Optimization of the Extraction Conditions of Bioactive Compounds from Ocimum basilicum Leaves Using Ultrasound-Assisted Extraction via a Sonotrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Polar and Other Compounds in Ocimum basilicum Extracts by HPLC-ESI-TOF-MS
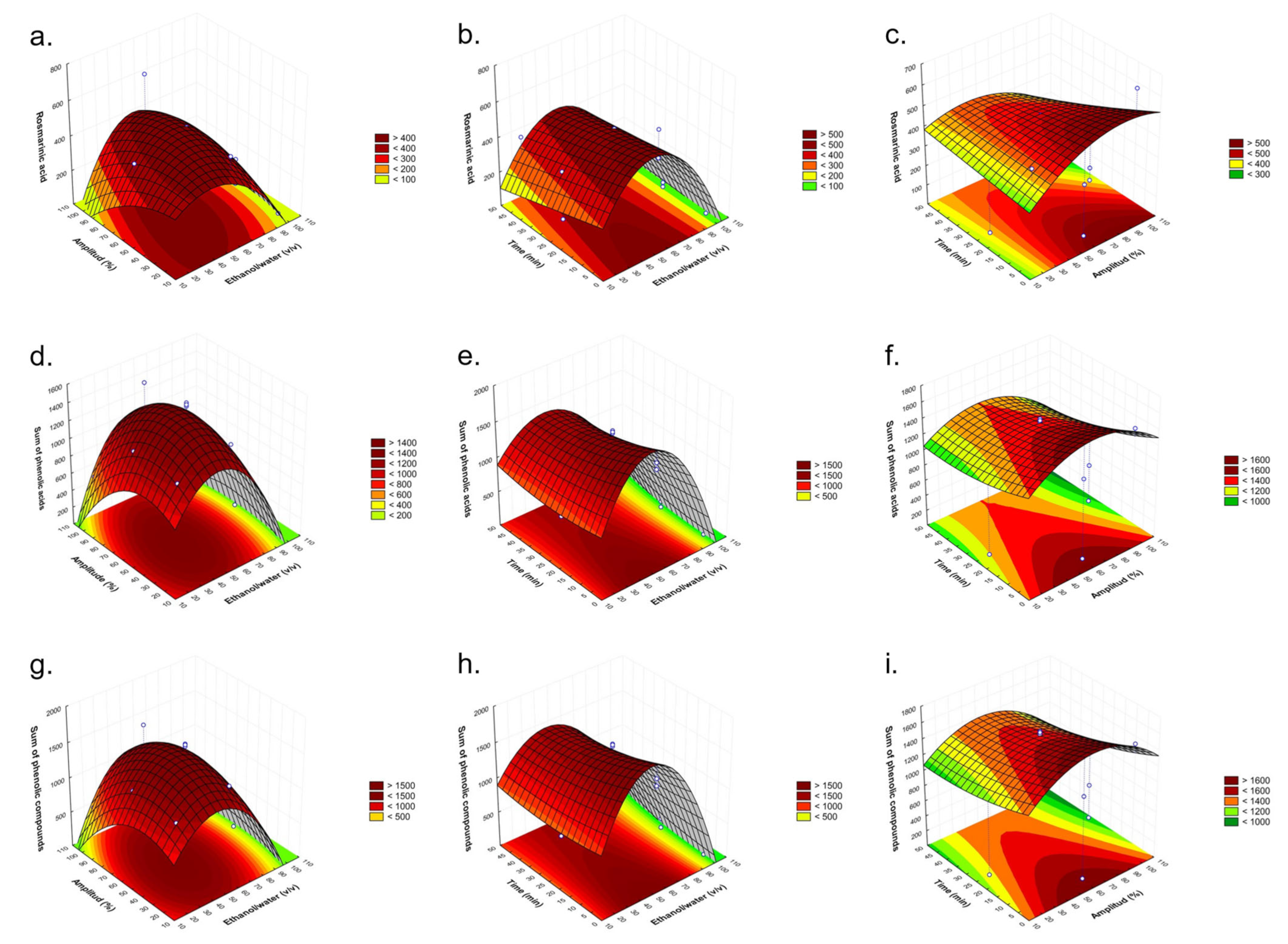
2.2. Fitting the Model
2.3. Establishment of Optimal Conditions
2.4. Confirmation of the Optimal Extraction Parameters by a Sonotrode in Ocimum basilicum Leaves
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Experimental Design
3.3. Ultrasound-Assisted Extraction via a Sonotrode Applied to Ocimum basilicum Leaves
3.4. Determination of Polar and Other Compounds in Ocimum basilicum Leaf Extracts by HPLC-ESI-TOF-MSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdel-Tawwab, M.; Adeshina, I.; Emikpe, B.O.; Jenyo-Oni, A.; Ajani, E.K.; Tiamiyu, L.O. Effect of Dietary Clove Basil, Ocimum Gratissimum, Leaves Extract on Healing of Artificially Wounded African Catfish, Clarias gariepinus (B.), Juveniles. J. Appl. Aquac. 2019, 31, 289–300. [Google Scholar] [CrossRef]
- Marwat, S.K.; Fazal, R.; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K. Phytochemical Constituents and Pharmacological Activities of Sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2014, 23, 3773–3782. [Google Scholar]
- Dahchour, A. Anxiolytic and Antidepressive Potentials of Rosmarinic Acid: A Review with a Focus on Antioxidant and Anti-Inflammatory Effects. Pharm. Res. 2022, 184, 106421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Chen, J.; Qin, Z.; Yu, S.; Zhou, J. Coordinating Caffeic Acid and Salvianic Acid A Pathways for Efficient Production of Rosmarinic Acid in Escherichia Coli. Metab. Eng. 2023, 76, 29–38. [Google Scholar] [CrossRef]
- Chadni, M.; Isidore, E.; Lagalle, F.; Langlait, M.; Dosso, A.; Ioannou, I. Optimization of the Supercritical Extraction of Rosmarinic Acid from Clary Sage Residue and the Antioxidant Activity of the Extracts. J. Supercrit. Fluids 2023, 193, 105830. [Google Scholar] [CrossRef]
- Domitrović, R.; Škoda, M.; Vasiljev Marchesi, V.; Cvijanović, O.; Pernjak Pugel, E.; Štefan, M.B. Rosmarinic Acid Ameliorates Acute Liver Damage and Fibrogenesis in Carbon Tetrachloride-Intoxicated Mice. Food Chem. Toxicol. 2013, 51, 370–378. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, Y.-Y.; Chen, W.-Y.; Liao, S.-L.; Chou, S.-T.; Yang, C.-P.; Chen, C.