Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease
Abstract
:1. The Burden of Chronic Kidney Disease
2. High Protein Diets May Be Harmful to Kidney Health
3. A Low Protein Diet Preserves Kidney Function
4. Plant-Based Foods Have a Favorable Impact on Kidney Health
5. Benefits of a Plant-Dominant Low Protein Diet
- (1)
- Reduction in nitrogenous compounds leads to less production of ammonia and uremic toxins as an effective strategy in controlling uremia and delaying dialysis initiation [28].
- (2)
- (3)
- Attenuation of metabolites derived from gut bacteria that are linked with CKD and CV disease: Animal protein ingredients including choline and carnitine are converted by gut flora into trimethylamine (TMA) and TMA N-oxide (TMAO) that are associated with atherosclerosis, renal fibrosis [68], and increased risk of CV disease and death [69]. The favorable impact on the gut microbiome [70] similarly leads to lower levels of other uremic toxins such as indoxyl sulfate and p-cresol sulfate [71].
- (4)
- Decreased acid load: plant foods have a lower acidogenicity in contrast to animal foods, and this alkalization may have additional effects beyond mere intake of natural alkali [72].
- (5)
- Reduced phosphorus burden: there is less absorbable phosphorus in plant-based proteins given the presence of indigestible phytate binding to plant-based phosphorus. Fruits and vegetables are less likely to have added phosphorus-based preservatives that are often used for meat processing [59,73,74,75].
- (6)
- (7)
- (8)
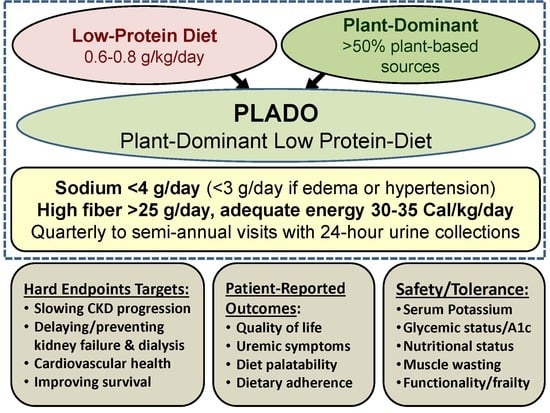
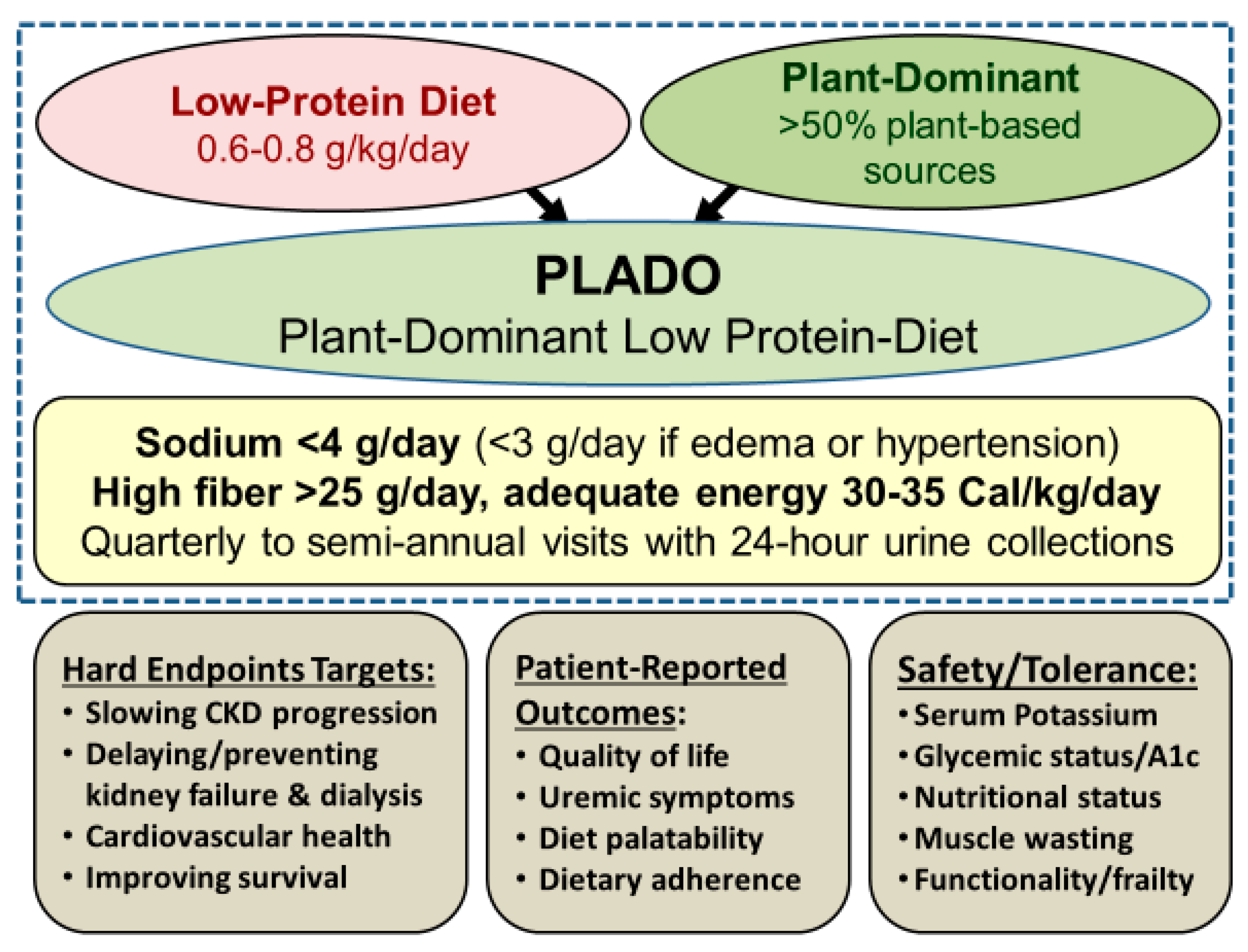
6. Features of PLADO Regimens
7. Safety and Adequacy of a Plant-Dominant Low-Protein Diet
8. Impact of PLADO on Microbiome in CKD
9. Similarities and Distinctions between PLADO and other CKD Diets
10. Role of Dietitians in PLADO
11. Recommendations for Practical Implementation of PLADO
12. Concurrent Pharmacotherapy and Other Interventions
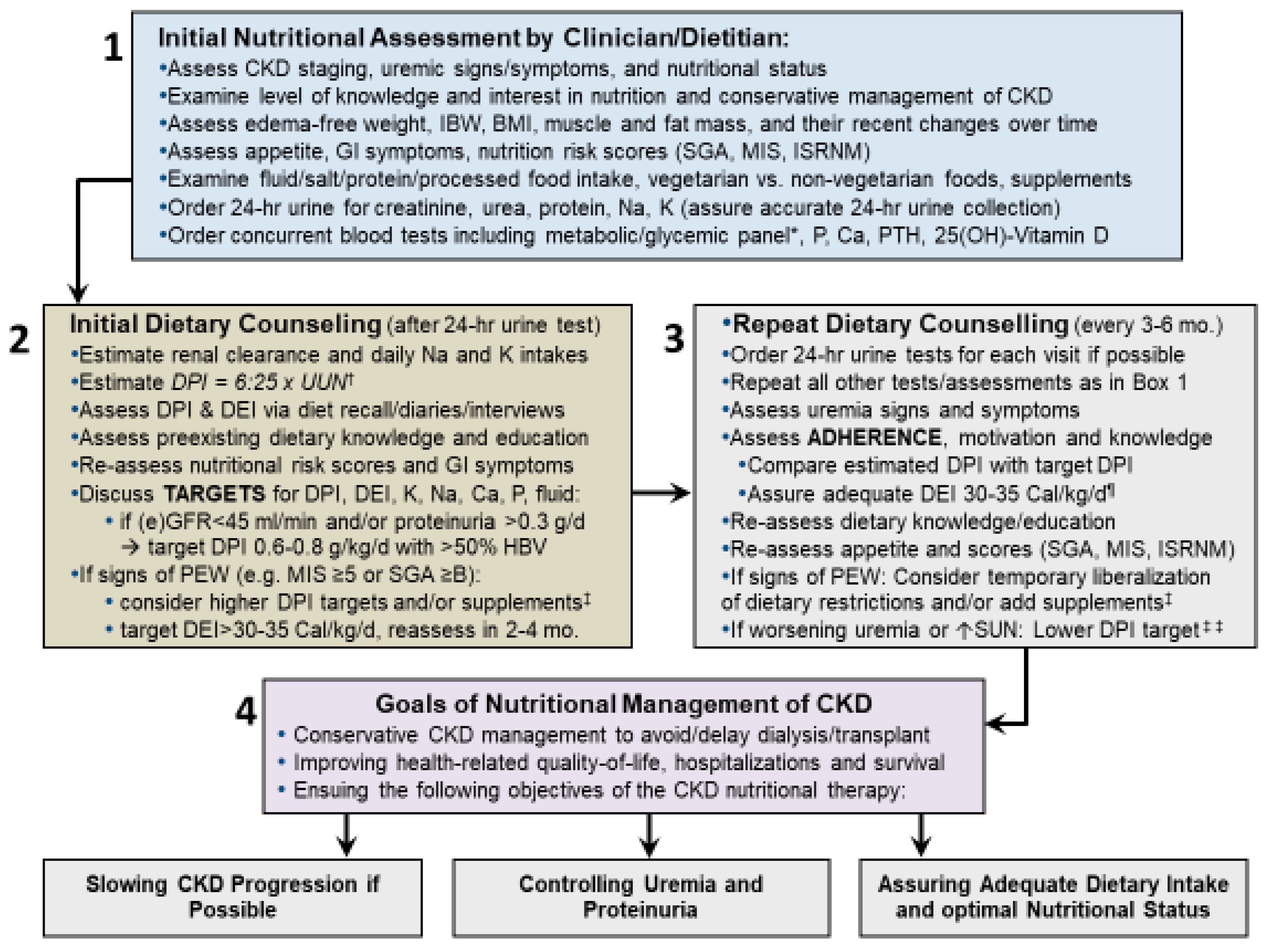
13. Laboratory Tests for Nutritional Management of CKD
- (1)
- Creatinine clearance: UCr*UV/SCr in ml/min, and to compare to eGFR;
- (2)
- Creatinine index: UCr/Weight (mg/kg), to identify 24-h urine collection inaccuracies including under- and over-collections by comparing it to the expected value of 1–2 mg/kg/d for women and 1.5–2.5 mg/kg/day for men;
- (3)
- (4)
- Estimated dietary Na intake: UNa in mmol/44 (g/day);
- (5)
- Estimated dietary K intake: UK in mmol/25 (g/day);
- (6)
- 24-h urinary protein and albumin excretion (mg/day).
