Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure to Obtain Quinoa Extract
2.2. Biosynthesis of QEx Series
2.3. Structural Properties: X-ray Diffraction Measurements
2.4. Morphological Properties: Transmission Electron Microscopy (TEM)
2.5. Dynamic Light Scattering and Zeta Potential Measurements
2.6. Fourier Transmission Infrared Spectrometry (FTIR)
2.7. Magnetic Properties: Vibrating Sample Magnetometer (VSM)
3. Results and Discussions
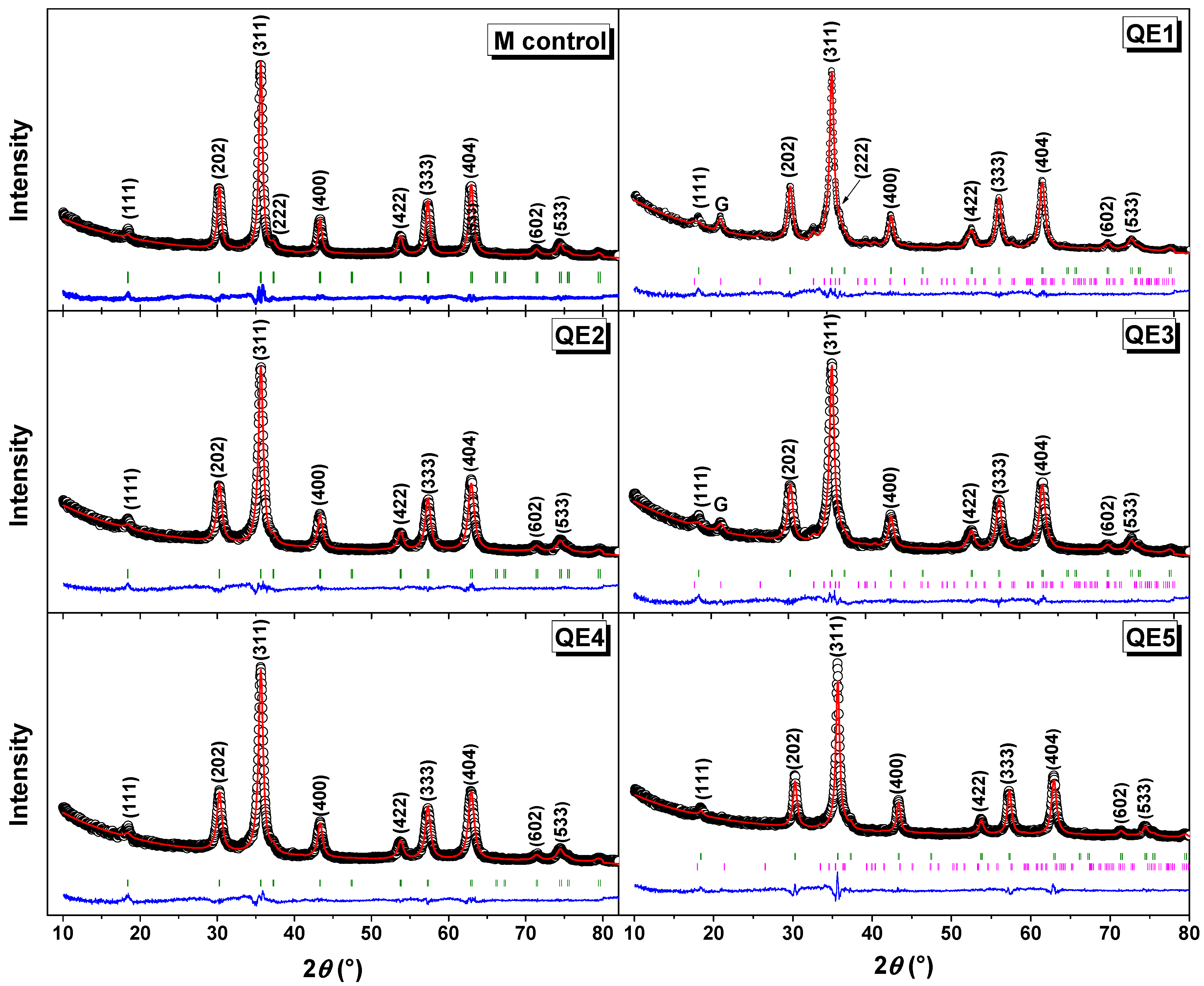
3.1. XRD Results and Rietveld Analysis
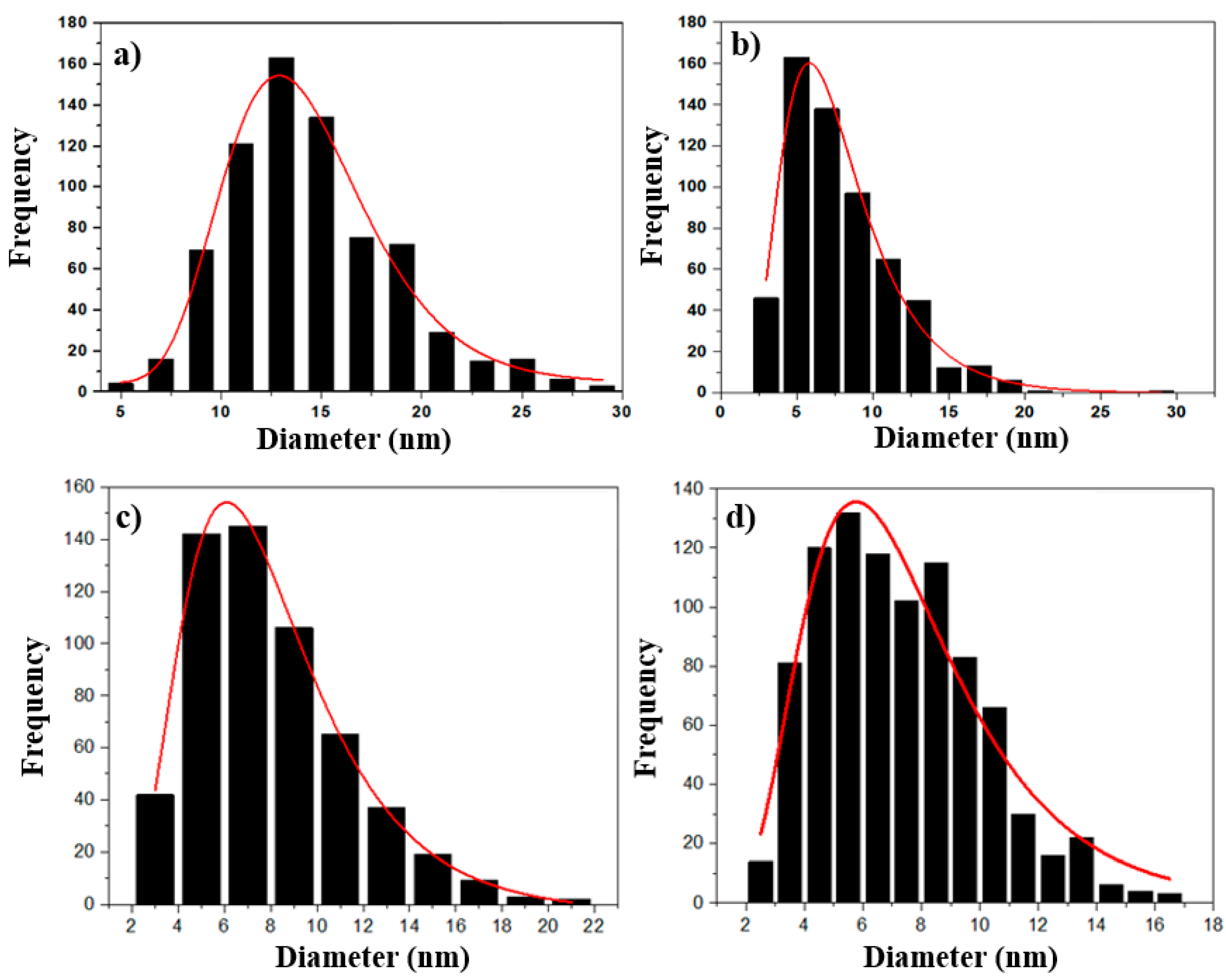
3.2. TEM Analysis
3.3. DLS and Zeta Potential Analysis
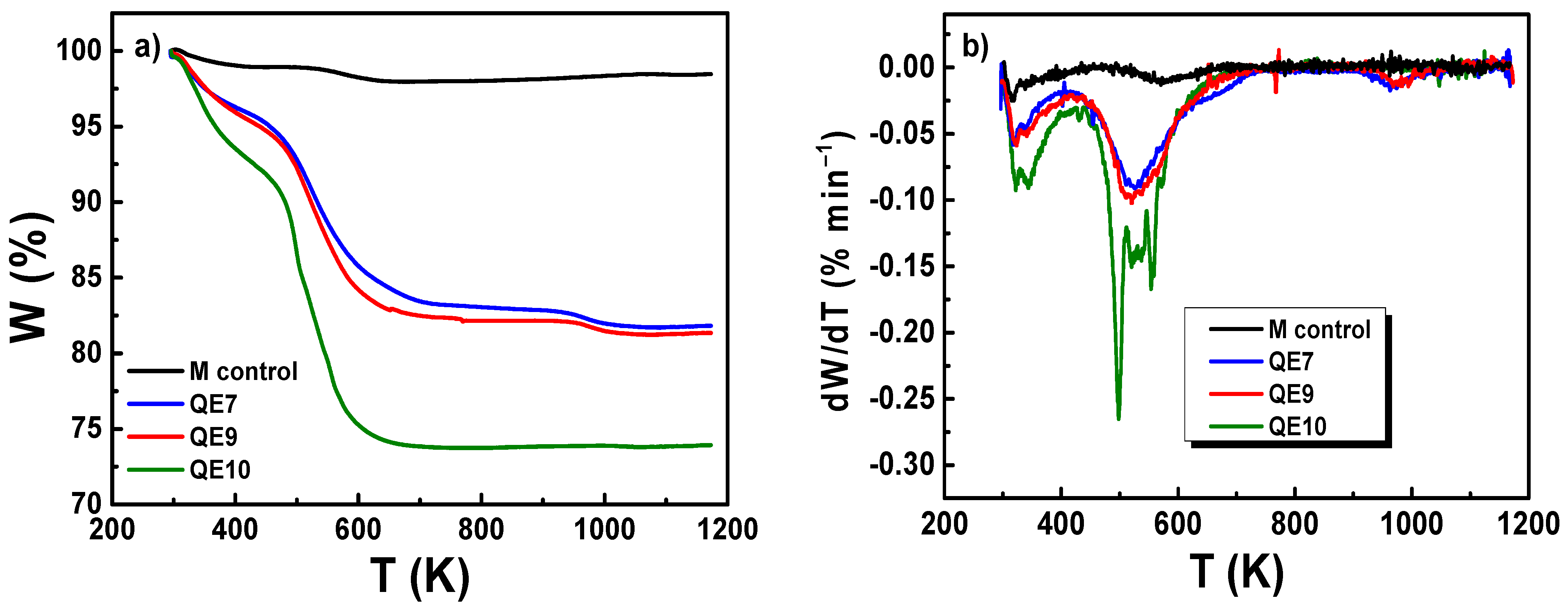
3.4. FTIR and Thermogravimetric Analysis
3.5. Magnetic Analysis
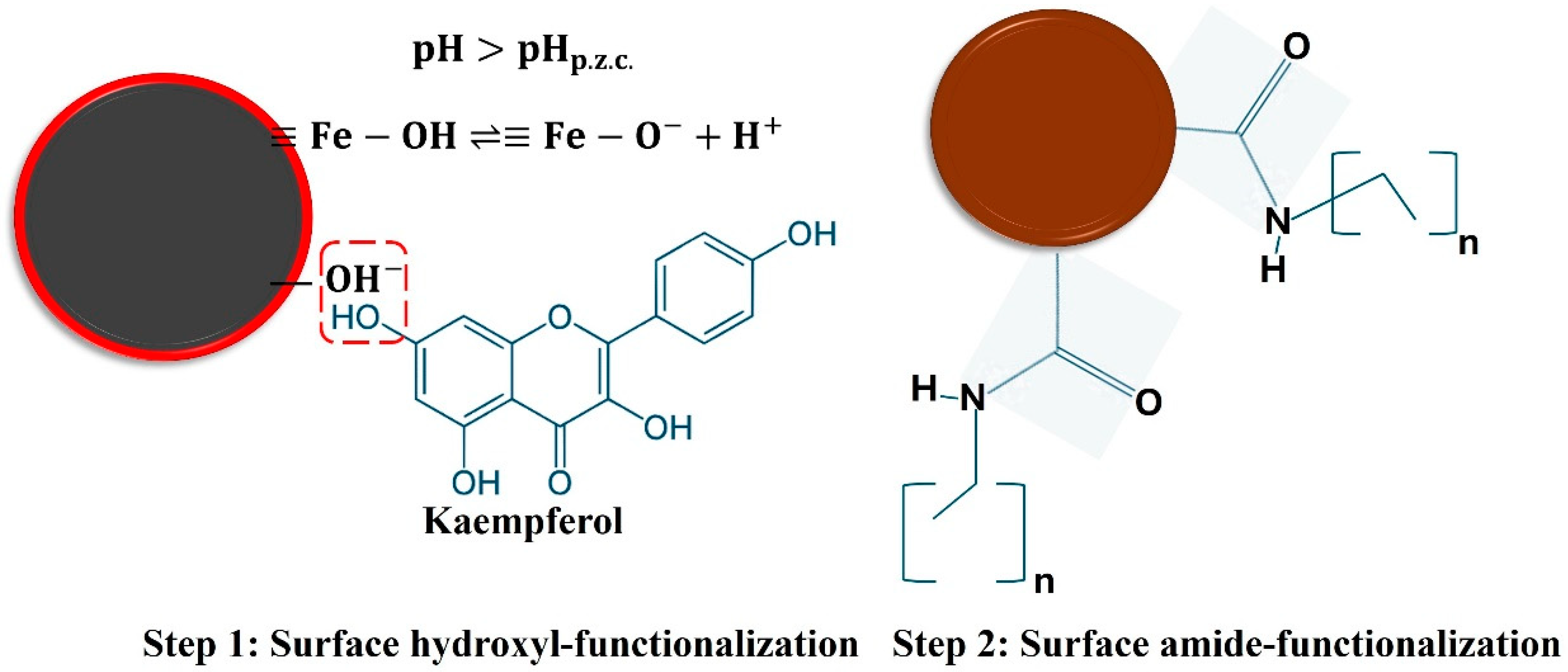