-J. Hepatoprotective Activities of Rosmarinic Acid against Extrahepatic Cholestasis in Rats. Food Chem. Toxicol. 2017, 108, 214–223. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; He, J.; Jiang, L.; Wei, M.; Cao, Q.; Guan, M.; Xie, X.; Deng, X.; He, J. Effects of a Natural Prolyl Oligopeptidase Inhibitor, Rosmarinic Acid, on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Molecules 2012, 17, 3586–3598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, Y.; Cao, J.; Liu, D.; Wan, L. Rosmarinic Acid Ameliorates Septic-Associated Mortality and Lung Injury in Mice via GRP78/IRE1α/JNK Pathway. J. Pharm. Pharmacol. 2021, 73, 916–921. [Google Scholar] [CrossRef]
- Quan, W.; Liu, H.; Zhang, W.; Lou, W.; Gong, Y.; Yuan, C.; Shao, Q.; Wang, N.; Guo, C.; Liu, F. Cardioprotective Effect of Rosmarinic Acid against Myocardial Ischaemia/Reperfusion Injury via Suppression of the NF-ΚB Inflammatory Signalling Pathway and ROS Production in Mice. Pharm. Biol. 2021, 59, 220–229. [Google Scholar] [CrossRef]
- Toutounchi, N.S.; Afrooziyan, A.; Rameshrad, M.; Rezabakhsh, A.; Vaez, H.; Hamedeyazdan, S.; Fathiazad, F.; Garjani, A. Cardioprotective Effects of Rosmarinic Acid on Isoproterenol-Induced Myocardial Infarction in Rats. Pharm. Sci. 2017, 23, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.B.; Oliveira Brito Pereira Bezerra Martins, A.; Cesário, F.R.A.S.; Ferreira e Castro, F.; de Albuquerque, T.R.; Martins Fernandes, M.N.; Fernandes da Silva, B.A.; Quintans Júnior, L.J.; da Costa, J.G.M.; Melo Coutinho, H.D.; et al. Anti-Inflammatory and Antiedematogenic Activity of the Ocimum basilicum Essential Oil and Its Main Compound Estragole: In Vivo Mouse Models. Chem. Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Mabood, F.; Gilani, S.A.; Hussain, J.; Alshidani, S.; Alghawi, S.; Albroumi, M.; Alameri, S.; Jabeen, F.; Hussain, Z.; Al-Harrasi, A.; et al. New Design of Experiment Combined with UV–Vis Spectroscopy for Extraction and Estimation of Polyphenols from Basil Seeds, Red Seeds, Sesame Seeds and Ajwan Seeds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 14–18. [Google Scholar] [CrossRef]