14. Suggested Self-Administered Questionnaires
15. Diet Safety and Transient Dietary Regimen Suspension
16. Challenges and Pitfalls of the Dietary Management of CKD
17. Anticipated Impact and Future Steps
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, P.K.; Garcia-Garcia, G.; Lui, S.F.; Andreoli, S.; Fung, W.W.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney health for everyone everywhere-from prevention to detection and equitable access to care. Kidney Int. 2020, 97, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Crowley, S.T.; Beddhu, S.; Chen, J.L.T.; Daugirdas, J.T.; Goldfarb, D.S.; Jin, A.; Kovesdy, C.P.; Leehey, D.J.; Moradi, H.; et al. Renal Replacement Therapy and Incremental Hemodialysis for Veterans with Advanced Chronic Kidney Disease. Semin. Dial. 2017, 30, 251–261. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef] [Green Version]
- Saran, R.; Shahinian, V.; Pearsonm, A.; Tilea, A.; Steffick, D.; Wyncott, A.; Bragg-Gresham, J.; Heung, M.; Morgenstern, M.; Hutton, D.; et al. Establishing a National Population Health Management System for Kidney Disease: The Veterans Health Administration Renal Information System (VA-REINS). Am. J. Kidney Dis. 2017, 5, A3. [Google Scholar]
- Saran, R.; Li, Y.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Ayanian, J.; Bragg-Gresham, J.; Balkrishnan, R.; Chen, J.L.; Cope, E.; et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2016, 67 (Suppl. 1), S1–S305. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Li, Y.; Robinson, B.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Chen, J.T.; Cope, E.; Gipson, D.; He, K.; et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2015, 66 (Suppl. 1), S1–S305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, V.; Maciejewski, M.L.; Patel, U.D.; Stechuchak, K.M.; Hynes, D.M.; Weinberger, M. Comparison of outcomes for veterans receiving dialysis care from VA and non-VA providers. BMC Health Serv. Res. 2013, 13, 26. [Google Scholar] [CrossRef] [Green Version]
- Streja, E.; Kovesdy, C.P.; Soohoo, M.; Obi, Y.; Rhee, C.M.; Park, C.; Chen, J.L.T.; Nakata, T.; Nguyen, D.V.; Amin, A.N.; et al. Dialysis Provider and Outcomes among United States Veterans Who Transition to Dialysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1055–1062. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Wightman, A.; Liao, S. Ensuring Choice for People with Kidney Failure—Dialysis, Supportive Care, and Hope. N. Engl. J. Med. 2020, in press. [Google Scholar]
- Kurella Tamura, M.; Covinsky, K.E.; Chertow, G.M.; Yaffe, K.; Landefeld, C.S.; McCulloch, C.E. Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 2009, 361, 1539–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Li, P.K. Strategies to prevent kidney disease and its progression. Nat. Rev. Nephrol. 2020, 16, 129–130. [Google Scholar] [CrossRef]
- Moore, L.W.; Kalantar-Zadeh, K. Implementing the “Advancing American Kidney Health Initiative” by Leveraging Nutritional and Dietary Management of Kidney Patients. J. Ren. Nutr. 2019, 29, 357–360. [Google Scholar] [CrossRef]
- Ko, G.J.; Kalantar-Zadeh, K.; Goldstein-Fuchs, J.; Rhee, C.M. Dietary Approaches in the Management of Diabetic Patients with Kidney Disease. Nutrients 2017, 9, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.W.; Byham-Gray, L.D.; Scott Parrott, J.; Rigassio-Radler, D.; Mandayam, S.; Jones, S.L.; Mitch, W.E.; Osama Gaber, A. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013, 83, 724–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiakos, S.M.; Lieberman, H.R.; Fulgoni, V.L., 3rd. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J. Nutr. 2015, 145, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, G.-J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Impact of High Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, in press. [Google Scholar]
- Kamper, A.L.; Strandgaard, S. Long-Term Effects of High-Protein Diets on Renal Function. Annu. Rev. Nutr. 2017, 37, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kramer, H.M.; Fouque, D. High-protein diet is bad for kidney health: Unleashing the taboo. Nephrol. Dial. Transplant. 2020, 35, 1–4. [Google Scholar] [CrossRef]
- Kime, P. VA Eyes Keto Diet-Based Diabetes Treatment, But Questions Remain. 2019. Available online: https://www.military.com/daily-news/2019/07/09/va-eyes-keto-diet-based-diabetes-treatment-questions-remain.html (accessed on 27 July 2019).
- Joshi, S.; Ostfeld, R.J.; McMacken, M. The Ketogenic Diet for Obesity and Diabetes-Enthusiasm Outpaces Evidence. JAMA Intern. Med. 2019, 179, 1163–1164. [Google Scholar] [CrossRef]
- Esmeijer, K.; Geleijnse, J.M.; de Fijter, J.W.; Kromhout, D.; Hoogeveen, E.K. Dietary protein intake and kidney function decline after myocardial infarction: The Alpha Omega Cohort. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhee, J.H.; Kee, Y.K.; Park, S.; Kim, H.; Park, J.T.; Han, S.H.; Kang, S.W.; Yoo, T.H. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: A community-based prospective cohort study. Nephrol. Dial. Transplant. 2020, 35, 98–106. [Google Scholar] [CrossRef]