3.6. Functionalization Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacinto, M.J.; Silva, V.C.; Valladao, D.M.; Souto, R.S. Biosynthesis of magnetic iron oxide nanoparticles: A review. Biotechnology 2021, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Flores, D.A.; Passamani, E.C. Differentiating nanomaghemite and nanomagnetite and discussing their importance in arsenic and lead removal from contaminated effluents: A critical review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Najafzadeh, H.; Darroudi, M.; Miri, M.J.; Kouhbanani, M.A.; Sarani, M. Iron oxide nanoparticles: Biosynthesis, magnetic behavior, cytotoxic effect. Chem. Open 2021, 10, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, D.; Roy, K.; Debnath, S.C. Green and sustainable biosynthesis of iron oxide nanoparticles (ION) from pomegranate seed and the development of highly reinforced ION based natural rubber (NR) nanocomposite in presence of epoxidized natural rubber (ENR) as compatibilizer. CRGSC 2022, 5, 100256. [Google Scholar] [CrossRef]
- Segura, R.; Vásquez, G.; Colson, E.; Gerbaux, P.; Frischmon, C.; Nesic, A.; García, D.E.; Cabrera-Barjas, G. Phytostimulant properties of highly stable silver nanoparticles obtained with saponin extract from Chenopodium quinoa. J. Sci. Food Agric. 2020, 100, 4987–4994. [Google Scholar] [CrossRef]
- Andrade-Zavaleta, K.; Chacon-Laiza, Y.; Asmat-Campos, D.; Raquel-Checca, N. Green synthesis of superparamagnetic iron oxide nanoparticles with Eucalyptus globulus extract and their application in the removal of heavy metals from agricultural soil. Molecules 2022, 27, 1367. [Google Scholar] [CrossRef]
- Piro, N.S.; Hamad, S.M.; Mohammed, A.S.; Barzinjy, A.A. Green Synthesis Magnetite (Fe₃O₄) Nanoparticles from Rhus coriaria Extract: A Characteristic Comparison with a Conventional Chemical Method. IEEE Trans. Nanobiosci. 2023, 22, 308–317. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Khelef, A.; Tedjani, M.L.; Guemari, F. Effect of Ferric Chloride Concentration on the Type of Magnetite (Fe3O4) Nanoparticles Biosynthesized by Aqueous Leaves Extract of Artemisia and Assessment of Their Antioxidant Activities. J. Clust. Sci. 2021, 32, 1033–1041. [Google Scholar] [CrossRef]
- Amutha, S.; Sridhar, S. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Clausena indica Leaf Extract and Evaluation of Antibacterial Activity. J. Biol. Chem. Res. 2016, 35, 441–448. [Google Scholar]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Selvaraj, R.; Pai, S.; Murugesan, G.; Pandey, S.; Bhole, R.; Gonsalves, D.; Varadavenkatesan, T.; Vinayagam, R. Green synthesis of magnetic α–Fe2O3 nanospheres using Bridelia retusa leaf extract for Fenton-like degradation of crystal violet dye. Appl. Nanosci. 2021, 11, 2227–2234. [Google Scholar] [CrossRef]
- Campos-Rodríguez, Y.; Acosta-Coral, K.; Paucar-Menacho, L.M. Quinoa (Chenopodium quinoa): Nutritional composition and bioactive compounds of grain and leaf, and impact of heat treatment and germination. Sci. Agropecu. 2022, 13, 209–220. [Google Scholar] [CrossRef]
- James, L.E.A. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef]
- Paz, P.C.; Janny, R.J.; Håkansson, Å. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef]
- International Dairy Foods Association Pasteurization. WWW Document. Available online: https://www.idfa.org/news-views/media-kits/milk/pasteurization (accessed on 1 October 2024).
- Ríos, K.C. Tecnología de Alimentos, 1st ed.; Ediciones de la U: Bogotá, Colombia, 2011; pp. 14–15. [Google Scholar]
- Putz, H. Brandeburg, “Match!—Phase Identification from Powder Diffraction”. Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/match (accessed on 19 September 2024).