- Teofilović, B.; Grujić-Letić, N.; Goločorbin-Kon, S.; Stojanović, S.; Vastag, G.; Gadžurić, S. Experimental and Chemometric Study of Antioxidant Capacity of Basil (Ocimum basilicum) Extracts. Ind. Crop. Prod. 2017, 100, 176–182. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The Effect of Different Solvents and Number of Extraction Steps on the Polyphenol Content and Antioxidant Capacity of Basil Leaves (Ocimum basilicum L.) Extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Mahmoud, E.; Starowicz, M.; Ciska, E.; Topolska, J.; Farouk, A. Determination of Volatiles, Antioxidant Activity, and Polyphenol Content in the Postharvest Waste of Ocimum basilicum L. Food Chem. 2022, 375, 131692. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, Q.Y.; Li, D.W.; Zhang, Z.M.; You, C.X. Antagonistic Storage Potential of Tagetes Minuta, Eupatorium Fortunei and Ocimum basilicum Oils with Volatile Secondary Metabolites against Tribolium castaneum and Lasioderma serricorne. Ind. Crop. Prod. 2022, 187, 115502. [Google Scholar] [CrossRef]
- Chenni, M.; el Abed, D.; Neggaz, S.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Solvent Free Microwave Extraction Followed by Encapsulation of O. basilicum L. Essential Oil for Insecticide Purpose. J. Stored Prod. Res. 2020, 86, 101575. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. RETRACTED: Preliminary Study: Kinetics of Oil Extraction from Basil (Ocimum basilicum) by Microwave-Assisted Hydrodistillation and Solvent-Free Microwave Extraction. S. Afr. J. Chem. Eng. 2016, 21, 49–53. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-Free Microwave Extraction of Essential Oil from Aromatic Herbs: Comparison with Conventional Hydro-Distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Moura, E.; Faroni, L.R.D.A.; Heleno, F.F.; Rodrigues, A.A.Z.; Prates, L.H.F.; De Queiroz, M.E.L.R. Optimal Extraction of Ocimum basilicum Essential Oil by Association of Ultrasound and Hydrodistillation and Its Potential as a Biopesticide against a Major Stored Grains Pest. Molecules 2020, 25, 2781. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Ghavidel, R.A.; Sheikholeslami, Z.; Elahi, M.; Elahi, S. Optimization of ultrasonic assisted extraction of antioxidants and phenolic compounds from red basil using response surface methodology. Agro Food Ind. Hi. Tech. 2015, 26, 28–32. [Google Scholar]
- Hashemi, S.M.B.; Ghorashi, S.; Hadizadeh, F.; Zarei, Z.; Yazdani, M.; Noormohammadi, M. Effect of Amplitude of Ultrasound-Assisted Solvent Extraction and Extraction Temperature on the Kinetics, Thermodynamics, Antioxidant and Antimicrobial Activity of Ocimum basilicum L. Extract. J. Agric. Sci. Technol. 2017, 19, 1517–1526. [Google Scholar]