- Malhotra, R.; Lipworth, L.; Cavanaugh, K.L.; Young, B.A.; Tucker, K.L.; Carithers, T.C.; Taylor, H.A.; Correa, A.; Kabagambe, E.K.; Ikizler, T.A. Protein Intake and Long-term Change in Glomerular Filtration Rate in the Jackson Heart Study. J. Ren. Nutr. 2018, 28, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Laville, M.; Boissel, J.P.; Chifflet, R.; Labeeuw, M.; Zech, P.Y. Controlled low protein diets in chronic renal insufficiency: Meta-analysis. BMJ 1992, 304, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chewcharat, A.; Takkavatakarn, K.; Wongrattanagorn, S.; Panrong, K.; Kittiskulnam, P.; Eiam-Ong, S.; Susantitaphong, P. The Effects of Restricted Protein Diet Supplemented with Ketoanalogue on Renal Function, Blood Pressure, Nutritional Status, and Chronic Kidney Disease-Mineral and Bone Disorder in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yang, L.; Li, Z.; Qin, W. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 409–418. [Google Scholar] [CrossRef]
- Metzger, M.; Yuan, W.L.; Haymann, J.P.; Flamant, M.; Houillier, P.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Bankir, L.; et al. Association of a Low-Protein Diet With Slower Progression of CKD. Kidney Int Rep. 2018, 3, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Tantisattamo, E.; Dafoe, D.C.; Reddy, U.G.; Ichii, H.; Rhee, C.M.; Streja, E.; Landman, J.; Kalantar-Zadeh, K. Current Management of Patients with Acquired Solitary Kidney. Kidney Int. Rep. 2019, 4, 1205–1218. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Bruton, J.L.; Perusek, M.C.; Lancaster, J.L.; Kopp, D.T.; DeFronzo, R.A. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1991, 324, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W. Does Kidney Longevity Mean Healthy Vegan Food and Less Meat or Is Any Low-Protein Diet Good Enough? J. Ren. Nutr. 2019, 29, 79–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.B.; ter Wee, P.M.; Meijer, S.; Piers-Becht, T.P.; Sluiter, W.J.; Donker, A.J. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 1984, 2, 1291–1296. [Google Scholar] [CrossRef]
- Rosman, J.B.; Langer, K.; Brandl, M.; Piers-Becht, T.P.; van der Hem, G.K.; ter Wee, P.M.; Donker, A.J. Protein-restricted diets in chronic renal failure: A four year follow-up shows limited indications. Kidney Int. Suppl. 1989, 27, S96–S102. [Google Scholar] [PubMed]
- Ihle, B.U.; Becker, G.J.; Whitworth, J.A.; Charlwood, R.A.; Kincaid-Smith, P.S. The effect of protein restriction on the progression of renal insufficiency. N. Engl. J. Med. 1989, 321, 1773–1777. [Google Scholar] [CrossRef]
- Lindenau, K.; Abendroth, K.; Kokot, F.; Vetter, K.; Rehse, C.; Frohling, P.T. Therapeutic effect of keto acids on renal osteodystrophy. A prospective controlled study. Nephron 1990, 55, 133–135. [Google Scholar] [CrossRef]
- Williams, P.S.; Stevens, M.E.; Fass, G.; Irons, L.; Bone, J.M. Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: A prospective, randomized, controlled trial. QJM 1991, 81, 837–855. [Google Scholar] [CrossRef]
- Locatelli, F.; Alberti, D.; Graziani, G.; Buccianti, G.; Redaelli, B.; Giangrande, A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Northern Italian Cooperative Study Group. Lancet 1991, 337, 1299–1304. [Google Scholar] [CrossRef]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Montes-Delgado, R.; Guerrero Riscos, M.A.; Garcia-Luna, P.P.; Martin Herrera, C.; Pereira Cunill, J.L.; Garrido Vazquez, M.; Lopez Munoz, I.; Suarez Garcia, M.J.; Martin-Espejo, J.L.; Soler Junco, M.L.; et al. Treatment with low-protein diet and caloric supplements in patients with chronic kidney failure in predialysis. Comparative study. Rev. Clin. Esp. 1998, 198, 580–586. [Google Scholar] [PubMed]
- Malvy, D.; Maingourd, C.; Pengloan, J.; Bagros, P.; Nivet, H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. J. Am. Coll. Nutr. 1999, 18, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Teplan, V.; Schuck, O.; Knotek, A.; Hajny, J.; Horackova, M.; Skibova, J.; Maly, J. Effects of low-protein diet supplemented with ketoacids and erythropoietin in chronic renal failure: A long-term metabolic study. Ann. Transplant. 2001, 6, 47–53. [Google Scholar]
- Prakash, S.; Pande, D.P.; Sharma, S.; Sharma, D.; Bal, C.S.; Kulkarni, H. Randomized, double-blind, placebo-controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. J. Ren. Nutr. 2004, 14, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunori, G.; Viola, B.F.; Parrinello, G.; De Biase, V.; Como, G.; Franco, V.; Garibotto, G.; Zubani, R.; Cancarini, G.C. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am. J. Kidney Dis. 2007, 49, 569–580. [Google Scholar] [CrossRef]
- Mircescu, G.; Garneata, L.; Stancu, S.H.; Capusa, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Pota, A.; Pisani, A.; Torraca, S.; Annecchini, R.; Lombardi, P.; Capuano, A.; Nazzaro, P.; Bellizzi, V.; Sabbatini, M. Metabolic effects of two low protein diets in chronic kidney disease stage 4-5--a randomized controlled trial. Nephrol. Dial. Transplant. 2008, 23, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, B.R.; Cucciniello, E.; Martino, R.; Frallicciardi, A.; Tortoriello, R.; Struzziero, G. Acute and persistent antiproteinuric effect of a low-protein diet in chronic kidney disease. G. Ital. Nefrol. 2009, 26, 608–615. [Google Scholar]