- Canchanya-Huaman, Y.; Mayta-Armas, A.F.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Guerra, J.A.; Ramos-Guivar, J.A. Strain and Grain Size Determination of CeO2 and TiO2 Nanoparticles: Comparing Integral Breadth Methods versus Rietveld, μ-Raman, and TEM. Nanomaterials 2021, 11, 2311. [Google Scholar] [CrossRef]
- Rueda-Vellasmin, R.; Checca-Huaman, N.R.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Mössbauer studies of core-single-shell and core-double-shell polymer functionalized magnetic nanoparticles. Hyperfine Interact. 2022, 243, 27. [Google Scholar] [CrossRef]
- Brown, W.F., Jr. Theory of the approach to magnetic saturation. Phys. Rev. 1940, 58, 736. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Taipe, K.; Schettino, M.A., Jr.; Silva, E.; Morales Torres, M.A.; Passamani, E.C.; Litterst, F.J. Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption. Nanomaterials 2020, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. A smart magnetically active nanovehicle for on-demand targeted drug delivery: Where van der waals force balances the magnetic interaction. ACS Appl. Mater. Interfaces 2015, 7, 24229–24241. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef]
- González-Muñoz, A.; Montero, B.; Enrione, J.; Matiacevich, S. Rapid prediction of moisture content of quinoa (Chenopodium quinoa Willd.) flour by Fourier transform infrared (FTIR) spectroscopy. J. Cereal Sci. 2016, 71, 246–249. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.; Passamani, E.; Litterst, F. Superspinglass state in functionalized zeolite 5A-maghemite nanoparticles. AIP Adv. 2021, 11, 035223. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sadrollahi, E.; Menzel, D.; Ramos Fernandes, E.G.; López, E.O.; Torres, M.M.; Arsuaga, J.M.; Arencibia, A.; Litterst, F.J. Magnetic, structural and surface properties of functionalized maghemite nanoparticles for copper and lead adsorption. RSC Adv. 2017, 7, 28763–28779. [Google Scholar] [CrossRef]
- El Mendili, Y.; Bardeau, J.F.; Randrianantoandro, N.; Grasset, F.; Greneche, J.M. Insights into the mechanism related to the phase transition from γ-Fe2O3 to α-Fe2O3 nanoparticles induced by thermal treatment and laser irradiation. J. Phys. Chem. C 2012, 116, 23785–23792. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Morales, M.A.; Litterst, F.J. Suppression of exchange bias effect in maghemite nanoparticles functionalized with H2Y. J. Magn. Magn. Mater. 2016, 420, 324–335. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Pereira, E.; Cadavez, V.; Barros, L.; Encina-Zelada, C.; Stojković, D.; Sokovic, M.; Calhelha, R.C.; Gonzales-Barron, U.; Ferreira, I.C. Chenopodium quinoa Willd.(quinoa) grains: A good source of phenolic compounds. Food Res. Int. 2020, 137, 109574. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Kuzniarz, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm.-Drug Res. 2001, 58, 415–420. [Google Scholar]