- De Vallejuelo, S.F.O.; Barrena, A.; Arana, G.; de Diego, A.; Madariaga, J.M. Ultrasound Energy Focused in a Glass Probe: An Approach to the Simultaneous and Fast Extraction of Trace Elements from Sediments. Talanta 2009, 80, 434–439. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.-L.; Li, X.-Y.; Tang, W.-K.; Zhang, J.-X.; Fang, C.-X.; Zheng, C.-Y. Coupling Continuous Ultrasound-Assisted Extraction with Ultrasonic Probe, Solid-Phase Extraction and High-Performance Liquid Chromatography for the Determination of Sodium Danshensu and Four Tanshinones in Salvia Miltiorrhiza Bunge. Anal. Chim. Acta 2007, 589, 231–238. [Google Scholar] [CrossRef]
- Silva, E.K.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Recovery of Cinnamyl Alcohol Glycosides from Rhodiola Rosea Roots: Effect of Probe Diameter on the Ultrasound Energy Performance for the Extraction of Bioactive Compounds. Food Bioprod. Process. 2020, 122, 245–253. [Google Scholar] [CrossRef]
- Ahmadi, A.H.R.; Salehinia, F.; Rastegar, H.; Allameh, A.A.; Rezazadeh, S. Differences in Growth Response of Human Hair Follicle Mesenchymal Stem Cells to Herbal Extracts and a Growth Factor. J. Med. Plants 2018, 17, 36–46. [Google Scholar] [CrossRef]
- Soares, V.P.; Fagundes, M.B.; Guerra, D.R.; Leães, Y.S.V.; Speroni, C.S.; Robalo, S.S.; Emanuelli, T.; Cichoski, A.J.; Wagner, R.; Barin, J.S.; et al. Ultrasound Assisted Maceration for Improving the Aromatization of Extra-Virgin Olive Oil with Rosemary and Basil. Food Res. Int. 2020, 135, 109305. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 421–442. [Google Scholar]
- Buser, C.; Matile, P. Malic Acid in Vacuoles Isolated from Bryophyllum Leaf Cells. Z. Für. Pflanzenphysiol. 1977, 82, 462–466. [Google Scholar] [CrossRef]
- Strack, D.; Sharma, V. Vacuolar Localization of the Enzymatic Synthesis of Hydroxycinnamic Acid Esters of Malic Acid in Protoplasts from Raphanus Sativus Leaves. Physiol. Plant 1985, 65, 45–50. [Google Scholar] [CrossRef]
- Si, P.; Shao, W.; Yu, H.; Xu, G.; Du, G. Differences in Microbial Communities Stimulated by Malic Acid Have the Potential to Improve Nutrient Absorption and Fruit Quality of Grapes. Front. Microbiol. 2022, 13, 850807. [Google Scholar] [CrossRef]
- Human Metabolome Database: Showing Metabocard for 2-Isopropylmalic Acid (HMDB0000402). Available online: https://hmdb.ca/metabolites/HMDB0000402 (accessed on 3 May 2023).