- Jiang, N.; Qian, J.; Sun, W.; Lin, A.; Cao, L.; Wang, Q.; Ni, Z.; Wan, Y.; Linholm, B.; Axelsson, J.; et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: A prospective, randomized trial. Nephrol. Dial. Transplant. 2009, 24, 2551–2558. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Qian, J.; Lin, A.; Fang, W.; Zhang, W.; Cao, L.; Wang, Q.; Ni, Z.; Yao, Q. Low-protein diet supplemented with keto acids is associated with suppression of small-solute peritoneal transport rate in peritoneal dialysis patients. Int. J. Nephrol. 2011, 2011, 542704. [Google Scholar] [CrossRef] [Green Version]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L., 3rd. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Hwang, S.Y.; House, J.D.; Ogborn, M.R.; Weiler, H.A.; Karmin, O.; Aukema, H.M. Long-term high intake of whole proteins results in renal damage in pigs. J. Nutr. 2010, 140, 1646–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Hashmi, S.; Shah, S.; Kalantar-Zadeh, K. Plant-based diets for prevention and management of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 16–21. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Hanna, R.M.; Reddy, U.G.; Ichii, H.; Dafoe, D.C.; Danovitch, G.M.; Kalantar-Zadeh, K. Novel options for failing allograft in kidney transplanted patients to avoid or defer dialysis therapy. Curr. Opin. Nephrol. Hypertens. 2020, 29, 80–91. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2020, in press. [Google Scholar]
- Chauveau, P.; Lasseur, C. Plant-based Protein Intake and Kidney Function in Diabetic Patients. Kidney Int. Rep. 2019, 4, 638–639. [Google Scholar] [CrossRef] [Green Version]
- Campbell, T.M.; Liebman, S.E. Plant-based dietary approach to stage 3 chronic kidney disease with hyperphosphataemia. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Moorthi, R.N.; Vorland, C.J.; Hill Gallant, K.M. Diet and Diabetic Kidney Disease: Plant Versus Animal Protein. Curr. Diab. Rep. 2017, 17, 15. [Google Scholar] [CrossRef] [Green Version]
- Clegg, D.J.; Hill Gallant, K.M. Plant-Based Diets in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 141–143. [Google Scholar] [CrossRef]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of diet with albuminuria and kidney function decline. Clin. J. Am. Soc. Nephrol. 2010, 5, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The Associations of Plant Protein Intake with All-Cause Mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Koppe, L.; Fouque, D. The Role for Protein Restriction in Addition to Renin-Angiotensin-Aldosterone System Inhibitors in the Management of CKD. Am. J. Kidney Dis. 2019, 73, 248–257. [Google Scholar] [CrossRef]
- Pignanelli, M.; Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; Spence, J.D. Moderate Renal Impairment and Toxic Metabolites Produced by the Intestinal Microbiome: Dietary Implications. J. Ren. Nutr. 2018. [Google Scholar] [CrossRef]
- Fogelman, A.M. TMAO is both a biomarker and a renal toxin. Circ. Res. 2015, 116, 396–397. [Google Scholar] [CrossRef]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2018. [Google Scholar] [CrossRef] [Green Version]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; de Carvalho Ferreira, D.; Rosado, A.; Carraro Eduardo, J.C.; Mafra, D. Does Low-Protein Diet Influence the Uremic Toxin Serum Levels From the Gut Microbiota in Nondialysis Chronic Kidney Disease Patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef]
- Angeloco, L.R.N.; Arces de Souza, G.C.; Almeida Romao, E.; Garcia Chiarello, P. Alkaline Diet and Metabolic Acidosis: Practical Approaches to the Nutritional Management of Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 215–220. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.T.; Barretti, P.; Caramori, J.C.T. Dietary Intervention in Phosphatemia Control-Nutritional Traffic Light Labeling. J. Ren. Nutr. 2018, 28, e45–e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.T.; Barretti, P.; Caramori, J.C.T. Attention to Food Phosphate and Nutrition Labeling. J. Ren. Nutr. 2018, 28, e29–e31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirci, B.G.; Tutal, E.; Eminsoy, I.O.; Kulah, E.; Sezer, S. Dietary Fiber Intake: Its Relation with Glycation End Products and Arterial Stiffness in End-Stage Renal Disease Patients. J. Ren. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Parpia, A.S.; L’Abbe, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P.B. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients With Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 83–90. [Google Scholar] [CrossRef]
- Picard, K. Potassium Additives and Bioavailability: Are We Missing Something in Hyperkalemia Management? J. Ren. Nutr. 2018. [Google Scholar] [CrossRef]
- Hirahatake, K.M.; Jacobs, D.R.; Gross, M.D.; Bibbins-Domingo, K.B.; Shlipak, M.G.; Mattix-Kramer, H.; Odegaard, A.O. The Association of Serum Carotenoids, Tocopherols, and Ascorbic Acid with Rapid Kidney Function Decline: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Ren. Nutr. 2018. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Chen, J.; Yang, W.; Appel, L.J.; Kusek, J.W.; Alper, A.; Delafontaine, P.; Keane, M.G.; Mohler, E.; Ojo, A.; et al. Sodium Excretion and the Risk of Cardiovascular Disease in Patients With Chronic Kidney Disease. JAMA 2016, 315, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.S.; Kim, Y.H.; Chung, W.; Park, S.K.; Choi, K.H.; Ahn, C.; Oh, K.H. Dietary Protein Intake, Protein Energy Wasting, and the Progression of Chronic Kidney Disease: Analysis from the KNOW-CKD Study. Nutrients 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.; Krishnan, N.; Kimani, P.K.; Lycett, D. Effect of Dietary Potassium Restriction on Serum Potassium, Disease Progression, and Mortality in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Bross, R.; Benner, D.; Kopple, J.D. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 683–692. [Google Scholar] [CrossRef] [Green Version]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P.; Meijers, B.K. Dietary fiber and protein: Nutritional therapy in chronic kidney disease and beyond. Kidney Int. 2012, 81, 227–229. [Google Scholar] [CrossRef]