- Yadav, B.; Yadav, R.K.; Srivastav, G.; Yadav, R.A. Experimental Raman, FTIR and UV-vis spectra, DFT studies of molecular structures and barrier heights, thermodynamic functions and bioactivity of kaempferol. J. Mol. Struct. 2022, 1258, 132637. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pittman, C.U., Jr.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Al-Harahsheh, M.; AlJarrah, M.; Mayyas, M.; Alrebaki, M. High-stability polyamine/amide-functionalized magnetic nanoparticles for enhanced extraction of uranium from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2018, 86, 148–157. [Google Scholar] [CrossRef]






| % w/v | Sample | QE (mL) | H2O | FeSO4•7H2O (g) | FeCl3 (g) | NH4OH (mL) |
|---|---|---|---|---|---|---|
| 0 | M control | 0 | 100 mL | 5.2 | 6 | 20 |
| 5 | QE1 | 100 | 0 | 5.2 | 6 | 10 |
| 5 | QE2 | 100 | 0 | 5.2 | 6 | 15 |
| 10 | QE3 | 100 | 0 | 5.2 | 6 | 10 |
| 10 | QE4 | 100 | 0 | 5.2 | 6 | 15 |
| 20 | QE5 | 100 | 0 | 5.2 | 6 | 10 |
| 20 | QE6 | 100 | 0 | 5.2 | 6 | 15 |
| 40 | QE7 | 100 | 0 | 5.2 | 6 | 10 |
| 40 | QE8 | 100 | 0 | 5.2 | 6 | 15 |
| 50 | QE9 | 100 | 0 | 5.2 | 6 | 10 |
| 50 | QE10 | 100 | 0 | 5.2 | 6 | 15 |
| Sample | Phase | Caglioti Parameters | Lattice Parameters (Å) | DXRD (nm) | Weight Fraction (%) | Statistical Parameters | ||
|---|---|---|---|---|---|---|---|---|
| U | V | W | ||||||
| M control | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 8.9(1) | 100.0 | Rp = 14.3% Rwp = 14.0% Rexp = 7.2% χ2 = 3.8 |
| QE1 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 7.2(1) | 90.0 | Rp = 13.5% Rwp = 13.6% Rexp = 8.3% χ2 = 2.7 |
| G | 0.113 | −0.584 | 0.561 | a = 9.952(1) b = 3.026(1) c = 4.616(1) α = β = γ = 90° | 10.4(1). | 10.0 | ||
| QE2 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 7.5(1) | 100.0 | Rp = 13.2% Rwp = 13.3% Rexp = 8.1% χ2 = 2.7 |
| QE3 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 7.5(1) | 93.0 | Rp = 13.3% Rwp = 13.3% Rexp = 8.0% χ2 = 2.8 |
| G | 0.702 | −0.670 | 0.626 | a = 9.945(1) b = 3.035(1) c = 4.606(1) α = β = γ = 90° | 9.4(1) | 7.0 | ||
| QE4 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 7.1(1) | 100.0 | Rp = 13.1% Rwp = 13.0% Rexp = 7.7% χ2 = 2.9 |
| QE5 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 9.4(1) | 95.0 | Rp = 19.9% Rwp = 17.3% Rexp = 9.1% χ2 = 3.6 |
| G | 0.436 | −0.729 | 0.669 | a = 9.892(1) b = 3.049(1) c = 4.587(1) α = β = γ = 90° | 10.9(1) | 5.0 | ||
| QE6 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 6.5(1) | 95.0 | Rp = 16.5% Rwp= 15.5% Rexp = 8.2% χ2 = 3.6 |
| G | 0.113 | −0.584 | 0.561 | a = 9.952(1) b = 3.026(1) c = 4.616(1) α = β = γ = 90° | 11.8(1) | 5.0 | ||
| QE7 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90° | 6.3(1) | 100.0 | Rp = 21.6% Rwp = 19.4% Rexp = 9.9% χ2 = 3.9 |
| QE8 | γ-Fe2O3 | 0.013 | −0.034 | 0.008 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90 | 6.9(1) | 100.0 | Rp = 15.4% Rwp = 14.6% Rexp = 10.0% χ2 = 2.1 |
| QE9 | γ-Fe2O3 | 0.009 | −0.005 | 0.001 | a = 8.355(1) b = 8.355(1) c = 8.355(1) α = β = γ = 90 | 7.6(1) | 100.0 | Rp = 26.3% Rwp = 23.3% Rexp = 10.2% χ2 = 5.2 |