- Lee, J.; Scagel, C.F. Chicoric Acid Found in Basil (Ocimum basilicum L.) Leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Kiferle, C.; Lucchesini, M.; Mensuali-Sodi, A.; Maggini, R.; Raffaelli, A.; Pardossi, A. Rosmarinic Acid Content in Basil Plants Grown in Vitro and in Hydroponics. Open Life Sci. 2011, 6, 946–957. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed Priming with Melatonin Effects on Growth, Essential Oil Compounds and Antioxidant Activity of Basil (Ocimum basilicum L.) under Salinity Stress. Ind. Crop. Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-Based Rapid Screening of Phenolics and Triterpenic Acids in Leaf Extracts of Ocimum Species and Their Interspecies Variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.D.M. Metabolic Profiling of the Oil of Sesame of the Egyptian Cultivar ‘Giza 32’ Employing LC-MS and Tandem MS-Based Untargeted Method. Foods 2021, 10, 298. [Google Scholar] [CrossRef]
- Matsuura, H.; Ohkubo, Y.; Yoshihara, T. Occurrence of 11-Hydroxyjasmonic Acid Glucoside in Leaflets of Potato Plants (Solanum tuberosum L.). Biosci. Biotechnol. Biochem. 2001, 65, 378–382. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of Phenolic and Other Polar Compounds in a Lemon Verbena Extract by Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef]
- Kitajima, J.; Ishikawa, T.; Urabe, A. A New Hydroxyjasmone Glucoside and Its Related Compounds from the Leaf of Thyme. Chem. Pharm. Bull. 2004, 52, 1013–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. HPLC Analysis of Sesaminol Glucosides in Sesame Seeds. J. Agric. Food Chem. 2006, 54, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Inamake, M.R.; Shelar, P.D.; Kulkarni, M.S.; Katekar, S.M.; Rashmi, T. Isolation and Analytical Characterization of Stevioside from Leaves of Stevia Rebaudiana Bert; (Asteraceae). Int. J. Res. Ayurveda Pharm. 2010, 1, 572–581. [Google Scholar]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-De-cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-de-cerio, E.; Tylewicz, U.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Romani, S. Design of Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium guajava L. Leaves. Food Anal. Methods 2017, 10, 2781–2791. [Google Scholar] [CrossRef]
- Le Man, H.; Behera, S.K.; Park, H.S. Optimization of Operational Parameters for Ethanol Production from Korean Food Waste Leachate. Int. J. Environ. Sci. Technol. 2010, 7, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green ultrasound assisted extraction of trans rosmarinic acid from Plectranthus scutellarioides (L.) R.Br. leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Extraction of rosmarinic acid from Melissa officinalis L. by heat-, microwave- and ultrasound-assisted extraction techniques: A comparative study through response surface analysis. Sep. Purif. Technol. 2017, 186, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Barrera Vázquez, M.F.; Andreatta, A.E.; Martini, R.E.; Núñez Montoya, S.C.; Cabrera, J.L.; Comini, L.R. Optimization of Pretreatment with Microwaves Prior the Pressurized Hot Water Extraction of Anthraquinones from Heterophyllaea Pustulata, Using Doehlert Experimental Design. Chem. Eng. Process. Process Intensif. 