- Xu, H.; Huang, X.; Riserus, U.; Krishnamurthy, V.M.; Cederholm, T.; Arnlov, J.; Lindholm, B.; Sjogren, P.; Carrero, J.J. Dietary fiber, kidney function, inflammation, and mortality risk. Clin. J. Am. Soc. Nephrol. 2014, 9, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Ikizler, T.A. Let them eat during dialysis: An overlooked opportunity to improve outcomes in maintenance hemodialysis patients. J. Ren. Nutr. 2013, 23, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer. Adher. 2013, 7, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Debard, C.; Drai, J.; Denis, L.; Pesenti, S.; Bienvenu, J.; Vidal, H.; Laville, M.; Fouque, D. Differential dose effect of fish oil on inflammation and adipose tissue gene expression in chronic kidney disease patients. Nutrition 2013, 29, 730–736. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Braglia, A.; Chow, J.; Kwon, O.; Kuwae, N.; Colman, S.; Cockram, D.B.; Kopple, J.D. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: A pilot/feasibility study. J. Ren. Nutr. 2005, 15, 318–331. [Google Scholar] [CrossRef]
- Rattanasompattikul, M.; Molnar, M.Z.; Lee, M.L.; Dukkipati, R.; Bross, R.; Jing, J.; Kim, Y.; Voss, A.C.; Benner, D.; Feroze, U.; et al. Anti-Inflammatory and Anti-Oxidative Nutrition in Hypoalbuminemic Dialysis Patients (AIONID) study: Results of the pilot-feasibility, double-blind, randomized, placebo-controlled trial. J. Cachexia Sarcopenia Muscle. 2013, 4, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Soohoo, M.; Ahmadi, S.F.; Qader, H.; Streja, E.; Obi, Y.; Moradi, H.; Rhee, C.M.; Kim, T.H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Goshtasbi, N.; Yuan, J.; Jellbauer, S.; Moradi, H.; Raffatellu, M.; Kalantar-Zadeh, K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am. J. Nephrol. 2012, 36, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D.; Yuan, J.; Nazertehrani, S.; Ni, Z.; Liu, S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am. J. Nephrol. 2013, 38, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnusson, M.; Magnusson, K.E.; Sundqvist, T.; Denneberg, T. Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: Effects of low- and high-protein diets. Nephron 1990, 56, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Magnusson, K.E.; Sundqvist, T.; Denneberg, T. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 1991, 32, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.L.; Liu, S.M.; Pahlevan, S.; Yuan, J.; Khazaeli, M.; Ni, Z.; Chan, J.Y.; Vaziri, N.D. Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig. Dis. Sci. 2015, 60, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 2012, 17, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, F.; Jiang, H.; Liu, H.; Wei, M.; Wang, Z.; Xie, L. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig. Dis. Sci. 2014, 59, 2109–2117. [Google Scholar] [CrossRef]
- Feroze, U.; Kalantar-Zadeh, K.; Sterling, K.A.; Molnar, M.Z.; Noori, N.; Benner, D.; Shah, V.; Dwivedi, R.; Becker, K.; Kovesdy, C.P.; et al. Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J. Ren. Nutr. 2012, 22, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Lai, K.B.; Chung, K.Y.; Leung, C.B.; Li, P.K. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 431–436. [Google Scholar] [CrossRef]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3-4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Harrison, L.E.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Kidney Foundation (NKF). Plant-Based Diet and Kidney Health. 2019. Available online: https://wwwkidneyorg/atoz/content/plant-based (accessed on 27 June 2020).
- Wang, M.; Chou, J.; Chang, Y.; Lau, W.L.; Reddy, U.; Rhee, C.M.; Chen, J.; Hao, C.; Kalantar-Zadeh, K. The role of low protein diet in ameliorating proteinuria and deferring dialysis initiation: What is old and what is new. Panminerva Med. 2017, 59, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.A.; Kusek, J.W.; Greene, T.; Agodoa, L.Y.; Jones, C.A.; Levey, A.S.; Breyer, J.A.; Faubert, P.; Rolin, H.A.; Wang, S.R. Effects of blood pressure control on progressive renal disease in blacks and whites. Modification of Diet in Renal Disease Study Group. Hypertension 1997, 30, 428–435. [Google Scholar] [CrossRef]
- Kramer, H.; Jimenez, E.Y.; Brommage, D.; Vassalotti, J.; Montgomery, E.; Steiber, A.; Schofield, M. Medical Nutrition Therapy for Patients with Non-Dialysis-Dependent Chronic Kidney Disease: Barriers and Solutions. J. Acad. Nutr. Diet. 2018, 118, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.; Asakura, K.; Masayasu, S.; Sasaki, S. Relationship of nutrition knowledge and self-reported dietary behaviors with urinary excretion of sodium and potassium: Comparison between dietitians and nondietitians. Nutr. Res. 2016, 36, 440–451. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W. Impact of Nutrition and Diet on COVID-19 Infection and Implications for Kidney Health and Kidney Disease Management. J. Ren. Nutr. 2020, 30, 179–181. [Google Scholar] [CrossRef]
- Kelly, J.T.; Campbell, K.L.; Hoffmann, T.; Reidlinger, D.P. Patient Experiences of Dietary Management in Chronic Kidney Disease: A Focus Group Study. J. Ren. Nutr. 2018, 28, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs. Chronic Kidney Disease Prevention, Early Recognition, and Management, Veterans Health Administration Transmittal Sheet. 2020. Available online: https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737 (accessed on 27 June 2020).
