic. Bioresour. Technol. 2008, 99, 4414–4419. [Google Scholar] [CrossRef]
- Lau, C.; Chua, L.; Lee, C.; Aziz, R. Optimization and kinetic modeling of rosmarinic acid extraction from Orthosiphon stamineus. Curr. Bioact. Compd. 2015, 10, 271–285. [Google Scholar]
- Parida, S.; Biswal, S. Kinetics and thermodynamics of lipids extraction from microalgae using n-hexane. Int. J. Energy Appl. Technol. 2020, 7, 69–73. [Google Scholar]
- Butiuk, A.P.; Maidana, S.A.; Adachi, O.; Akakabe, Y.; Martos, M.A.; Hours, R.A. Optimization and modeling of the chlorogenic acid extraction from a residue of yerba mate processing. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100329. [Google Scholar]
- Lai, H.T.N.; Nguyen, P.V.; Tran, H.T.; Dao, V.H.T.; Hoang, H.H. Optimization of chlorogenic acid extraction from green coffee beans using response surface methodology. Vietnam J. Agric. Sci. 2019, 2, 332–342. [Google Scholar] [CrossRef]
- Dibert, K.; Cros, E.; Andrieu, J. Solvent extraction of oil and chlorogenic acid from green coffee. Part II: Kinetic data. J. Food Eng. 1989, 10, 199–214. [Google Scholar]
- Liu, Q.M.; Yang, X.M.; Zhang, L.; Majetich, G. Optimization of ultrasonic-assisted extraction of chlorogenic acid from Folium eucommiae and evaluation of its antioxidant activity. J. Med. Plants Res. 2010, 4, 2503–2511. [Google Scholar]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Norma Mexicana NMX-F-083-1986; Alimentos. Determinación De Humedad En Productos Alimenticios. Foods. Moisture in Food Products Detetermination. Diario Oficial de la Federación: CDMX, Mexico, 1986; pp. 1–3.
- Meinhart, A.D.; Silveira, T.F.F.; Silva, R.A.; Damin, F.M.; Bruns, R.E.; Godoy, H.T. Multivariate optimization of chlorogenic acid extraction from Brazilian coffee. Food Anal. Methods 2017, 10, 2943–2951. [Google Scholar]
- Ruiz-Palomino, P.; Guatemala-Morales, G.; Mondragón-Cortéz, P.M.; Zúñiga-González, E.A.; Corona-González, R.I.; Arriola-Guevara, E. Empirical model of the chlorogenic acid degradation kinetics during coffee roasting in a spouted bed. Rev. Mex. Ing. Quim. 2019, 18, 387–396. [Google Scholar]
- Geankopolis, C.J. Transport Process and Unit Operations, 3rd ed.; Prentice Hall International Inc.: Hoboken, NJ, USA, 1993; pp. 715–733. [Google Scholar]


| Run | Variables a | 5-CQA Extraction Yield (mg/g) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental Data b | RSM-Predicted | |
| 1 | 30 | 15 | 75 | 0 | 22.56 ± 0.61 | 23.71 |
| 2 | 45 | 30 | 50 | 35 | 49.49 ± 2.27 | 43.33 |
| 3 | 45 | 30 | 75 | 35 | 38.8 ± 1.68 | 36.47 |
| 4 | 60 | 45 | 75 | 0 | 23.32 ± 0.06 | 20.71 |
| 5 | 45 | 30 | 50 | 35 | 43.60 ± 0.22 | 43.33 |
| 6 | 30 | 45 | 75 | 0 | 15.11 ± 1.23 | 17.43 |
| 7 | 45 | 15 | 50 | 35 | 39.47 ± 3.60 | 40.92 |
| 8 | 45 | 30 | 25 | 35 | 46.58 ± 2.39 | 50.51 |
| 9 | 45 | 30 | 50 | 35 | 43.61 ± 1.46 | 43.33 |
| 10 | 30 | 15 | 25 | 70 | 39.04 ± 0.85 | 39.02 |
| 11 | 30 | 45 | 25 | 70 | 39.78 ± 2.40 | 42.76 |
| 12 | 60 | 45 | 25 | 70 | 59.62 ± 2.36 | 55.85 |
| 13 | 45 | 30 | 50 | 70 | 43.8 ± 1.83 | 42.49 |
| 14 | 30 | 30 | 50 | 35 | 42.28 ± 2.62 | 41.29 |
| 15 | 60 | 30 | 50 | 35 | 44.31 ± 0.51 | 46.90 |