2020, 155, 10855. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and Kinetic Modeling of Ultrasonic-Assisted Extraction of Fucoxanthin from Edible Brown Algae Sargassum Fusiforme Using Green Solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M. Ultrasound-Assisted Extraction of Asiaticoside from Centella Asiatica Using Betaine-Based Natural Deep Eutectic Solvent. Ind. Crop. Prod. 2023, 192, 116069. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Wang, C.; Chang, T.; Jiang, H. Ultrasound-Homogenization-Assisted Extraction of Polyphenols from Coconut Mesocarp: Optimization Study. Ultrason. Sonochem. 2021, 78, 105739. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Rathod, V.K. Green Extraction of Glycyrrhizic Acid from Glycyrrhiza Glabra Using Choline Chloride Based Natural Deep Eutectic Solvents (NADESs). Process Biochem. 2021, 102, 22–32. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green Extraction of Phenolic Compounds from Foxtail Millet Bran by Ultrasonic-Assisted Deep Eutectic Solvent Extraction: Optimization, Comparison and Bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response Surface Optimization of Ultrasound-Assisted Polysaccharides Extraction from Pomegranate Peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-Assisted Extraction and Properties of Polysaccharide from Ginkgo Biloba Leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-Assisted Extraction of Phenolics with Maximal Antioxidant Activities in Tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Yang, Y.C.; Li, J.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Wu, N.; Liu, X.L. Optimisation of Microwave-Assisted Enzymatic Extraction of Corilagin and Geraniin from Geranium Sibiricum Linne and Evaluation of Antioxidant Activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of Microwave-Assisted Extraction of Polyphenols from Myrtus Communis L. Leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of Ultrasound-Assisted Extraction of Phenolic Antioxidants from Turkey Berry (Solanum torvum Sw) Fruits Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100387. [Google Scholar] [CrossRef]
- Shahidi, S.A. Effect of Solvent Type on Ultrasound-Assisted Extraction of Antioxidant Compounds from Ficaria Kochii: Optimization by Response Surface Methodology. Food Chem. Toxicol. 2022, 163, 112981. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of Ultrasound-Assisted Extraction and Solvent Composition on Bioactive Compounds and in Vitro Biological Activities of Thyme and Rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential Extraction of Carnosic Acid, Rosmarinic Acid and Pigments (Carotenoids and Chlorophylls) from Rosemary by Online Supercritical Fluid Extraction-Supercritical Fluid Chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The Effect of Pulsed Electric Field as a Pre-Treatment Step in Ultrasound Assisted Extraction of Phenolic Compounds from Fresh Rosemary and Thyme by-Products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of Ultrasound-Assisted Extraction via Sonotrode of Phenolic Compounds from Orange by-Products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]