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Kovesdy, C.P.; Bross, R.; Lee, M.; Oreopoulos, A.; Benner, D.; Mehrotra, R.; Kopple, J.D.; Kalantar-Zadeh, K. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am. J. Kidney Dis. 2011, 57, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori, N.; Kopple, J.D.; Kovesdy, C.P.; Feroze, U.; Sim, J.J.; Murali, S.B.; Luna, A.; Gomez, M.; Luna, C.; Bross, R.; et al. Mid-Arm Muscle Circumference and Quality of Life and Survival in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 2258–2268. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Dunne, E.; Nixon, K.; Kahn, K.; Lee, G.H.; Kleiner, M.; Luft, F.C. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol. Dial. Transplant. 1999, 14, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Block, G.; Kelly, M.P.; Schroepfer, C.; Rodriguez, R.A.; Humphreys, M.H. Near infra-red interactance for longitudinal assessment of nutrition in dialysis patients. J. Ren. Nutr. 2001, 11, 23–31. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kuwae, N.; Wu, D.Y.; Shantouf, R.S.; Fouque, D.; Anker, S.D.; Block, G.; Kopple, J.D. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am. J. Clin. Nutr. 2006, 83, 202–210. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Rambod, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Malnutrition-Inflammation Score for risk stratification of patients with CKD: Is it the promised gold standard? Nat. Clin. Pract. Nephrol. 2008, 4, 354–355. [Google Scholar] [CrossRef]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Molnar, M.Z.; Keszei, A.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Haromszeki, B.; Kosa, J.P.; Lakatos, P.; Sarvary, E.; Beko, G.; et al. Evaluation of the malnutrition-inflammation score in kidney transplant recipients. Am. J. Kidney Dis. 2010, 56, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Bross, R.; Chandramohan, G.; Kovesdy, C.P.; Oreopoulos, A.; Noori, N.; Golden, S.; Benner, D.; Kopple, J.D.; Kalantar-Zadeh, K. Comparing Body Composition Assessment Tests in Long-term Hemodialysis Patients. Am. J. Kidney Dis. 2010. [Google Scholar] [CrossRef] [Green Version]
- Heimburger, O.; Qureshi, A.R.; Blaner, W.S.; Berglund, L.; Stenvinkel, P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am. J. Kidney Dis. 2000, 36, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Amin, A.N. Toward more accurate detection and risk stratification of chronic kidney disease. JAMA 2012, 307, 1976–1977. [Google Scholar] [CrossRef] [PubMed]
- Genoni, A.; Lo, J.; Lyons-Wall, P.; Devine, A. Compliance, Palatability and Feasibility of PALEOLITHIC and Australian Guide to Healthy Eating Diets in Healthy Women: A 4-Week Dietary Intervention. Nutrients 2016, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.V.; Dwyer, J.T.; Larive, B.; Greene, T.; Cockram, D.B.; Chumlea, W.C.; Kusek, J.W.; Leung, J.; Burrowes, J.D.; McLeroy, S.L.; et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int. 2004, 65, 2321–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbord, S.D.; Fried, L.F.; Arnold, R.M.; Rotondi, A.J.; Fine, M.J.; Levenson, D.J.; Switzer, G.E. Development of a symptom assessment instrument for chronic hemodialysis patients: The Dialysis Symptom Index. J. Pain Symptom Manag. 2004, 27, 226–240. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Wishart, P.M.; Wasil, B.I.; Robinson, J.W. The Self-Perception and Relationships Tool (S-PRT): A novel approach to the measurement of subjective health-related quality of life. Health Qual Life Outcomes. 2004, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Bross, R.; Benner, D.; Noori, N.; Murali, S.B.; Block, T.; Norris, J.; Kopple, J.D.; Block, G. Design and development of a dialysis food frequency questionnaire. J. Ren. Nutr. 2011, 21, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, B.M.; Moore, L.W.; Benner, D.; Biruete, A.; Boaz, M.; Brunori, G.; Chen, J.; Drechsler, C.; Guebre-Egziabher, F.; Hensley, M.K.; et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI Clinical Practice Guideline for Nutrition in Chronic Kidney Disease. J. Ren. Nutr. 2020, in press. [Google Scholar]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgoni, V., 3rd; Drewnowski, A. An Economic Gap Between the Recommended Healthy Food Patterns and Existing Diets of Minority Groups in the US National Health and Nutrition Examination Survey 2013-2014. Front. Nutr. 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Study (Year) | Cohort, [N] (Country) | Duration Of Follow Up | Findings |
|---|---|---|---|
| Esmeijer [22] (2020) | Alpha Omega Cohort (2255) (Netherlands) | 41 mo | ↑ DPI 0.1 g/kg/day associated with ↑ eGFR decline of −0.12 ml/min/year |
| Jhee [23] (2020) | South Korea (9226) | 14 yrs | 3.5-fold ↑ risk of hyperfiltration. 1.3-fold ↑ faster decline |
| Malhotra [24] (2018) | Jackson Heart (USA) (5301) | 8 yrs | ↑ DPI density associated with ↑ eGFR decline |
| Farhadnejad [24] (2018) | Healthy Iranian adults (1797) | 6.1 yrs | 48% ↑ risk of incident CKD in high DPI |
| Study (Year) | Participants | Diet (g/kg/day) | Duration of Follow Up | Results |
|---|---|---|---|---|
| Rosman (1984) [35,36]. | 247 CKD 3–5 pts | 0.90–0.95 vs. 0.70–0.80 vs. unrestricted | 4 yrs | Significant CKD slowing in LPD in male pts. |