| 16 | 45 | 30 | 50 | 0 | 28.4 ± 3.69 | 31.31 |
| 17 | 60 | 15 | 75 | 0 | 22.58 ± 0.09 | 21.83 |
| 18 | 30 | 45 | 75 | 70 | 34.94 ± 0.24 | 33.21 |
| 19 | 30 | 15 | 25 | 0 | 39.8 ± 0.06 | 38.52 |
| 20 | 30 | 45 | 25 | 0 | 39.94 ± 3.12 | 37.19 |
| 21 | 60 | 15 | 25 | 70 | 47.06 ± 0.68 | 46.96 |
| 22 | 60 | 45 | 75 | 70 | 39.07 ± 1.84 | 42.57 |
| 23 | 60 | 45 | 25 | 0 | 42.28 ± 2.47 | 44.19 |
| 24 | 45 | 30 | 50 | 35 | 41.43 ± 0.14 | 43.33 |
| 25 | 60 | 15 | 75 | 70 | 38.50 ± 1.28 | 38.63 |
| 26 | 30 | 15 | 75 | 70 | 34.11 ± 0.84 | 34.42 |
| 27 | 60 | 15 | 25 | 0 | 41.27 ± 0.44 | 40.37 |
| 28 | 45 | 45 | 50 | 35 | 42.08 ± 0.71 | 42.23 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2153.36 | 14 | 153.81 | 13.43 | <0.0001 |
| X1 a | 141.40 | 1 | 141.40 | 12.35 | 0.0038 |
| X2 a | 7.67 | 1 | 7.67 | 0.6698 | 0.4279 |
| X3 a | 887.33 | 1 | 887.33 | 77.48 | <0.0001 |
| X4 a | 562.91 | 1 | 562.91 | 49.15 | <0.0001 |
| X1X2 | 26.57 | 1 | 26.57 | 2.32 | 0.1516 |
| X1X3 | 13.91 | 1 | 13.91 | 1.21 | 0.2904 |
| X1X4 | 37.03 | 1 | 37.03 | 3.23 | 0.0954 |
| X2X3 | 24.40 | 1 | 24.40 | 2.13 | 0.1681 |
| X2X4 | 25.65 | 1 | 25.65 | 2.24 | 0.1583 |
| X3X4 | 104.24 | 1 | 104.24 | 9.10 | 0.0099 |
| X12 | 1.53 | 1 | 1.53 | 0.1333 | 0.7209 |
| X22 | 7.91 | 1 | 7.91 | 0.6903 | 0.4211 |
| X32 | 0.0697 | 1 | 0.0697 | 0.0061 | 0.9390 |
| X42 | 106.50 | 1 | 106.50 | 9.30 | 0.0093 |
| Residual | 148.88 | 13 | 11.45 | ||
| Lack of Fit | 112.95 | 10 | 11.30 | 0.9433 | 0.5912 |
| Pure Error | 35.92 | 3 | 11.97 | ||
| Cor Total | 2302.24 | 27 |
| Fit Statistics | Yield (mg/g) |
|---|---|
| R2 | 0.9353 |
| Adjusted R2 | 0.8657 |
| Predicted R2 | 0.6690 |
| C.V. % | 8.75 |
| Adeq Precision | 15.5083 |
| T (°C) | T (K) | R2 | ||
|---|---|---|---|---|
| 45 | 318.15 | 0.266 | 46.0356 | 0.978 |
| 60 | 333.15 | 0.289 | 61.8535 | 0.987 |
| 75 | 348.15 | 0.320 | 71.1760 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Vallejo, M.; Arriola-Guevara, E.; Corona-González, R.I.; Flores-Méndez, D.A.; Padilla-de la Rosa, J.D.; Esquivel-Solis, H.; Pelayo-Ortiz, C.; Guatemala-Morales, G.M. Optimization of 5-CQA Extraction Conditions from Green Coffee By-Product (Coffea arabica) Using a Response-Surface Design and the Study of Its Extraction Kinetics. Molecules 2022, 27, 5704. https://doi.org/10.3390/molecules27175704
Granados-Vallejo M, Arriola-Guevara E, Corona-González RI, Flores-Méndez DA, Padilla-de la Rosa JD, Esquivel-Solis H, Pelayo-Ortiz C, Guatemala-Morales GM. Optimization of 5-CQA Extraction Conditions from Green Coffee By-Product (Coffea arabica) Using a Response-Surface Design and the Study of Its Extraction Kinetics. Molecules. 2022; 27(17):5704. https://doi.org/10.3390/molecules27175704
Chicago/Turabian StyleGranados-Vallejo, Miriam, Enrique Arriola-Guevara, Rosa Isela Corona-González, David Antonio Flores-Méndez, José Daniel Padilla-de la Rosa, Hugo Esquivel-Solis, Carlos Pelayo-Ortiz, and Guadalupe María Guatemala-Morales. 2022. "Optimization of 5-CQA Extraction Conditions from Green Coffee By-Product (Coffea arabica) Using a Response-Surface Design and the Study of Its Extraction Kinetics" Molecules 27, no. 17: 5704. https://doi.org/10.3390/molecules27175704