| Peak | Time (min) | Experimental m/z | Calculated m/z | Error (ppm) | Score (%) | Molecular Formula | m/z in the Source Fragments | Compound Name |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.42 | 133.0139 | 133.0137 | 1.5 | 100 | C4H6O5 | - | Malic acid |
| 2 | 0.57 | 191.0551 | 191.0556 | −2.6 | 98.91 | C7H12O6 | 111.0082 | Quinic acid isomer a |
| 3 | 1.09 | 191.0549 | 191.0556 | −3.7 | 100 | C7H12O6 | 133.0282 | Quinic acid isomer b |
| 4 | 1.19 | 175.0599 | 175.0606 | −4.0 | 99.95 | C7H12O5 | 133.0271 | Isopropylmalic acid isomer a |
| 5 | 2.15 | 175.0605 | 175.0606 | −0.6 | 100 | C7H12O5 | 133.0293 | Isopropylmalic acid isomer b |
| 6 | 4.30 | 179.0340 | 179.0344 | −2.2 | 100 | C9H8O4 | 133.0287 | Caffeic acid |
| 7 | 5.43 | 387.1653 | 387.1655 | −0.5 | 100 | C18H28O9 | 207.1017; 179.0712 | Tuberonic acid glucoside |
| 8 | 6.33 | 855.2519 | 855.2559 | −4.7 | 95.08 | C38H48O22 | 179.0699; 161.0605 | Sesaminol triglucoside |
| 9 | 8.29 | 473.0705 | 473.0720 | −3.2 | 99.95 | C22H18O12 | 293.0287 | Chicoric acid isomer a |
| 10 | 8.87 | 359.0768 | 359.0767 | 0.3 | 100 | C18H16O8 | 133.0292 | Rosmarinic acid isomer a |
| 11 | 9.04 | 609.1469 | 609.1456 | 2.1 | 100 | C27H30O16 | 300.0279 | Quercetin-rutinoside |
| 12 | 9.51 | 473.0740 | 473.0720 | 4.2 | 99.17 | C22H18O12 | 293.0308 | Chicoric acid isomer b |
| 13 | 9.74 | 359.0778 | 359.0767 | 3.1 | 100 | C18H16O8 | 133.0289 | Rosmarinic acid isomer b |
| 14 | 9.78 | 717.1451 | 717.1456 | −0.7 | 97.38 | C36H30O16 | 161.0246 | Salvianolic acid |
| 15 | 10.21 | 593.1498 | 593.1506 | −1.3 | 99.96 | C27H30O15 | 285.0395 | Luteolin hexoside deoxyhexoside |
| 16 | 13.38 | 803.3701 | 803.3701 | 0.0 | 100 | C38H60O18 | 503.3382; 357.1906; 315.1796; 161.0463 | Stevioside |
| 17 | 13.58 | 313.0707 | 313.0712 | −1.6 | 100 | C17H14O6 | 161.0235 | Cirsimaritin isomer a |
| 18 | 13.82 | 313.0702 | 313.0712 | −3.2 | 100 | C17H14O6 | 161.0233 | Cirsimaritin isomer b |
| 19 | 14.74 | 345.1696 | 345.1702 | −1.7 | 100 | C20H26O5 | 283.1692 | Rosmanol |
| 20 | 14.92 | 491.0983 | 491.0978 | 1.0 | 100 | C26H20O10 | 179.0345 | Isosalvianolic acid |
| Independent Factors | Response Variables | |||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| Run | Ethanol/ Water (v/v) | Amplitude (%) | Time (min) | Rosmarinic Acid (µg/g d.w.) | The Sum of Phenolic Acids (µg/g d.w.) | The Sum of Phenolic Compounds (µg/g d.w.) |
| 1 | 20 | 60 | 5 | 206.59 ± 0.28 | 1053.93 ± 1.41 | 1091.82 ± 1.48 |
| 2 | 100 | 60 | 5 | 18.19 ± 0.06 | 18.19 ± 0.06 | 24.63 ± 0.09 |
| 3 | 20 | 60 | 45 | 238.12 ± 0.32 | 1177.45 ± 1.56 | 1224.58 ± 1.64 |
| 4 | 100 | 60 | 45 | 149.26 ± 0.21 | 330.65 ± 0.50 | 383.61 ± 0.60 |
| 5 | 20 | 20 | 25 | 254.64 ± 0.34 | 1142.60 ± 1.50 | 1185.53 ± 1.58 |
| 6 | 100 | 20 | 25 | 8.26 ± 0.03 | 8.26 ± 0.03 | 8.44 ± 0.03 |
| 7 | 20 | 100 | 25 | 61.24 ± 0.11 | 570.75 ± 0.78 | 580.83 ± 0.80 |
| 8 | 100 | 100 | 25 | 33.92 ± 0.07 | 92.39 ± 0.20 | 142.17 ± 0.29 |
| 9 | 60 | 20 | 5 | 317.40 ± 0.41 | 1302.98 ± 1.69 | 1358.20 ± 1.78 |
| 10 | 60 | 20 | 45 | 253.07 ± 0.34 | 1108.33 ± 1.47 | 1149.89 ± 1.55 |
| 11 | 60 | 100 | 5 | 412.44 ± 0.53 | 1396.61 ± 1.82 | 1459.35 ± 1.93 |