| QE10 | γ-Fe2O3 | 0.009 | −0.005 | 0.001 | a = 8.354(1) b = 8.354(1) c = 8.354(1) α = β = γ = 90 | 3.6(1) | 100.0 | Rp = 15.7% Rwp = 15.3% Rexp = 9.1% χ2 = 2.8 |
| Sample | D | σ | PDI | n |
|---|---|---|---|---|
| Control | 13.8(2) | 0.27 | 0.02 | 726 |
| QE7 | 7.2(2) | 0.45 | 0.06 | 587 |
| QE9 | 7.5(4) | 0.45 | 0.06 | 570 |
| QE10 | 7.0(2) | 0.40 | 0.06 | 914 |
| Nanosystem | Extract | Particle Diameter (nm) | Reference |
|---|---|---|---|
| Superparamagnetic iron-oxide nanoparticles | Eucalyptus globulus | 2.3 | [6] |
| nanomagnetite (Fe3O4) | Rhus Coriaria | 7.9 | [7] |
| Fe3O4 | Artemisia leaves | 19–24 | [8] |
| Iron-oxide NPs (Fe3O4) | Clausena indica | 47.5–59.5 | [9] |
| Iron-oxide NPs | Carica papaya | 21.6 | [10] |
| Magnetic nanospheres of α-Fe2O3 | Bridelia retusa | 38.6 | [11] |
| Sample | Concentration (g L−1) | DH (nm) | p.z.c. |
|---|---|---|---|
| M control | 1.0 | 136 | 5.5 |
| QE1 | 7.5 | 242 | 6.4 |
| QE2 | 5.8 | 263 | 6.5 |
| QE3 | 7.3 | 349 | 6.4 |
| QE4 | 7.4 | 1310 | 5.8 |
| QE5 | 3.6 | 417 | 5.6 |
| QE6 | 3.5 | 1101 | 5.7 |
| QE7 | 6.4 | 305 | 4.0 |
| QE8 | 4.5 | 649 | 4.0 |
| QE9 | 0.5 | 396 | 3.4 |
| QE10 | 0.5 | 383 | 3.8 |
| Functional Groups | Wavenumber (cm−1) (Literature) | Wavenumber (cm−1) (This Work) | Band Intensity |
|---|---|---|---|
| –CH | 2915 | 2923(8) | Weak |
| –CH2 | 2850 | 2850(0) | Weak |
| –COOH | 998 | 1001(3) | Strong (characteristic) |
| O–H | 855 | 860(5) | medium |
| C=O | 1737 | - | weak |
| Amide I | 1640 | 1639(1) | medium |
| Amide II | 1545 | - | weak |
| C–N | 1237 | 1240(3) | weak |
| QE7 | QE9 | QE10 | IR Band |
|---|---|---|---|
| 545 | 545 | 565 | γ-Fe2O3 (strong) |
| 624 | 624 | 694 | γ-Fe2O3 (strong) |
| 1026 | 1026 | 1026 | Quinoa (-COOH) (strong) |
| 1647 | 1637 | 1628 | Quinoa (amide I) (strong) |
| 1261 | 1263 | 1263 | C–N (amide III) |
| Sample | T (K) | Hc (kOe) | Ms (emu g−1) | Keff (104 J m−3) |
|---|---|---|---|---|
| M control | 5 | 0.27(1) | 76 | 4.0 |
| 300 | - | 69 | 3.2 | |
| QE7 | 5 | 0.22(1) | 64 | 3.3 |
| 300 | - | 51 | 2.8 | |
| QE9 | 5 | 0.21(1) | 51 | 2.9 |
| 300 | - | 42 | 2.6 | |
| QE10 | 5 | 0.36(1) | 17 | 1.8 |
| 300 | - | 11 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Carrillo, M.d.P.; Checca-Huaman, N.-R.; Passamani, E.C.; Ramos-Guivar, J.A. Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract. Nanomaterials 2024, 14, 1607. https://doi.org/10.3390/nano14191607
Marcos-Carrillo MdP, Checca-Huaman N-R, Passamani EC, Ramos-Guivar JA. Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract. Nanomaterials. 2024; 14(19):1607. https://doi.org/10.3390/nano14191607
Chicago/Turabian StyleMarcos-Carrillo, Mercedes del Pilar, Noemi-Raquel Checca-Huaman, Edson C. Passamani, and Juan A. Ramos-Guivar. 2024. "Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract" Nanomaterials 14, no. 19: 1607. https://doi.org/10.3390/nano14191607
APA StyleMarcos-Carrillo, M. d. P., Checca-Huaman, N.-R., Passamani, E. C., & Ramos-Guivar, J. A. (2024). Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract. Nanomaterials, 14(19), 1607. https://doi.org/10.3390/nano14191607