| Ihle (1989) [37] | 72 CKD 4–5 pts | LPD (0.6) vs. higher DPI (0.8) | 18 mo | Loss of GFR in control vs. LPD (p < 0.05). Wt loss |
| Lindenau (1990) [38] | 40 CKD 5 pts | LPD vs. sVLPD (0.4) w KA | 12 mo | Decreased phos. with sVLPD and improved bone health |
| Williams (1991) [39] | 95 CKD 4–5 | LPD (0.7) vs. 1.02–1.14 | 18 mo | No differences, minor Wt loss |
| Locatelli (1991) [40] | 456 CKD 3–4 | 0.78 vs. 0.9 | 2 yrs | Trend for difference in renal outcomes (p = 0.059). |
| MDRD Klahr (1994) [41] | 585 CKD 3–4 | 1.3 vs. 0.6 | 27 mo | No difference in GFR decline at 3 years. |
| Montes-Delgado (1998) [42] | 33 CKD 3–5 | LPD vs. sLPD | 6 mo | Slower eGFR decline with supplements |
| Malvy (1999) [43] | 50 CKD 4–5 | sVLPD (0.3) KA vs. LPD (0.65) | 3 yrs | Decreased SUN lean body mass and fat in sVLPD |
| Teplan (2001) [44] | 105 CKD 3b–4 | LPD w vs. w/o KA | 3 yrs | Slower CKD progression |
| Prakash (2004) [45] | 34 CKD 3b–4 | 0.6 vs. 0.3 w KA | 9 mo | Faster decline in LPD |
| Brunori (2007) [46] | 56 > 70 yrs old CKD 5 | sVLPD (0.30) w KA vs. dialysis | 27 mo | Similar survival but more hospitalizations in dialysis |
| Mircescu (2007) [47] | 53 CKD 4–5 | sVLPD (0.3) vegan w KA vs. LPD | 48 wks | Less dialysis initiation in sVLDP |
| Cianciaruso (2008) [48] | 423 CKD 4–5 | 0.55 vs. 0.80 | 18 mo | Reduced urinary urea, Na, phos |
| Di Iorio (2009) [49] | 32 CKD w proteinuria | VLPD vs. LPD | 6 mo | 58% greater reduction in proteinuria |
| Jiang (2009 and 2011) [50,51]. | 60 PD w RKF | LPD vs. sLPD w KA vs. HPD | 12 mo | RKF decreased in the LPD and HPD. |
| Garneata (2016) [52] | 207 CKD 4–5 | LPD (0.6) vs. sVLPD w KA | 15 mo | Less dialysis initiation |
| Benefits of LPD with >50% Plant Sources | Potential Challenges of LPD |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
|
| Protein Metric | Standard Diet | LPD >50% Plant-based Sources (PLADO) |
|---|---|---|
| Proportion of plant-based protein, % | 20–30% | 50–70% * |
| Total protein per kg IBW, g/kg/day | >0.8, usually 1.2–1.4 | 0.6–0.8 |
| Total protein intake, g/day | 96 to 112 g | 48 to 64 g |
| Protein density, g/100 Cal | 4.4–5.1 | 2.2–2.9 |
| Proportion of energy from protein, % | 16–19% | 8–11% |
| Total plant-based protein, g/day | 24–34 | 24–45 |
| Total animal-based protein, g/day | 68–83 | 14–32 (or none *) |
| Timeline of for PLADO Therapy Visits | “Run-in” Period | Year 1 (Quarterly) | Years 2+ (Semi-Annual) | Needed Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PALDO Months | 0 | 1 | 3 | 6 | 9 | 12 | 18 | 24 | 30 | 36 | ||
| History and physical examination with updates on clinical and dietary status | X | x | x | x | x | X | x | x | X | x | 10–20 min | |
| Lab tests | Routine lab panel: CMP/LFT, anemia, MBD, A1c | X | x | x | x | X | x | x | X | x | <10 min | |
| Spot urine, urinalysis, protein, albumin, creatinine | X | x | x | x | X | x | x | X | x | <5 min | ||
| 24 hr urine: Nitrogen, Na, K, creatinine, alb, prot. | X | x | x | x | X | x | x | X | x | Collected at home | ||
| eGFR assessment and creatinine and urea clearance | X | x | x | x | X | x | x | X | x | |||
| Dietitian visit | Dietary education for LPD >50% plant based | X | x | x | x | x | X | x | x | X | X | 10–20 min |
| Dietary assessment, three-day diet diary with interview | X | x | x | x | x | X | x | x | X | X | 10–20 min | |
| Anthropometry: triceps and biceps skinfolds, mid-arm circumference * | X | x | x | x | X | x | x | X | X | 2–4 min | ||
| Body fat estimation * | X | x | x | x | X | x | x | X | X | 1–2 min | ||
| Malnutrition-inflammation score * | X | x | x | x | X | x | x | X | x | 2–5 min | ||
| Handgrip strength test * | X | x | x | x | X | x | x | X | x | 1–2 min | ||
| Phone calls to reinforce PLADO education, adherence, and meal preparation | x | x | x | x | x | X | x | x | X | x | 10–30 min | |
| Questionnaires | Diet palatability and appetite questionnaire | x | x | x | X | x | X | x | x | X | x | 15–30 min |
| Food Frequency Questionnaire * | x | x | X | x | x | X | x | 15–30 min | ||||
| Quality of life: KDQOL™ including SF36 quest * | x | x | x | x | X | x | x | X | x | 10–15 min | ||
| Uremic symptoms questionnaire | x | x | x | x | X | x | x | X | x | 10–15 min | ||
| Self-Perception and Relationship Questionnaire * | x | x | x | x | X | x | x | X | x | 10–15 min | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. https://doi.org/10.3390/nu12071931
Kalantar-Zadeh K, Joshi S, Schlueter R, Cooke J, Brown-Tortorici A, Donnelly M, Schulman S, Lau W-L, Rhee CM, Streja E, et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients. 2020; 12(7):1931. https://doi.org/10.3390/nu12071931
Chicago/Turabian StyleKalantar-Zadeh, Kamyar, Shivam Joshi, Rebecca Schlueter, Joanne Cooke, Amanda Brown-Tortorici, Meghan Donnelly, Sherry Schulman, Wei-Ling Lau, Connie M. Rhee, Elani Streja, and et al. 2020. "Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease" Nutrients 12, no. 7: 1931. https://doi.org/10.3390/nu12071931
APA StyleKalantar-Zadeh, K., Joshi, S., Schlueter, R., Cooke, J., Brown-Tortorici, A., Donnelly, M., Schulman, S., Lau, W. -L., Rhee, C. M., Streja, E., Tantisattamo, E., Ferrey, A. J., Hanna, R., Chen, J. L. T., Malik, S., Nguyen, D. V., Crowley, S. T., & Kovesdy, C. P. (2020). Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients, 12(7), 1931. https://doi.org/10.3390/nu12071931