| 12 | 60 | 100 | 45 | 142.60 ± 0.21 | 863.03 ± 1.17 | 909.48 ± 1.26 |
| 13 | 60 | 60 | 25 | 420.13 ± 0.46 | 1458.90 ± 1.87 | 1559.45 ± 2.04 |
| 14 | 60 | 60 | 25 | 406.35 ± 0.53 | 1419.25 ± 1.56 | 1522.60 ± 2.03 |
| 15 | 60 | 60 | 25 | 415.72 ± 0.51 | 1437.80 ± 1.23 | 1545.94 ± 1.94 |
| Y1 | Y2 | Y3 | ||||
|---|---|---|---|---|---|---|
| Regression Coefficients | Rosmarinic Acid (µg/g d.w.) | The Sum of Phenolic Acids (µg/g d.w.) | The Sum of Phenolic Compounds (µg/g d.w.) | |||
| Effect | p-Value | Effect | p-Value | Effect | p-Value | |
| β0 | 254.74 | 0.0004 * | 755.43 | 0.0001 * | 793.21 | 0.0000 * |
| Linear | ||||||
| β1 | −226.90 | 0.0035 * | −851.32 | 0.0003 * | −856.61 | 0.0003 * |
| β2 | −75.48 | 0.0305 * | −187.85 | 0.0061 * | −180.20 | 0.0059 * |
| β3 | 1.66 | 0.9133 | 23.95 | 0.2467 | 37.55 | 0.1140 |
| Crossed | ||||||
| β12 | 149.79 | 0.0143 * | 327.99 | 0.0036 * | 369.21 | 0.0025 * |
| β13 | 7.74 | 0.7108 | 94.47 | 0.0414 * | 113.11 | 0.0261 * |
| β23 | −134.62 | 0.0176 * | −169.47 | 0.0134 * | −170.78 | 0.0117 * |
| Quadratic | ||||||
| β11 | 267.63 | 0.0012 * | 753.92 | 0.0002 * | 800.74 | 0.0001 * |
| β22 | 66.23 | 0.0197 * | 231.24 | 0.0020 * | 262.67 | 0.0014 * |
| β33 | −22.82 | 0.1365 | 39.68 | 0.0615 | 60.76 | 0.0246 * |
| R2 | 0.9855 | 0.9980 | 0.9967 | |||
| p-model | 0.0010 * | 0.0000 * | 0.0000 * | |||
| p lack of fit | 0.3525 | 0.2467 | 0.1140 | |||
| Optimal Conditions | |||
|---|---|---|---|
| Ethanol/water (v/v) | 50 | ||
| Amplitude (%) | 50 | ||
| Time (min) | 5 | ||
| Rosmarinic acid | Sum of phenolic acids | Sum of phenolic compounds | |
| Predicted value (µg/g d.w.) | 529.97 ± 59.37 | 1657.15 ± 86.09 | 1749.04 ± 80.91 |
| Obtained value (µg/g d.w.) | 531.00 ± 2.65 | 1695.33 ± 5.03 | 1762.00 ± 19.29 |
| CV (%) | 0.14 | 1.61 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisio, C.; Razola-Díaz, M.d.C.; Aznar-Ramos, M.J.; Longhi, M.R.; Andreatta, A.E.; Verardo, V. Optimization of the Extraction Conditions of Bioactive Compounds from Ocimum basilicum Leaves Using Ultrasound-Assisted Extraction via a Sonotrode. Molecules 2023, 28, 5286. https://doi.org/10.3390/molecules28135286
Aloisio C, Razola-Díaz MdC, Aznar-Ramos MJ, Longhi MR, Andreatta AE, Verardo V. Optimization of the Extraction Conditions of Bioactive Compounds from Ocimum basilicum Leaves Using Ultrasound-Assisted Extraction via a Sonotrode. Molecules. 2023; 28(13):5286. https://doi.org/10.3390/molecules28135286
Chicago/Turabian StyleAloisio, Carolina, María del Carmen Razola-Díaz, María José Aznar-Ramos, Marcela R. Longhi, Alfonsina E. Andreatta, and Vito Verardo. 2023. "Optimization of the Extraction Conditions of Bioactive Compounds from Ocimum basilicum Leaves Using Ultrasound-Assisted Extraction via a Sonotrode" Molecules 28, no. 13: 5286. https://doi.org/10.3390/molecules28135286
APA StyleAloisio, C., Razola-Díaz, M. d. C., Aznar-Ramos, M. J., Longhi, M. R., Andreatta, A. E., & Verardo, V. (2023). Optimization of the Extraction Conditions of Bioactive Compounds from Ocimum basilicum Leaves Using Ultrasound-Assisted Extraction via a Sonotrode. Molecules, 28(13), 5286. https://doi.org/10.3390/molecules28135286










